Changes of Airway Space and Flow in Patients Treated with Rapid Palatal Expander (RPE): An Observational Pilot Study with Comparison with Non-Treated Patients
Abstract
1. Introduction
- -
- correction and monitoring of bad habits contributing to the etiology:
- -
- removal of dental interferences or creation of a cusp guide, to prevent the patient from chewing on the side with the functional crossbite (usually considered for unilateral crossbites associated with functional shifts with canine guidance);
- -
- actively expansion of the contracted maxillary arch using removable or fixed appliances.
2. Materials and Methods
2.1. Study Design
2.2. Inclusion and Exclusion Criteria
- ○
- age between 8 and 12 years;
- ○
- ○
- ○
- treatment with RPE;
- ○
- complete clinical documentation, including CBCT, photographs, study models, and anamnesis forms, at baseline (T0) and at 1-year follow-up (T1);
- ○
- good oral hygiene.
- ○
- age between 8 and 12 years;
- ○
- complete clinical documentation, including CBCT scans of the facial structure and anamnesis forms, at T0 and at T1; in this group of patients, CBCT was performed for diagnosis and follow-up of juvenile idiopathic arthritis [23,24] affecting temporomandibular joint; these patients were regularly followed at the Pediatric Rheumatology Department at G.B. Rossi Hospital and at the Civile Maggiore Hospital in Verona.
- ○
- patients who had already undergone orthodontic treatment;
- ○
- history of nasal or tonsils surgery;
- ○
- genetic diseases or conditions interfering with treatment;
- ○
- craniofacial syndromes;
- ○
- cleft lip and palate;
- ○
- significant skeletal anomalies or facial asymmetries;
- ○
- severe adeno-tonsillar hypertrophy;
- ○
- bilateral cross-bite;
- ○
- post-pubertal developmental stage.
2.3. Orthodontic Treatment with RPE for Group A
2.4. CBCT Evaluation
2.5. Volumetric Measurement Protocol
2.6. Airflow Velocity Calculation Protocol
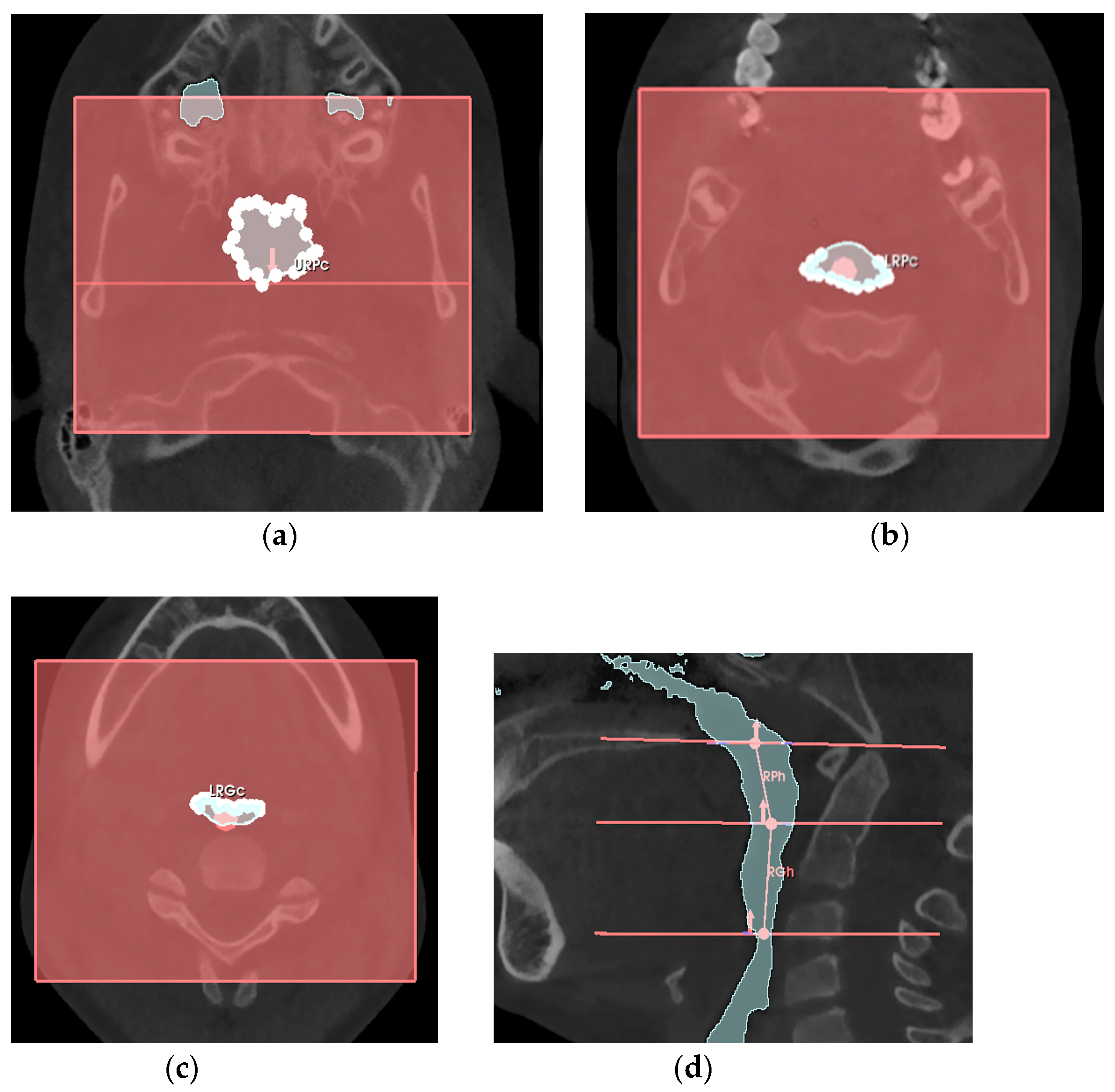
- -
- the retro-palatal volume section was modeled as a right truncated cone, F, with an upper circumference URPc (from which the diameter URPd = URPc/π was derived), a lower circumference LRPc (from which the diameter LRPd = LRPc/π was derived), and a height RPh;
- -
- the retro-glossal volume section was modeled as a right truncated cone, G, with an upper circumference LRPc (from which the diameter LRPd = LRPc/π was derived), a lower circumference LRGc (from which the diameter LRGd = LRGc/π was derived), and a height RGh.
2.7. Sample Size, Data Collection and Analysis
3. Results
- -
- group A consisted in 16 patients: 8 females and 8 males; 8 patients treated with the McNamara device and 8 with the Hyrax device, with an average age of 9.34 ± 1.3 years;
- -
- group B consisted in 8 patients: 3 females and 5 males, with an average age of 11.11 ± 1.47 years.
- -
- a statistically significant increase can be observed for volumes VN, VRP and VRG;
- -
- a statistically significant decrease can be observed for all variables related to airflow velocity.
- -
- a statistically significant increase can be observed for VN;
- -
- a statistically significant decrease can be observed for all variables related to airflow velocity;
- -
- a not significant increase can be observed for VRP and VRG.
- -
- age ranges (two age-intervals of 8–9 years and 10–12 years);
- -
- patients’ growth, considering if stage CS varied or not between T0 and T1 (from CS1 to CS2, or from CS2 to CS3), only for group A (as in this group CS was evaluated at the first visit).
