Predictors of Atrial Fibrillation in Heart Failure Patients with Indications for ICD Implantation
Abstract
1. Introduction
2. Materials and Methods
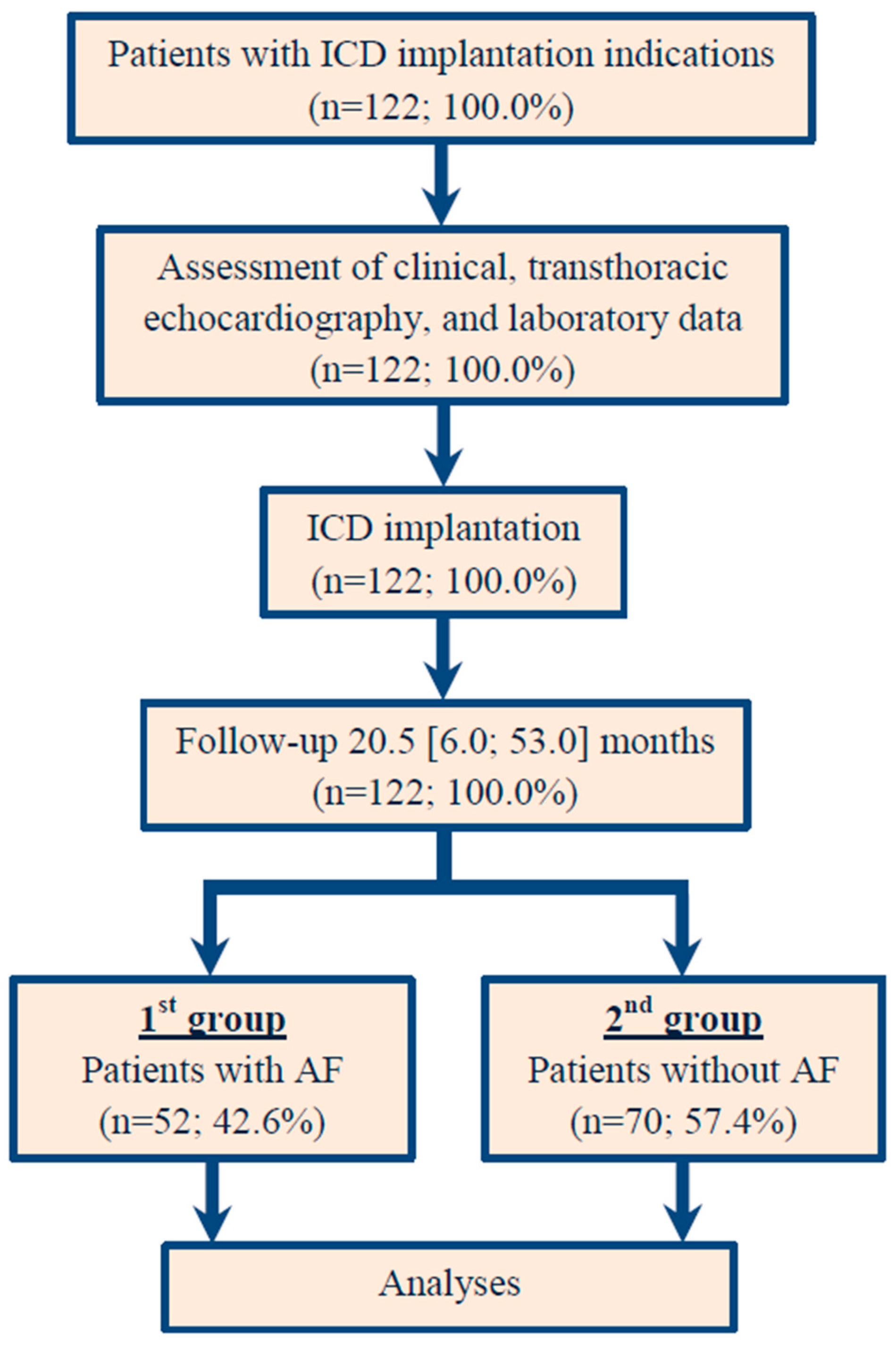
2.1. Study Population
2.2. Ethical Aspects
2.3. Clinical Assessment
2.4. ICD Implantation and Programming
2.5. Clinical Assessment, End Point, and Design of the Study
2.6. Statistical Analysis, Risk Stratification, and Score Development
3. Results
3.1. Patient Baseline Demographic and Clinical Data
3.2. Patient Baseline Echocardiographic Characteristics
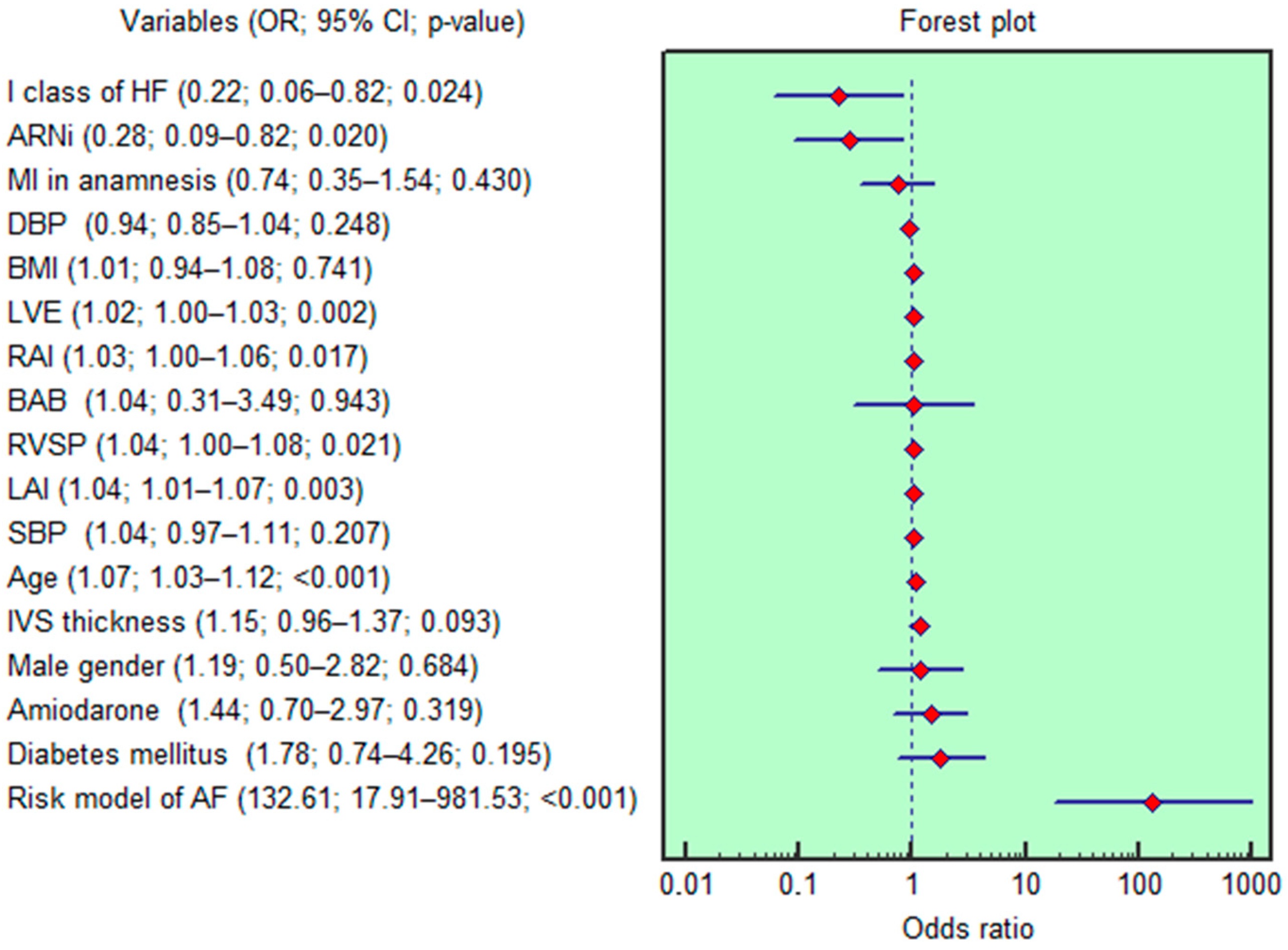
3.3. Analysis for AF Risk Stratification
3.4. Development of the AF Risk Score
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 6MWT | Six Minute Walk Test |
| AF | Atrial Fibrillation |
| ARNi | Angiotensin Receptor Neprilysin inhibitor |
| AUC | Area Under the ROC Curve |
| CI | Confidence Interval |
| ECG | Electrocardiography |
| FC | Functional Class |
| HF | Heart Failure |
| ICD | Implantable Cardiac–Defibrillator |
| LAI | Left Atrial Index |
| LAV | Left Atrial Volume |
| LVA | Left Ventricle Active Filling Rate |
| LVE | Left Ventricle Early Diastolic Filling Rate |
| LVEF | Left Ventricle Ejection Fraction |
| M | Mean |
| Me | Median |
| MI | Myocardial Infarction |
| NYHA | New York Heart Association |
| OR | Odds ratio |
| RAI | Right Atrial Index |
| RAV | Right Atrial Volume |
| RV | Right Ventricle |
| RVSP | Right Ventricle Systolic Pressure |
| SD | Standard Deviation |
| TE | Transthoracic Echocardiography |
| VT | Ventricular Tachycardia |
References
- Kroshian, G.; Joseph, J.; Kinlay, S.; Peralta, A.O.; Hoffmeister, P.S.; Singh, J.P.; Yuyun, M.F. Atrial fibrillation and risk of adverse outcomes in heart failure with reduced, mildly reduced, and preserved ejection fraction: A systematic review and meta-analysis. J. Cardiovasc. Electrophysiol. 2024, 35, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Van Rees, J.B.; Borleffs, C.J.; de Bie, M.K.; Stijnen, T.; van Erven, L.; Bax, J.J.; Schalij, M.J. Inappropriate implantable cardioverter-defibrillator shocks: Incidence, predictors, and impact on mortality. J. Am. Coll. Cardiol. 2011, 57, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, B.; Burri, H.; Buehler, A.; Reek, S.; Sticherling, C.; Schaer, B.; Linka, A.; Ammann, P.; Müller, A.S.; Dzemali, O.; et al. High incidence of inappropriate alarms in patients with wearable cardioverter-defibrillators: Findings from the Swiss WCD registry. J. Clin. Med. 2021, 10, 3811. [Google Scholar] [CrossRef] [PubMed]