- -
- between patients of 8–9 years and 10–12 years;
- -
- for group A, between patients in which CS varied or patients in which CS did not vary.
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix A.1. Orthodontic Treatment with RPE for Group A
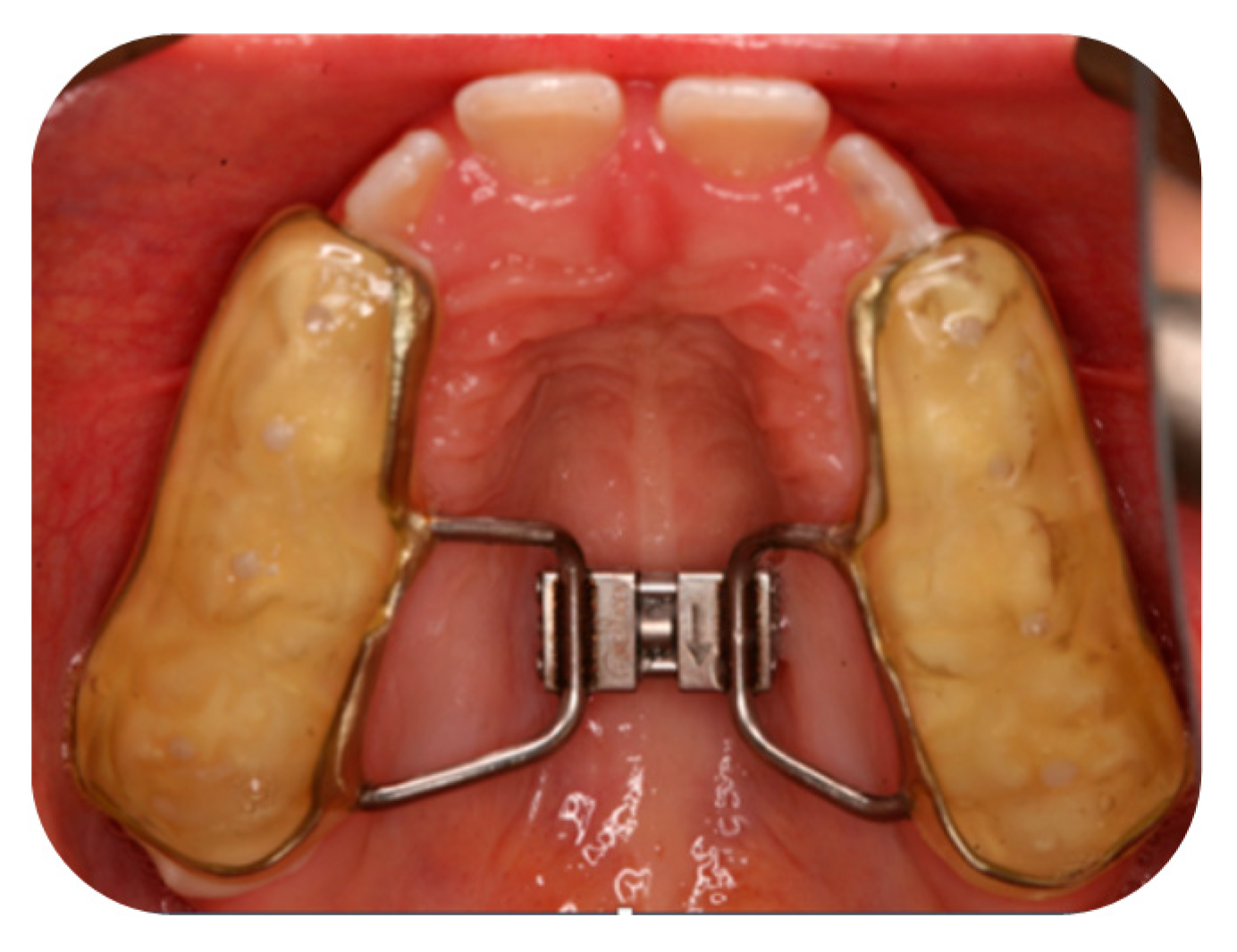

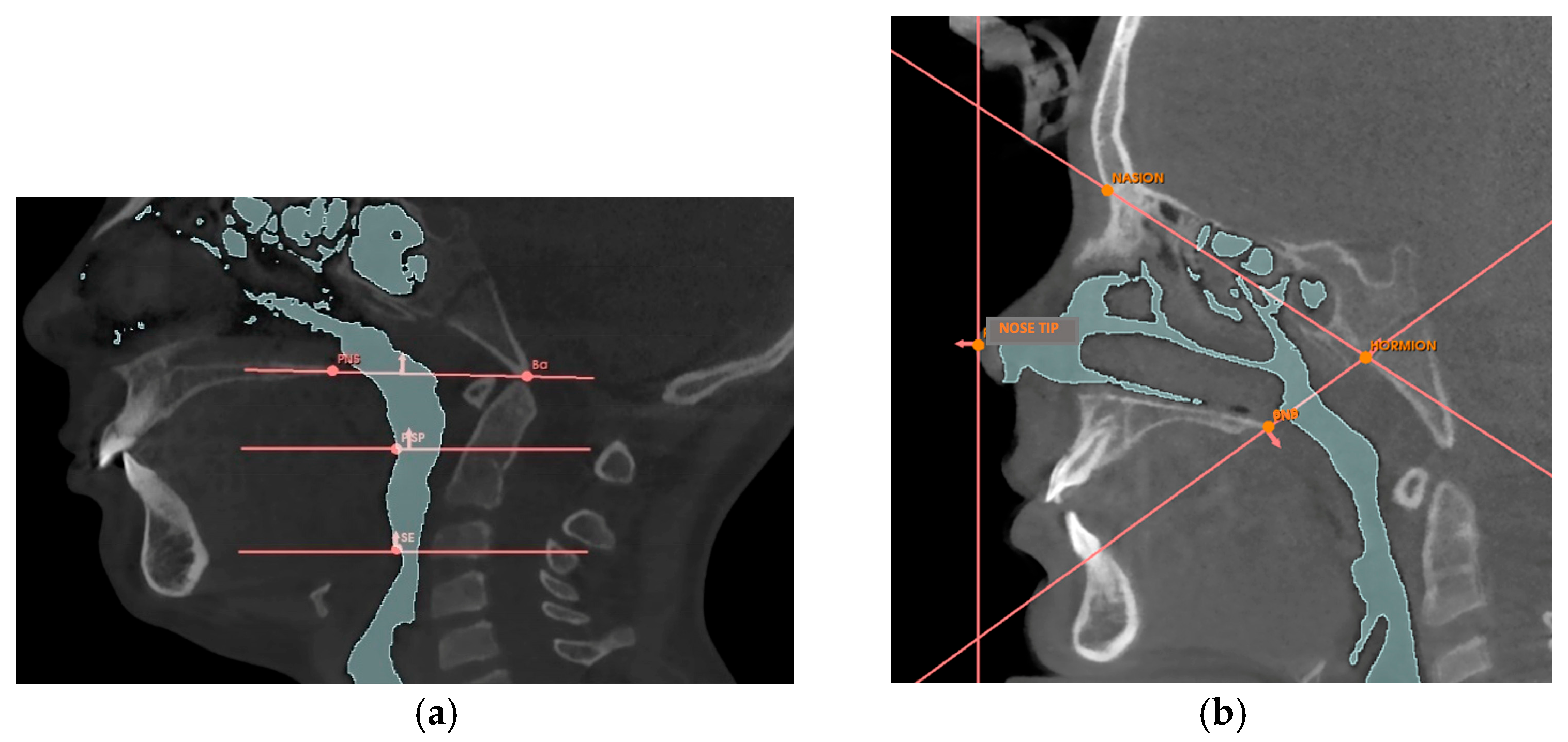
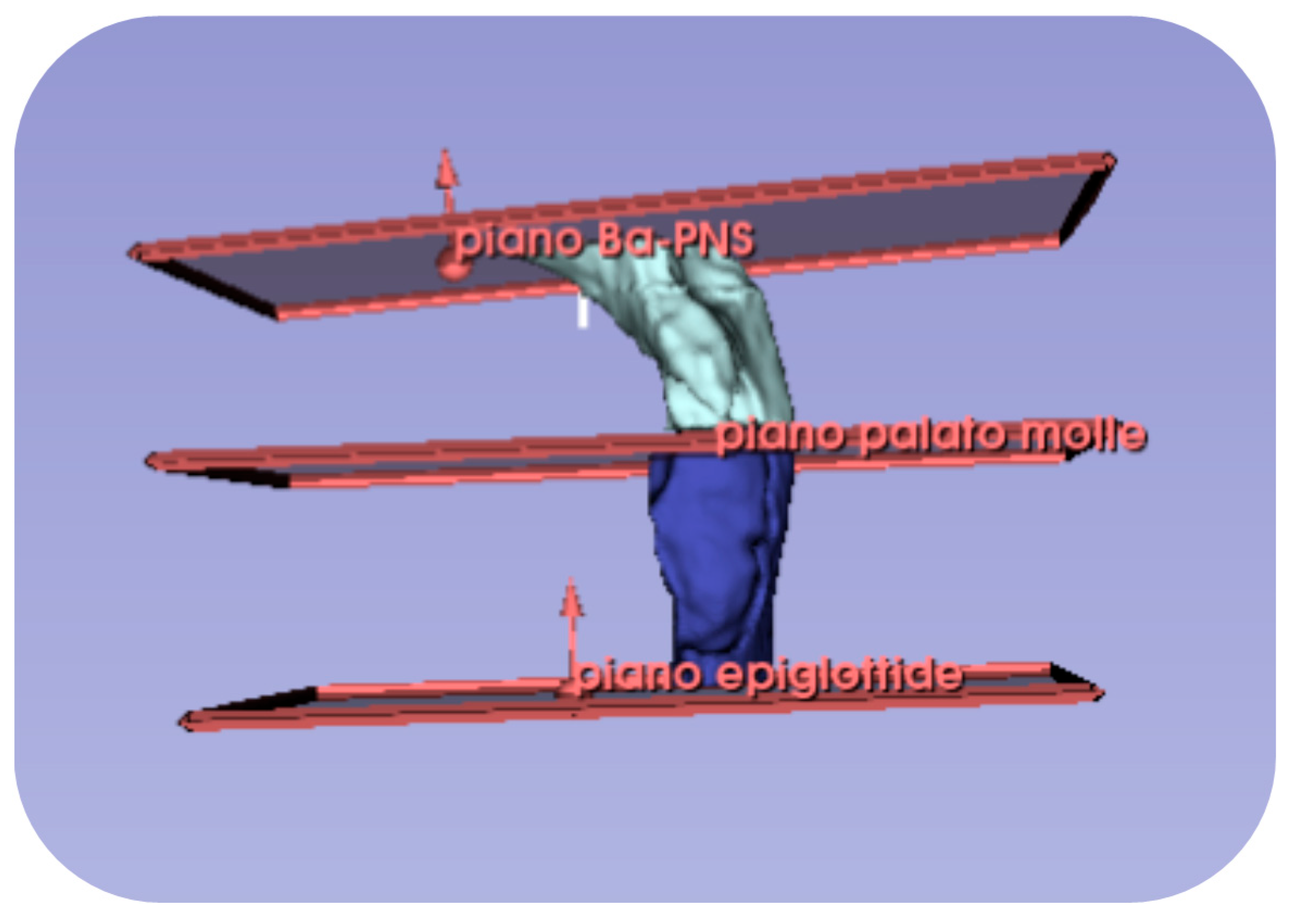
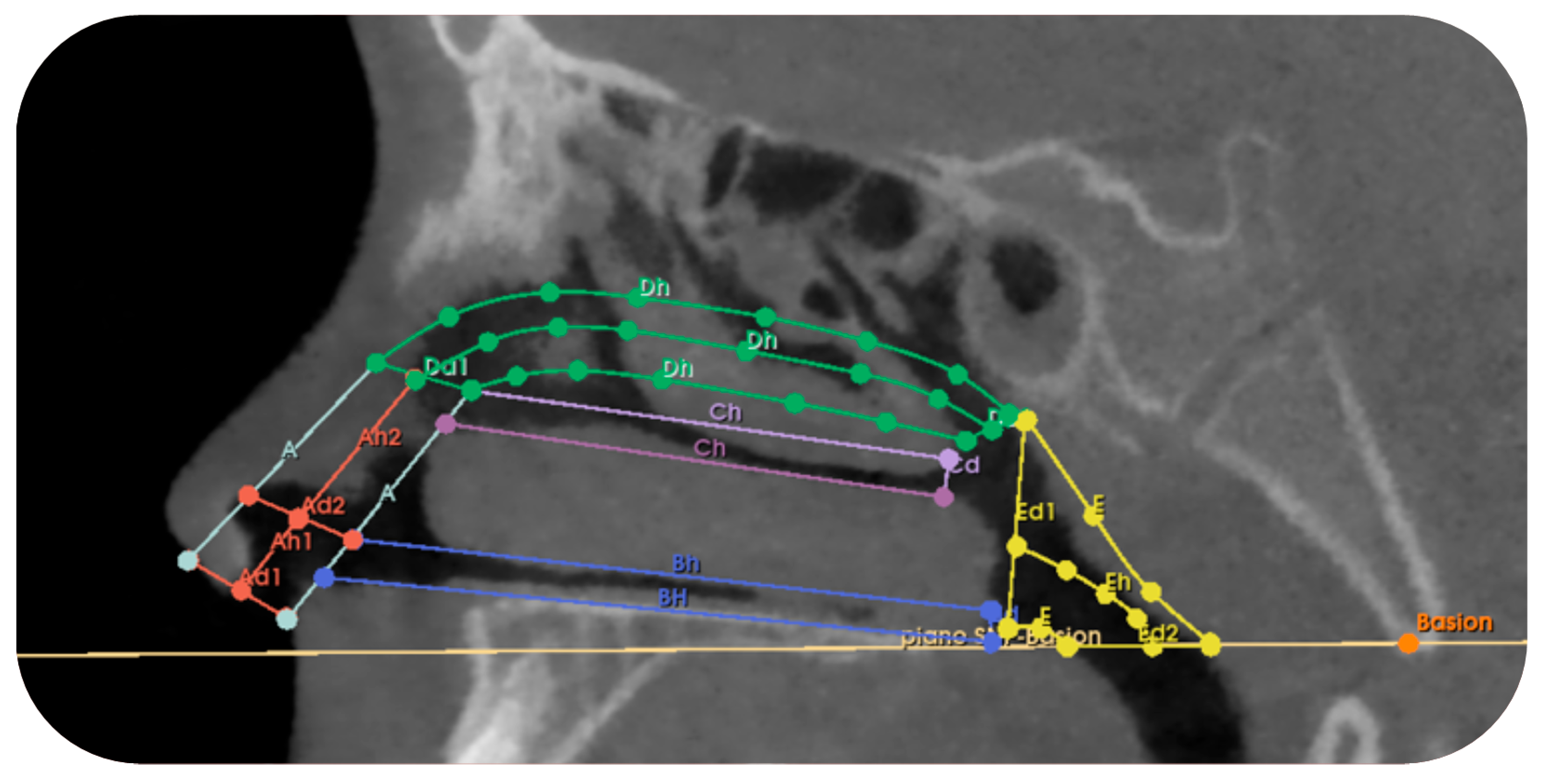
Appendix A.2. Volumetric Measurement Protocol
| Reference Point | Description |
|---|---|
| Nose tip | The most anterior point of the nose |
| PNS | Posterior nasal spine, the most posterior point of the hard palate |
| Hormion [42] | Point where the posterior border of the vomer articulates with the sphenoid bone |
| Nasion | The most anterior point of the fronto-nasal suture |
| BA | Basion, the lowest point of the anterior border of the foramen magnum |
| PISP | Posteroinferior point of the soft palate, the most posterior and inferior point of the soft palate |
| SE | Superior point of the epiglottis, the uppermost point of the epiglottis |
| Planes | Description |
|---|---|
| Nasal Perpendicular | Vertical plane passing through the tip of the nose |
| Hormion-PNS | Plane passing through the Hormion point and the PNS point |
| Hormion-Nasion | Plane passing through the Hormion point and the Nasion point |
| Ba-SPN [43] | Plane passing through the Ba point and the PNS point |
| Soft palate plane [43] | Horizontal plane passing through the PISP point |
| Epiglottis plane [43] | Horizontal plane passing through the SE point |
| Section of the Nasal Cavity | Dimensions | Section Definition |
|---|---|---|
| TRUNK-CONE A1 Left and right | Diameter 1 upper base and Diameter 1 lower base | It includes the vertical section of the nose that extends vertically from the entrance of the right/left nostril and ends at the upper margin of the inferior nasal concha. |
| Height 1 | ||
| TRUNK CONE A2 Left and Right | Diameter 2 upper base and Diameter 2 lower base | It includes the vertical section of the nose that extends from the end of section A1 to the upper margin of the middle nasal concha. |
| Height 2 | ||
| CYLINDER B Left and right | Diameter Bd | It includes the section of the nose that extends horizontally from the end of section A1 to the beginning of the choana, corresponding to the inferior nasal concha. |
| Height Bh | ||
| CYLINDER C Left and right | Diameter Cd | It includes the section that extends horizontally from the end of section A2 to the beginning of the choana, corresponding to the middle nasal concha. |
| Height Ch | ||
| TRUNK CONE D Left and Right | Diameter Dd1 | It includes the section that extends horizontally from the upper margin of section A2 to the beginning of the choana, corresponding to the superior nasal concha. |
| Diameter Dd2 | ||
| Height Dh | ||
| TRUNK CONE E | Diameter Ed1 | It includes the section that extends from the junction point of sections B-C-D to the reference plane Basion-PNS. |
| Diameter Ed2 | ||
| Height Eh |
| Variable | Definition |
|---|---|
| URPc (mm) | Upper circumference of the retro-palatal volume, measured on the axial slice where the Ba-PNS plane superiorly defines the retro-palatal volume. |
| LRPc (mm) | Lower circumference of the retro-palatal volume, measured on the axial slice where the horizontal plane of the soft palate inferiorly defines the retro-palatal volume and superiorly defines the retro-glossal volume. |
| LRGc (mm) | Lower circumference of the retro-glossal volume, measured on the axial slice where the horizontal plane of the epiglottis inferiorly defines the retro-glossal volume. |
| RPh (mm) | Height of the retro-palatal volume, the distance measured on the median sagittal slice of the retro-palatal volume from the midpoint of the upper limit defined by the Ba-PNS plane to the midpoint of the lower limit defined by the horizontal plane of the soft palate. |
| RGh (mm) | Height of the retro-glossal volume, the distance measured on the median sagittal slice of the retro-glossal volume from the midpoint of the upper limit defined by the horizontal plane of the soft palate to the midpoint of the lower limit defined by the horizontal plane of the epiglottis. |
References
- Calvo-Henriquez, C.; Capasso, R.; Chiesa-Estomba, C.; Liu, S.Y.; Martins-Neves, S.; Castedo, E.; O’Connor-Reina, C.; Ruano-Ravina, A.; Kahn, S. The role of pediatric maxillary expansion on nasal breathing. A systematic review and meta-analysis. Int. J. Pediatr. Otorhinolaryngol. 2020, 135, 110139. [Google Scholar] [CrossRef] [PubMed]
- Petré, S.; Bondemark, L.; Björk, O.; Derfeldt, S. A systematic review concerning early orthodontic treatment of unilateral posterior crossbite. Angle Orthod. 2003, 73, 588–595. [Google Scholar]
- Malandris, M.; Mahoney, E.K. Aetiology, diagnosis and treatment of posterior cross-bites in the primary dentition. Int. J. Paediatr. Dent. 2004, 14, 155–166. [Google Scholar] [CrossRef]
- Andrucioli, M.C.D.; Matsumoto, M.A.N. Transverse maxillary deficiency: Treatment alternatives in face of early skeletal maturation. Dental Press J. Orthod. 2020, 25, 70–79. [Google Scholar] [CrossRef]
- Primožič, J.; Franchi, L.; Perinetti, G.; Richmond, S.; Ovsenik, M. Influence of sucking habits and breathing pattern on palatal constriction in unilateral posterior crossbite—A controlled study. Eur. J. Orthod. 2013, 35, 706–712. [Google Scholar] [CrossRef]
- Melink, S.; Vagner, M.V.; Hocevar-Boltezar, I.; Ovsenik, M. Posterior crossbite in the deciduous dentition period, its relation with sucking habits, irregular orofacial functions, and otolaryngological findings. Am. J. Orthod. Dentofac. Orthop. 2010, 138, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Brizuela, M.; Palla, A.; N, D.K. Posterior Crossbite; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Bin Dakhil, N.; Bin Salamah, F. The diagnosis methods and management modalities of maxillary transverse discrepancy. Cureus 2021, 13, e20482. [Google Scholar] [CrossRef]
- Oliveira, N.L.; Da Silveira, A.C.; Kusnoto, B.; Viana, G. Three-dimensional assessment of morphologic changes of the maxilla: A comparison of 2 kinds of palatal expanders. Am. J. Orthod. Dentofac. Orthop. 2004, 126, 354–362. [Google Scholar] [CrossRef]
- da Silva Filho, O.G.; Prado Montes, L.A.; Torelly, L.F. Rapid maxillary expansion in the deciduous and mixed dentition evaluated through posteroanterior cephalometric analysis. Am. J. Orthod. Dentofac. Orthop. 1995, 107, 268–275. [Google Scholar] [CrossRef]
- Milad, S.A.A.; Hussein, F.A.; Mohammed, A.D.A.; Hashem, M.I. Three-dimensional assessment of transverse dentoskeletal mandibular dimensions after utilizing two designs of fixed mandibular expansion appliance: A prospective clinical investigation. Saudi J. Biol. Sci. 2020, 27, 727–735. [Google Scholar] [CrossRef]
- Franchi, L.; Baccetti, T.; Lione, R.; Fanucci, E.; Cozza, P. Modifications of midpalatal sutural density induced by rapid maxillary expansion: A low-dose computed-tomography evaluation. Am. J. Orthod. Dentofac. Orthop. 2010, 137, 486–488. [Google Scholar] [CrossRef] [PubMed]
- La Luce, M. Terapie Ortodontiche, 3rd ed.; Edra: Milan, Italy, 2015. [Google Scholar]
- Bucci, R.; D’Antò, V.; Rongo, R.; Valletta, R.; Martina, R.; Michelotti, A. Dental and skeletal effects of palatal expansion techniques: A systematic review of the current evidence from systematic reviews and meta-analyses. J. Oral. Rehabil. 2016, 43, 543–564. [Google Scholar] [CrossRef] [PubMed]
- Olmez, H.; Akin, E.; Karacay, S. Multitomographic evaluation of the dental effects of two different rapid palatal expansion appliances. Eur. J. Orthod. 2007, 29, 379–385. [Google Scholar] [CrossRef]
- Asanza, S.; Cisneros, G.J.; Nieberg, L.G. Comparison of Hyrax and bonded expansion appliances. Angle Orthod. 1997, 67, 15–22. [Google Scholar]
- Bazargani, F.; Magnuson, A.; Ludwig, B. Effects on nasal airflow and resistance using two different RME appliances: A randomized controlled trial. Eur. J. Orthod. 2018, 40, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Hershey, H.G.; Stewart, B.L.; Warren, D.W. Changes in nasal airway resistance associated with rapid maxillary expansion. Am. J. Orthod. 1976, 69, 274–284. [Google Scholar] [CrossRef]