- Daubert, J.P.; Zareba, W.; Cannom, D.S.; McNitt, S.; Rosero, S.Z.; Wang, P.; Schuger, C.; Steinberg, J.S.; Higgins, S.L.; Wilber, D.J.; et al. Inappropriate implantable cardioverter-defibrillator shocks in MADIT II: Frequency, mechanisms, predictors, and survival impact. J. Am. Coll. Cardiol. 2008, 51, 1357–1365. [Google Scholar] [CrossRef]
- Younis, A.; Heist, E.K.; McNitt, S.; Aktas, M.K.; Rosero, S.; Goldenberg, I.; Kutyifa, V. Predictors and outcomes of atrial tachyarrhythmia among patients with implantable defibrillators. Heart Rhythm 2020, 17, 553–559. [Google Scholar] [CrossRef]
- Blum, S.; Meyre, P.; Aeschbacher, S.; Berger, S.; Auberson, C.; Briel, M.; Osswald, S.; Conen, D. Incidence and predictors of atrial fibrillation progression: A systematic review and meta-analysis. Heart Rhythm 2019, 16, 502–510. [Google Scholar] [CrossRef]
- Rakhimova, I.R.; Khaibullin, T.N.; Kovalchuk, V.V.; Semenova, Y.M.; Abdrakhmanov, A.S. Predictors of atrial fibrillation in patients with ischemic stroke of undetermined etiology. Kardiologiia 2022, 62, 40–45. [Google Scholar] [CrossRef]
- Sama, C.; Fongwen, N.T.; Chobufo, M.D.; Hamirani, Y.S.; Mills, J.D.; Roberts, M.; Greathouse, M.; Zeb, I.; Kazienko, B.; Balla, S. A systematic review and meta-analysis of the prevalence, incidence, and predictors of atrial fibrillation in cardiac sarcoidosis. Int. J. Cardiol. 2023, 391, 131285. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: Executive summary: A report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e876–e894. [Google Scholar] [CrossRef]
- Yap, J.; Lim, F.Y.; Gao, F.; Teo, L.L.; Lam, C.S.; Yeo, K.K. Correlation of the New York Heart Association classification and the 6-min walk distance: A systematic review. Clin. Cardiol. 2015, 38, 621–628. [Google Scholar] [CrossRef]