- Compadretti, G.C.; Tasca, I.; Bonetti, G.A. Nasal airway measurements in children treated by rapid maxillary expansion. Am. J. Rhinol. 2006, 20, 385–393. [Google Scholar] [CrossRef]
- Baratieri, C.; Alves, M.; De Souza, M.M.G.; De Souza Araújo, M.T.; Maia, L.C. Does rapid maxillary expansion have long-term effects on airway dimensions and breathing? Am. J. Orthod. Dentofac. Orthop. 2011, 140, 146–156. [Google Scholar] [CrossRef]
- Baccetti, T.; Franchi, L.; McNamara, J.A., Jr. An improved version of the cervical vertebral maturation (CVM) method for the assessment of mandibular growth. Angle Orthod. 2002, 72, 316–323. [Google Scholar]
- Sandler, P.J.; Duterloo, H.S. European Board of Orthodontists: A professional challenge. J. Orthod. 2003, 30, 59–71. [Google Scholar] [CrossRef]
- Donelli, M.; Lanteri, V.; Ugolini, A.; Bruni, A.; Cressoni, P.; Abate, A.; Maspero, C. Cone-Beam Computed Tomography (CBCT) Analysis of Mandibular Condyles’ Diameters in Patients with Juvenile Idiopathic Arthritis and Temporomandibular Joint Affection: A Cross-Sectional Investigation. J. Clin. Med. 2024, 13, 5104. [Google Scholar] [CrossRef]
- Gibson, M.; Cron, R.Q.; Stoll, M.L.; Kinard, B.E.; Patterson, T.; Kau, C.H. A 3D CBCT Analysis of Airway and Cephalometric Values in Patients Diagnosed with Juvenile Idiopathic Arthritis Compared to a Control Group. Appl. Sci. 2022, 12, 4286. [Google Scholar] [CrossRef]
- Lione, R.; Huanca Ghislanzoni, L.T.; Defraia, E.; Franchi, L.; Cozza, P. Bonded versus banded rapid palatal expander followed by facial mask therapy: Analysis on digital dental casts. Eur. J. Orthod. 2016, 38, 217–222. [Google Scholar] [CrossRef]
- Faccioni, P.; Butera, A.; Bazzanella, S.; Albanese, M.; Gallo, S.; Pascadopoli, M.; Scribante, A.; Pardo, A. 3D Evaluation of Upper Airway Morphological Changes in Growing Patients with Class II Malocclusion Using Sander Bite Jumping Appliance. Appl. Sci. 2023, 13, 3908. [Google Scholar] [CrossRef]
- Teuscher, U. A growth-related concept for skeletal Class II treatment. Am. J. Orthod. 1978, 74, 258–275. [Google Scholar] [CrossRef]
- Sarver, D.M.; Johnston, M.W. Skeletal changes in vertical and anterior displacement of the maxilla with bonded rapid palatal expansion appliances. Am. J. Orthod. Dentofac. Orthop. 1989, 95, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Guest, S.S.; McNamara, J.A., Jr.; Baccetti, T.; Franchi, L. Improving Class II malocclusion as a side-effect of rapid maxillary expansion: A prospective clinical study. Am. J. Orthod. Dentofac. Orthop. 2010, 138, 582–591. [Google Scholar] [CrossRef]
- McNamara, J.A.; Sigler, L.M.; Franchi, L.; Guest, S.S.; Baccetti, T. Changes in occlusal relationships in mixed dentition patients treated with rapid maxillary expansion. Angle Orthod. 2010, 80, 230–238. [Google Scholar] [CrossRef]
- Toygar Memikoğlu, D.T.U. Effects of a bonded rapid maxillary expansion appliance during orthodontic treatment. Angle Orthod. 1999, 69, 251–256. [Google Scholar]
- Farronato, G.; Giannini, L.; Galbiati, G.; Maspero, C. Comparison of the dental and skeletal effects of two different rapid palatal expansion appliances for the correction of the maxillary asymmetric transverse discrepancies. Minerva Stomatol. 2012, 61, 45–55. [Google Scholar]
- Proffit, W.R.; Fields, H.W.; Larson, B.E.; Sarver, D.M. Ortodonzia Moderna, 6th ed.; Edra: Milano, Italy, 2020; p. 736. [Google Scholar]
- Lione, R.; Franchi, L.; Fanucci, E.; Laganá, G.; Cozza, P. Three-dimensional densitometric analysis of maxillary sutural changes induced by rapid maxillary expansion. Dentomaxillofac. Radiol. 2013, 42, 20120433. [Google Scholar] [CrossRef] [PubMed]
- Aboudara, C.; Nielsen, I.; Huang, J.C.; Maki, K.; Miller, A.J.; Hatcher, D. Comparison of airway space with conventional lateral headfilms and 3-dimensional reconstruction from cone-beam computed tomography. Am. J. Orthod. Dentofac. Orthop. 2009, 135, 468–479. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kwon, Y.H.; Park, J.H.; Baek, S.H.; Lee, K.J.; Kwon, T.G. Assessment of changes in the nasal airway after nonsurgical miniscrew-assisted rapid maxillary expansion in young adults. Angle Orthod. 2018, 88, 435–441. [Google Scholar] [CrossRef]
- Chang, Y.; Koenig, L.J.; Pruszynski, J.E.; Bradley, T.G.; Bosio, J.A.; Liu, D. Dimensional changes of upper airway after rapid maxillary expansion: A prospective cone-beam computed tomography study. Am. J. Orthod. Dentofac. Orthop. 2013, 143, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, A.; Beichel, R.; Kalpathy-Cramer, J.; Finet, J.; Fillion-Robin, J.-C.; Pujol, S.; Bauer, C.; Jennings, D.; Fennessy, F.M.; Sonka, M.; et al. 3D Slicer as an Image Computing Platform for the Quantitative Imaging Network. Magn. Reson. Imaging. Nov. 2012, 30, 1323–1341. [Google Scholar] [CrossRef] [PubMed]
- Karbowski, K.; Kopiczak, B.; Chrzan, R.; Gawlik, J.; Szaleniec, J. Accuracy of virtual rhinomanometry. Pol. J. Med. Phys. Eng. 2023, 29, 59–72. [Google Scholar] [CrossRef]
- Patil, N.; Jain, S. Rhinomanometry: A Comprehensive Review of Its Applications and Advancements in Rhinology Practice. Cureus. 2024, 16, e61370. [Google Scholar] [CrossRef]
- Kaneda, S.; Iida, M.; Yamamoto, H.; Sekine, M.; Ebisumoto, K.; Sakai, A.; Takakura, Y. Evaluation of Nasal Airflow and Resistance: Computational Modeling for Experimental Measurements. Tokai J. Exp. Clin. Med. 2019, 44, 59–67. [Google Scholar]
- Iwasaki, T.; Papageorgiou, S.N.; Yamasaki, Y.; Ali Darendeliler, M.; Papadopoulou, A.K. Nasal ventilation and rapid maxillary expansion (RME): A randomized trial. Eur. J. Orthod. 2021, 43, 283–292. [Google Scholar] [CrossRef]
- Wertz, R.A. Changes in nasal airflow incident to rapid maxillary expansion. Angle Orthod. 1968, 33, 1–11. [Google Scholar]
- Chen, S.; Wang, J.; Xi, X.; Zhao, Y.; Liu, H.; Liu, D. Rapid maxillary expansion has a beneficial effect on the ventilation in children with nasal septal deviation: A computational fluid dynamics study. Front. Pediatr. 2022, 9, 718735. [Google Scholar] [CrossRef] [PubMed]
- Fastuca, R.; Meneghel, M.; Zecca, P.A.; Mangano, F.; Antonello, M.; Nucera, R.; Caprioglio, A. Multimodal airway evaluation in growing patients after rapid maxillary expansion. Eur. J. Paediatr. Dent. 2015, 16, 129–134. [Google Scholar] [PubMed]
- Prescott, C.A.J.; Prescott, K.E. Peak nasal inspiratory flow measurement: An investigation in children. Int. J. Pediatr. Otorhinolaryngol. 1995, 32, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.; Ghoneima, A.; Stewart, K.; Liu, S.; Eckert, G.; Halum, S.; Kula, K. Three-dimensional computed tomography analysis of airway volume changes after rapid maxillary expansion. Am. J. Orthod. Dentofac. Orthop. 2012, 141, 618–626. [Google Scholar] [CrossRef]
- Zeng, J.; Gao, X. A prospective CBCT study of upper airway changes after rapid maxillary expansion. Int. J. Pediatr. Otorhinolaryngol. 2013, 77, 1805–1810. [Google Scholar] [CrossRef]
- Faccioni, P.; Gumirato, E.; Ambrosi, G.; Bazzanella, S.; Pancera, P.; Lonardi, F.; Lobbia, G.; Pardo, A.; Zangani, A.; Signoriello, A.; et al. CBCT evaluation of upper airway volumetric changes in patients treated with Hyrax-type and McNamara-type rapid maxillary expanders. J. Appl. Cosmetol. 2025, 43, 88–105. [Google Scholar] [CrossRef]
- Iwasaki, T.; Takemoto, Y.; Inada, E.; Sato, H.; Suga, H.; Saitoh, I.; Kakuno, E.; Kanomi, R.; Yamasaki, Y. The effect of rapid maxillary expansion on pharyngeal airway pressure during inspiration evaluated using computational fluid dynamics. Int. J. Pediatr. Otorhinolaryngol. 2014, 78, 1258–1264. [Google Scholar] [CrossRef]
- Di Carlo, G.; Cappabianca, S.; Cornacchia, C.; Quinzi, V.; Marzo, G.; Laganà, D. Rapid maxillary expansion and upper airway morphology: A systematic review on the role of cone beam computed tomography. Biomed. Res. Int. 2017, 2017, 5460429. [Google Scholar] [CrossRef]
- Görgülü, S.; Gokce, S.M.; Olmez, H.; Sagdic, D.; Ors, F. Nasal cavity volume changes after rapid maxillary expansion in adolescents evaluated with 3-dimensional simulation and modeling programs. Am. J. Orthod. Dentofac. Orthop. 2011, 140, 633–640. [Google Scholar] [CrossRef]
- Izuka, E.N.; Feres, M.F.N.; Pignatari, S.S.N. Immediate impact of rapid maxillary expansion on upper airway dimensions and on the quality of life of mouth breathers. Dental Press J. Orthod. 2015, 20, 43–49. [Google Scholar] [CrossRef]