- Douglas, P.S.; Carabello, B.A.; Lang, R.M.; Lopez, L.; Pellikka, P.A.; Picard, M.H.; Thomas, J.D.; Varghese, P.; Wang, T.Y.; Weissman, N.J.; et al. 2019 ACC/AHA/ASE key data elements and definitions for transthoracic echocardiography: A report of the American College of Cardiology/American Heart Association task force on clinical data standards (writing committee to develop cardiovascular endpoints data standards) and the American Society of Echocardiography. J. Am. Coll. Cardiol. 2019, 74, 403–469. [Google Scholar] [CrossRef]
- Stiles, M.K.; Fauchier, L.; Morillo, C.A.; Wilkoff, B.L. 2019 HRS/EHRA/APHRS/LAHRS focused update to 2015 expert consensus statement on optimal implantable cardioverter-defibrillator programming and testing. Heart Rhythm 2020, 17, e220–e228. [Google Scholar] [CrossRef] [PubMed]
- Potpara, T.; Romiti, G.F.; Sohns, C. The 2024 European Society of Cardiology guidelines for diagnosis and management of atrial fibrillation: A viewpoint from a practicing clinician’s perspective. Thromb Haemost. 2024, 124, 1087–1094. [Google Scholar] [CrossRef] [PubMed]
- Van Gelder, I.C.; Rienstra, M.; Bunting, K.V.; Casado-Arroyo, R.; Caso, V.; Crijns, H.J.G.M.; De Potter, T.J.R.; Dwight, J.; Guasti, L.; Hanke, T.; et al. 2024 ESC Guidelines for the management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2024, 45, 3314–3414. [Google Scholar] [CrossRef] [PubMed]
- Fauchier, L.; Laborie, G.; Clementy, N.; Babuty, D. Beta-blockers or digoxin for atrial fibrillation and heart failure? Card. Fail. Rev. 2016, 2, 35–39. [Google Scholar] [CrossRef]
- Aronson, D.; Shalev, V.; Katz, R.; Chodick, G.; Mutlak, D. Risk score for prediction of 10-year atrial fibrillation: A community-based study. Thromb. Haemost. 2018, 118, 1556–1563. [Google Scholar] [CrossRef]
- Nagarakanti, R.; Ezekowitz, M. Diastolic dysfunction and atrial fibrillation. J. Interv. Card. Electrophysiol. 2008, 22, 111–118. [Google Scholar] [CrossRef]
- Tsang, T.S.; Gersh, B.J.; Appleton, C.P.; Tajik, A.J.; Barnes, M.E.; Bailey, K.R.; Oh, J.K.; Leibson, C.; Montgomery, S.C.; Seward, J.B. Left ventricular diastolic dysfunction as a predictor of the first diagnosed nonvalvular atrial fibrillation in 840 elderly men and women. J. Am. Coll. Cardiol. 2002, 40, 1636–1644. [Google Scholar] [CrossRef]
- Liao, Y.C.; Liao, J.N.; Lo, L.W.; Lin, Y.J.; Chang, S.L.; Hu, Y.F.; Chao, T.F.; Chung, F.P.; Tuan, T.C.; Te, A.L.; et al. Left atrial size and left ventricular end-systolic dimension predict the progression of paroxysmal atrial fibrillation after catheter ablation. J. Cardiovasc. Electrophysiol. 2017, 28, 23–30. [Google Scholar] [CrossRef]