- Kavand, G.; Lagravère, M.; Kula, K.; Stewart, K.; Ghoneima, A. Retrospective CBCT analysis of airway volume changes after bone-borne vs. tooth-borne rapid maxillary expansion. Angle Orthod. 2019, 89, 566–574. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Di Carlo, G.; Cornelis, M.A.; Cattaneo, P.M. Three-dimensional analyses of short- and long-term effects of rapid maxillary expansion on nasal cavity and upper airway: A systematic review and meta-analysis. Orthod. Craniofac. Res. 2020, 23, 250–276. [Google Scholar] [CrossRef] [PubMed]
- Buck, L.M.; Dalci, O.; Darendeliler, M.A.; Papageorgiou, S.N.; Papadopoulou, A.K. Volumetric upper airway changes after rapid maxillary expansion: A systematic review and meta-analysis. Eur. J. Orthod. 2017, 39, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Si, J.; Hu, X.; Du, Y.; Wei, M.; Xu, L.; Li, B.; Chen, X.; Li, X. Rapid maxillary expansion treatment increases mid-facial depth in early mixed dentition. Front. Pediatr. 2023, 10, 1028968. [Google Scholar] [CrossRef]
- Iwasaki, T.; Shimizu, Y.; Shibata, M.; Inoue, N.; Seki, T.; Suzuki, K. Tongue posture improvement and pharyngeal airway enlargement as secondary effects of rapid maxillary expansion: A cone-beam computed tomography study. Am. J. Orthod. Dentofac. Orthop. 2013, 143, 235–245. [Google Scholar] [CrossRef]
- El, H.; Palomo, J.M. Three-dimensional evaluation of upper airway following rapid maxillary expansion: A CBCT study. Angle Orthod. 2014, 84, 265–273. [Google Scholar] [CrossRef]
- Aljawad, H.; Lee, K.M.; Lim, H.J. Three-dimensional evaluation of upper airway changes following rapid maxillary expansion: A retrospective comparison with propensity score matched controls. PLoS ONE 2021, 16, e0246969. [Google Scholar] [CrossRef]
- Feng, X.; Lie, S.A.; Hellén-Halme, K.; Shi, X.Q. Effect of rapid maxillary expansion on upper airway morphology: A retrospective comparison of normal patients versus patients with enlarged adenoid tissue. J. Clin. Pediatr. Dent. 2021, 45, 208–215. [Google Scholar] [CrossRef]
- Ribeiro, A.N.C.; da Silva Filho, O.G.; Dalstra, M.; Melsen, B. Upper airway expansion after rapid maxillary expansion evaluated with cone beam computed tomography. Angle Orthod. 2012, 82, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Nishimura, M.; Goseki, E.; Matsumoto, J.K.; Shimizu, G.; Owens, R.E. Oropharyngeal airway changes after rapid palatal expansion evaluated with cone-beam computed tomography. Am. J. Orthod. Dentofac. Orthop. 2010, 137, 486–494. [Google Scholar] [CrossRef]
- Pangrazio-Kulbersh, V.; Wine, P.; Haughey, M.; Pajtas, B.; Kaczynski, R. Cone beam computed tomography evaluation of changes in the naso-maxillary complex associated with two types of maxillary expanders. Angle Orthod. 2012, 82, 448–457. [Google Scholar] [CrossRef]
- Abdalla, Y.; Brown, L.; Sonnesen, L. Effects of rapid maxillary expansion on upper airway volume: A three-dimensional cone-beam computed tomography study. Angle Orthod. 2019, 89, 917–923. [Google Scholar] [CrossRef]
- Kabalan, O.; Gordon, J.; Heo, G.; Lagravère, M.O. Nasal airway changes in bone-borne and tooth-borne rapid maxillary expansion treatments. Int. Orthod. 2015, 13, 1–15. [Google Scholar] [CrossRef]
- Warren, D.W. A quantitative technique for assessing nasal airway impairment. Am. J. Orthod. 1984, 86, 306–314. [Google Scholar] [CrossRef]
- Song, B.; Li, Y.; Sun, J.; Qi, Y.; Li, P.; Li, Y.; Gu, Z. Computational fluid dynamics simulation of changes in the morphology and airflow dynamics of the upper airways in OSAHS patients after treatment with oral appliances. PLoS ONE 2019, 14, e0219642. [Google Scholar] [CrossRef]
- Warren, D.W.; Hairfield, W.M.; Dalston, E.T. Effect of age on nasal cross-sectional area and respiratory mode in children. Laryngoscope 1990, 100, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Itikawa, C.E.; Valera, F.C.P.; Matsumoto, M.A.N.; Lima, W.T.A. Effect of rapid maxillary expansion on the dimension of the nasal cavity and on facial morphology assessed by acoustic rhinometry and rhinomanometry. Dental Press J. Orthod. 2012, 17, 129–133. [Google Scholar] [CrossRef]
- Jorge, E.P.; de Oliveira, R.C.; Pereira, R.A.; de Souza, M.L.; de Souza, M.M.G. Evaluation of the effect of rapid maxillary expansion on the respiratory pattern using active anterior rhinomanometry: Case report and description of the technique. Int. J. Pediatr. Otorhinolaryngol. 2010, 74, 784–789. [Google Scholar]
- Hartgerink, D.V.; Vig, P.S.; Abbott, D.W. The effect of rapid maxillary expansion on nasal airway resistance. Am. J. Orthod. Dentofac. Orthop. 1987, 92, 381–389. [Google Scholar] [CrossRef]
- De Felippe, N.L.O.; Bhushan, N.; Da Silveira, A.C.; Viana, G.; Smith, B. Long-term effects of orthodontic therapy on the maxillary dental arch and nasal cavity. Am. J. Orthod. Dentofac. Orthop. 2009, 136, 490.e1–490.e8. [Google Scholar] [CrossRef]
- Langer, M.R.E.; Itikawa, C.E.; Pereira Valera, F.C.; Matsumoto, M.A.N.; Anselmo-Lima, W.T. Does rapid maxillary expansion increase nasopharyngeal space and improve nasal airway resistance? Int. J. Pediatr. Otorhinolaryngol. 2011, 75, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Oliveira De Felippe, N.L.; Da Silveira, A.C.; Viana, G.; Kusnoto, B.; Smith, B.; Evans, C.A. Relationship between rapid maxillary expansion and nasal cavity size and airway resistance: Short- and long-term effects. Am. J. Orthod. Dentofac. Orthop. 2008, 134, 370–382. [Google Scholar] [CrossRef]
- Torre, H.; Alarcón, J.A. Changes in nasal air flow and school grades after rapid maxillary expansion in oral breathing children. Med. Oral Patol. Oral Cir. Bucal 2012, 17, e865–e870. [Google Scholar] [CrossRef]
- Cistulli, P.A.; Palmisano, R.G.; Poole, M.D. Treatment of Obstructive Sleep Apnea Syndrome by Rapid Maxillary Expansion. Sleep 1998, 21, 831–835. [Google Scholar] [CrossRef]
- Pirelli, P.; Saponara, M.; Guilleminault, C. Rapid maxillary expansion in children with obstructive sleep apnea syndrome. Sleep 2004, 27, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Guilleminault, C.; Huang, Y.S.; Monteyrol, P.J.; Sato, R.; Quo, S.; Lin, C.H. Adeno-tonsillectomy and rapid maxillary distraction in pre-pubertal children, a pilot study. Sleep Breath 2011, 15, 173–177. [Google Scholar] [CrossRef]
- Camacho, M.; Chang, E.T.; Song, S.A.; Abdullatif, J.; Duggan, C.; Guilleminault, C.; Capasso, R.; Kushida, C.A. Rapid maxillary expansion for pediatric obstructive sleep apnea: A systematic review and meta-analysis. Laryngoscope 2017, 127, 1712–1719. [Google Scholar] [CrossRef]
- Faccioni, P.; Pardo, A.; Montini, E.; Bazzanella, S.; Pancera, P.; Beccherle, M.; Caroprese, M.; Lonardi, F.; Signoriello, A.; Montagna, P.; et al. Cephalometric variation of vertical dimension in patients treated with hyrax-type and McNamara-type rapid palatal expander. Study on latero-lateral teleradiography. J. Appl. Cosmetol. 2024, 42, 51–66. [Google Scholar] [CrossRef]
- Faccioni, P.; Sacchetto, L.; Sinigaglia, S.; Marchiori, M.; Pardo, A.; Zangani, A.; Luciano, U.; Melloni, F.; Albanese, M.; De Santis, D.; et al. An improvement of upper airway flow in patients treated with rapid maxillary expansion: A Cone Beam Computed Tomography study. J. Appl. Cosmetol. 2023, 41, 99–106. [Google Scholar] [CrossRef]
- Xu, C.; Yu, Y.; Wang, Y.; McDonough, J.M.; Kimbell, J.S.; Schlosser, R.J.; Tawhai, M.H.; Mi, Y. Computational fluid dynamics modeling of the upper airway of children with obstructive sleep apnea syndrome in steady flow. J. Biomech. 2006, 39, 2043–2054. [Google Scholar] [CrossRef]


| Variable | Group A (Treated with RPE) | ||||
|---|---|---|---|---|---|
| Average Values at T0 | Average Variations Between T0 and T1 | Comparison Between T0 and T1 | |||
| Mean | SD | Mean/Median | SD/(min; max) | p Value | |
| VN (mm3) | 10,097.22 | 2225.9 | 1971.01 | 1275.8; 3431.64 | <0.001 * |
| VRP (mm3) | 3533.17 | 516.37 | 340.26 | −173.13; 1664.13 | 0.001 * |
| VRG (mm3) | 2679.81 | 470.21 | 503.77 | −124; 1892.81 | 0.002 * |
| S_A1_R (m/s) | 10.68 | 2.53 | −2.89 | 1.46 | <0.001 * |
| S_A1_L (m/s) | 10.79 | 2.58 | −3.31 | 2.21 | <0.001 * |
| S_A2_R (m/s) | 8.26 | 1.74 | −2.32 | 1.19 | <0.001 * |