- Kang, M.K.; Joung, B.; Shim, C.Y.; Cho, I.J.; Yang, W.I.; Moon, J.; Jang, Y.; Chung, N.; Chang, B.C.; Ha, J.W. Post-operative left atrial volume index is a predictor of the occurrence of permanent atrial fibrillation after mitral valve surgery in patients who undergo mitral valve surgery. Cardiovasc. Ultrasound 2018, 16, 5. [Google Scholar] [CrossRef]
- Caputo, M.; Urselli, R.; Capati, E.; Navarri, R.; Sinesi, L.; Furiozzi, F.; Ballo, P.; Palazzuoli, A.; Favilli, R.; Mondillo, S. Usefulness of left ventricular diastolic dysfunction assessed by pulsed tissue Doppler imaging as a predictor of atrial fibrillation recurrence after successful electrical cardioversion. Am. J. Cardiol. 2011, 108, 698–704. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.I.; Shim, C.Y.; Kim, Y.J.; Kim, S.A.; Rhee, S.J.; Choi, E.Y.; Choi, D.; Jang, Y.; Chung, N.; Cho, S.Y.; et al. Left atrial volume index: A predictor of adverse outcome in patients with hypertrophic cardiomyopathy. J. Am. Soc. Echocardiogr. 2009, 22, 1338–1343. [Google Scholar] [CrossRef] [PubMed]
- Freedenberg, V.; Thomas, S.A.; Friedmann, E. Anxiety and depression in implanted cardioverter-defibrillator recipients and heart failure: A review. Heart Fail. Clin. 2011, 7, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Tateishi, R.; Suzuki, M.; Shimizu, M.; Shimada, H.; Tsunoda, T.; Miyazaki, H.; Misu, Y.; Yamakami, Y.; Yamaguchi, M.; Kato, N.; et al. Risk prediction of inappropriate implantable cardioverter-defibrillator therapy using machine learning. Sci. Rep. 2023, 13, 19586. [Google Scholar] [CrossRef]
- Slyngstad, T.; Huth Ruwald, A.C.; Kutyifa, V.; McNitt, S.; Polonsky, B.; Solomon, S.D.; Foster, E.; Goldenberg, I.; Wang, P.J.; Klein, H.; et al. Cardiac resynchronization therapy is associated with reductions in left atrial volume and inappropriate implantable cardioverter-defibrillator therapy in MADIT-CRT. Heart Rhythm 2014, 11, 1001–1007. [Google Scholar] [CrossRef]
- Lebedeva, V.K.; Levinova, O.E. Analysis of electrotherapy in patients with implantable cardioverter-defibrillator for primary prevention of sudden cardiac death according to remote monitoring data. Sib. J. Clin. Exp. Med. 2023, 38, 106–115. (In Russian) [Google Scholar] [CrossRef]