| S_A2_L (m/s) | 7.92 | 1.54 | −2.18 | 1.2 | <0.001 * |
| S_B_R (m/s) | 7.9 | 1.36 | −2.54 | 0.67 | <0.001 * |
| S_B_L (m/s) | 7.98 | 1.31 | −2.56 | 0.77 | <0.001 * |
| S_C_R (m/s) | 7.01 | 1.17 | −2.31 | 0.69 | <0.001 * |
| S_C_L (m/s) | 6.9 | 1.19 | −2.16 | 0.76 | <0.001 * |
| S_D_R (m/s) | 11.45 | 2.05 | −3.93 | 0.98 | <0.001 * |
| S_D_L (m/s) | 11.33 | 2.14 | −3.72 | 1.02 | <0.001 * |
| S_E (m/s) | 4.3 | 0.71 | −0.8 | 0.61 | <0.001 * |
| S_RP (m/s) | 4.53 | 1.01 | −0.75 | −2.29; 0.06 | <0.001 * |
| S_RG (m/s) | 6.24 | 1.99 | −0.99 | −4.33; −0.09 | <0.001 * |
| Variable | Group B (Control) | ||||
|---|---|---|---|---|---|
| Average Values at T0 | Average Variations Between T0 and T1 | Comparison Between T0 and T1 | |||
| Mean | SD | Mean/Median | SD/(min; max) | p Value | |
| VN (mm3) | 9854.72 | 1445.70 | 1005.39 | 560.7; 1283.95 | 0.01 * |
| VRP (mm3) | 3973.25 | 876.63 | 64.135 | −112.57; 784.44 | 0.26 |
| VRG (mm3) | 2977.32 | 751.83 | 83.13 | −100.95; 920.10 | 0.12 |
| S_A1_R (m/s) | 12.93 | 1.96 | −3.39 | 1.81 | 0.01 * |
| S_A1_L (m/s) | 13.2 | 1.96 | −3.65 | 2.1 | 0.001 * |
| S_A2_R (m/s) | 9.81 | 1.36 | −2.58 | 1.29 | <0.001 * |
| S_A2_L (m/s) | 9.59 | 1.64 | −2.38 | 1.27 | <0.001 * |
| S_B_R (m/s) | 7.84 | 1.38 | −2.23 | 0.79 | <0.001 * |
| S_B_L (m/s) | 8.9 | 0.95 | −2.14 | 0.76 | <0.001 * |
| S_C_R (m/s) | 8.00 | 0.96 | −2.00 | 0.67 | <0.001 * |
| S_C_L (m/s) | 6.89 | 1.22 | −2.16 | 0.76 | <0.001 * |
| S_D_R (m/s) | 12.83 | 1.74 | −3.93 | 0.98 | <0.001 * |
| S_D_L (m/s) | 12.32 | 1.54 | −3.72 | 1.02 | <0.001 * |
| S_E (m/s) | 4.01 | 0.58 | −0.8 | 0.61 | <0.001 * |
| S_RP (m/s) | 3.58 | 0.54 | −0.74 | −1.05; −0.16 | 0.01 * |
| S_RG (m/s) | 4.73 | 0.87 | −1.29 | −1.87; 0.38 | 0.02 * |
| Inter-Group Analysis (p Value) | |||
|---|---|---|---|
| Variable (Average Variations T0–T1) | Comparison Between Group A and Group B | Comparison Between 8–9 Years and 10–12 Years | Comparison (Only for Group A) Between Varied or Not Varied CS Stage |
| VN (mm3) | 0.001 * | 0.62 | 0.61 |
| VRP (mm3) | 0.08 | 0.88 | 0.95 |
| VRG (mm3) | 0.14 | 0.5 | 0.39 |
| S_A1_R (m/s) | 0.47 | 0.54 | 0.33 |
| S_A1_L (m/s) | 0.72 | 0.36 | 0.86 |
| S_A2_R (m/s) | 0.62 | 0.62 | 0.39 |
| S_A2_L (m/s) | 0.71 | 0.83 | 0.33 |
| S_B_R (m/s) | 0.32 | 0.7 | 0.12 |
| S_B_L (m/s) | 0.21 | 0.46 | 0.23 |
| S_C_R (m/s) | 0.30 | 0.36 | 0.28 |
| S_C_L (m/s) | 0.36 | 0.54 | 0.19 |
| S_D_R (m/s) | 0.06 | 0.36 | 0.95 |
| S_D_L (m/s) | 0.09 | 0.25 | 0.46 |
| S_E (m/s) | 0.4 | 0.66 | 0.4 |
| S_RP (m/s) | 0.75 | 0.89 | 0.56 |
| S_RG (m/s) | 0.66 | 0.34 | 0.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faccioni, P.; Pardo, A.; Matteazzi, G.; Zoccatelli, E.; Bazzanella, S.; Montini, E.; Lonardi, F.; Olivato, B.; Albanese, M.; Montagna, P.; et al. Changes of Airway Space and Flow in Patients Treated with Rapid Palatal Expander (RPE): An Observational Pilot Study with Comparison with Non-Treated Patients. J. Clin. Med. 2025, 14, 4357. https://doi.org/10.3390/jcm14124357
Faccioni P, Pardo A, Matteazzi G, Zoccatelli E, Bazzanella S, Montini E, Lonardi F, Olivato B, Albanese M, Montagna P, et al. Changes of Airway Space and Flow in Patients Treated with Rapid Palatal Expander (RPE): An Observational Pilot Study with Comparison with Non-Treated Patients. Journal of Clinical Medicine. 2025; 14(12):4357. https://doi.org/10.3390/jcm14124357
Chicago/Turabian StyleFaccioni, Paolo, Alessia Pardo, Giorgia Matteazzi, Erika Zoccatelli, Silvia Bazzanella, Elena Montini, Fabio Lonardi, Benedetta Olivato, Massimo Albanese, Pietro Montagna, and et al. 2025. "Changes of Airway Space and Flow in Patients Treated with Rapid Palatal Expander (RPE): An Observational Pilot Study with Comparison with Non-Treated Patients" Journal of Clinical Medicine 14, no. 12: 4357. https://doi.org/10.3390/jcm14124357
APA StyleFaccioni, P., Pardo, A., Matteazzi, G., Zoccatelli, E., Bazzanella, S., Montini, E., Lonardi, F., Olivato, B., Albanese, M., Montagna, P., Lombardo, G., Gualtieri, M., Signoriello, A., Conti, G., & Zangani, A. (2025). Changes of Airway Space and Flow in Patients Treated with Rapid Palatal