| Demographic and Clinical Characteristics | Overall Population (n = 122) | 1st Group Pts with AF (n = 52) | 2nd Group Pts Without AF (n = 70) | p 2–3 |
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| Age, year, Me [Q1; Q3] | 64.0 [58.0; 71.0] | 69.0 [62.0; 75.5] | 60.0 [56.0; 67.0] | <0.001 |
| Male gender, n (%) | 94 (77.0) | 41 (78.8) | 53 (75.7) | 0.687 |
| Myocardial infarction in anamnesis, n (%) | 73 (59.8) | 29 (55.7) | 44 (62.8) | 0.433 |
| Coronary artery stenting in anamnesis, n (%) | 48 (39.3) | 25 (48.1) | 23 (32.8) | 0.091 |
| Systolic blood pressure, mmHg, M ± SD | 120.7 ± 5.7 | 121.5 ± 4.6 | 120.2 ± 6.4 | 0.255 |
| Diastolic blood pressure, mmHg, M ± SD | 75.0 ± 3.7 | 74.5 ± 3.7 | 75.3 ± 3.6 | 0.237 |
| Baseline body mass index, kg/m2, M ± SD | 28.9 ± 5.1 | 29.1 ± 5.1 | 28.7 ± 5.2 | 0.954 |
| Baseline eGFR, mL/min/1.73 m2, M ± SD | 70.9 ± 19.2 | 67.1 ± 17.9 | 73.8 ± 19.8 | 0.109 |
| Baseline New York Heart Association class: | ||||
| I, n (%) | 18 (14.7) | 3 (5.7) | 15 (21.4) | 0.016 |
| II, n (%) | 66 (54.1) | 30 (57.7) | 36 (51.4) | 0.496 |
| III, n (%) | 38 (31.1) | 19 (36.5) | 19 (27.1) | 0.271 |
| Baseline 6 min walk test, m, M ± SD | 353.9 ± 85.3 | 336.9 ± 78.1 | 366.5 ± 88.8 | 0.079 |
| Bundle branch blocks and arrhythmias registered prior to ICD implantation: | ||||
| Left bundle branch block, n (%) | 37 (30.3) | 11 (21.1) | 26 (37.1) | 0.058 |
| Right bundle branch block, n (%) | 4 (3.3) | 1 (1.9) | 3 (4.3) | 0.475 |
| Sustained ventricular tachycardia, n (%) | 70 (56.6) | 35 (67.3) | 35 (50.0) | 0.057 |
| Ventricular fibrillation, n (%) | 6 (4.9) | 1 (1.9) | 5 (7.1) | 0.191 |
| ICD implantation indications: | ||||
| Primary prevention of SCD, n (%) | 52 (42.6) | 17 (32.7) | 35 (50.0) | 0.057 |
| Secondary prevention of SCD, n (%) | 70 (57.4) | 35 (67.3) | 35 (50.0) | 0.057 |
| Comorbidities: | ||||
| Diabetes mellitus, n (%) | 26 (21.3) | 14 (26.9) | 12 (17.1) | 0.195 |
| Dyslipidemia, n (%) | 61 (50.0) | 27 (51.9) | 34 (48.6) | 0.717 |
| Chronic obstructive pulmonary disease, n (%) | 16 (13.1) | 8 (15.4) | 8 (11.4) | 0.526 |
| Stroke, n (%) | 3 (2.4) | 1 (1.9) | 2 (2.8) | 0.750 |
| Baseline electrocardiographic findings: | ||||
| Corrected QT interval, ms, M ± SD | 425.3 ± 33.0 | 431.7 ± 34.5 | 420.5 ± 31.2 | 0.109 |
| QRS duration, ms, M ± SD | 123.7 ± 36.1 | 116.5 ± 28.8 | 129.1 ± 40.0 | 0.156 |
| Baseline therapy: | ||||
| Beta-adrenoblockers, n (%) | 106 (86.9) | 44 (84.6) | 62 (88.6) | 0.947 |
| Loop diuretics, n (%) | 50 (41.0) | 24 (46.1) | 26 (37.1) | 0.320 |
| Mineralocorticoid receptor antagonists, n (%) | 79 (64.7) | 36 (69.2) | 43 (61.4) | 0.376 |
| Angiotensin-converting enzyme inhibitors, n (%) | 72 (59.0) | 34 (65.4) | 38 (54.3) | 0.092 |
| Antiplatelet agents, n (%) | 72 (59.0) | 16 (30.8) | 56 (80.0) | <0.001 |
| Lipid-lowering treatment, n (%) | 107 (87.7) | 46 (88.5) | 61 (87.1) | 0.831 |
| Angiotensin II receptor blocker, n (%) | 24 (19.7) | 12 (23.1) | 12 (17.1) | 0.419 |
| Angiotensin receptor neprilysin inhibitors, n (%) | 24 (19.7) | 5 (4.1) | 19 (15.6) | 0.016 |
| Amiodarone, n (%) | 64 (52.4) | 30 (57.7) | 34 (48.6) | 0.322 |
| Sotalol, n (%) | 4 (3.3) | 3 (5.7) | 1 (1.4) | 0.187 |
| Anticoagulants, n (%) | 52 (42.6) | 45 (86.5) | 7 (10.0) | <0.001 |
| Hypoglycemic drugs, n (%) | 16 (13.1) | 9 (17.3) | 7 (10.0) | 0.241 |
| Sodium glucose co-transporter 2 inhibitors, n (%) | 30 (24.6) | 9 (17.3) | 21 (30.0) | 0.109 |
| Indicators | Overall Population (n = 122) | 1st Group Pts with AF (n = 52) | 2nd Group Pts Without AF (n = 70) | p 2–3 |
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| RAI, mL/m2 | 39.4 [34.1; 45.7] | 40.9 [35.8; 50.2] | 37.9 [31.2; 43.1] | 0.019 |
| LAI, mL/m2 | 52.4 [45.2; 59.7] | 55.3 [49.3; 66.1] | 50.1 [40.6; 55.9] | 0.002 |
| LVEDI, mL/m2 | 85.9 [66.1; 118.6] | 83.7 [65.8; 111.1] | 86.7 [66.3; 124.9] | 0.581 |
| LVESI, mL/m2 | 51.5 [30.1; 77.5] | 49.0 [30.2; 69.9] | 55.0 [30.1; 87.8] | 0.499 |
| LVEF, % | 40.0 [32.0; 55.0] | 42.0 [32.5; 56.0] | 40.0 [31.0; 53.0] | 0.711 |
| LVEDD, mm | 60.5 [52.0; 67.0] | 60.5 [51.5; 67.5] | 60.5 [53.0; 66.0] | 0.831 |
| LVESD, mm | 48.7 [38.0; 57.0] | 48.2 [38.0; 56.5] | 49.5 [38.0; 57.0] | 0.781 |
| LVEDV, mL | 175.5 [128.0; 223.0] | 171.0 [127.0; 219.0] | 178.0 [130.0; 225.0] | 0.541 |
| LVESV, mL | 105.0 [59.0; 150.0] | 104.5 [59.5; 143.5] | 111.0 [58.0; 154.0] | 0.510 |
| SV, mL | 70.0 [59.0; 80.0] | 69.0 [59.0; 79.5] | 70.5 [59.0; 80.0] | 0.652 |
| MMI, g/m2 | 115.5 [99.0; 138.0] | 116.0 [104.0; 142.0] | 115.0 [97.0; 137.0] | 0.294 |
| CIB, L/min/m2 | 2.2 [1.8; 2.5] | 2.1 [1.8; 2.6] | 2.2 [1.9; 2.5] | 0.688 |
| LA, mm | 45.0 [42.0; 50.0] | 46.5 [43.0; 51.5] | 45.0 [41.0; 48.0] | 0.031 |
| RV, mm | 25.0 [22.0; 27.0] | 25.5 [23.0; 28.0] | 24.0 [22.0; 26.0] | 0.016 |
| RAV, mL | 82.7 [67.3; 94.2] | 85.5 [72.5; 102.3] | 79.7 [65.4; 90.4] | 0.028 |
| LAV, mL | 108.6 [92.0; 123.1] | 113.7 [99.4; 129.9] | 104.6 [86.1; 117.5] | 0.011 |
| LVSI | 0.60 [0.55; 0.67] | 0.61 [0.56; 0.66] | 0.60 [0.54; 0.68] | 0.995 |
| LVE, cm/s | 65.5 [50.0; 85.0] | 74.5 [54.0; 97.5] | 58.5 [48.0; 74.0] | 0.006 |
| LVA, cm/s | 68.5 [57.0; 81.0] | 68.0 [55.0; 82.0] | 69.5 [60.0; 80.0] | 0.663 |
| LVE/LVA | 0.90 [0.67; 1.42] | 1.19 [0.69; 1.75] | 0.79 [0.65; 1.18] | 0.028 |
| IVS, mm | 10.5 [9.0; 12.0] | 11.0 [10.0; 12.0] | 10.0 [9.0; 11.5] | 0.016 |
| LVPW, mm | 10.0 [9.0; 11.0] | 10.5 [9.5; 11.1] | 10.0 [9.0; 11.0] | 0.295 |
| RAA, cm/m2 | 1.8 [1.7; 1.9] | 1.8 [1.7; 1.9] | 1.7 [1.6; 1.9] | 0.051 |
| RVS, cm/m2 | 1.8 [1.7; 1.9] | 1.8 [1.7; 1.9] | 1.7 [1.6; 1.9] | 0.084 |
| RVSP, mmHg | 30.0 [25.0; 36.0] | 31.5 [27.0; 42.0] | 30.0 [25.0; 33.0] | 0.017 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atabekov, T.; Batalov, R.; Archakov, E.; Silivanova, I.; Khlynin, M.; Kisteneva, I.; Krivolapov, S.; Popov, S. Predictors of Atrial Fibrillation in Heart Failure Patients with Indications for ICD Implantation. J. Clin. Med. 2025, 14, 4358. https://doi.org/10.3390/jcm14124358
Atabekov T, Batalov R, Archakov E, Silivanova I, Khlynin M, Kisteneva I, Krivolapov S, Popov S. Predictors of Atrial Fibrillation in Heart Failure Patients with Indications for ICD Implantation. Journal of Clinical Medicine. 2025; 14(12):4358. https://doi.org/10.3390/jcm14124358
Chicago/Turabian StyleAtabekov, Tariel, Roman Batalov, Evgenii Archakov, Irina Silivanova, Mikhail Khlynin, Irina Kisteneva, Sergey Krivolapov, and Sergey Popov. 2025. "Predictors of Atrial Fibrillation in Heart Failure Patients with Indications for ICD Implantation" Journal of Clinical Medicine 14, no. 12: 4358. https://doi.org/10.3390/jcm14124358
APA StyleAtabekov, T., Batalov, R., Archakov, E., Silivanova, I., Khlynin, M., Kisteneva, I., Krivolapov, S., & Popov, S. (2025). Predictors of Atrial Fibrillation in Heart Failure Patients with Indications for ICD Implantation. Journal of Clinical Medicine, 14(12), 4358. https://doi.org/10.3390/jcm14124358






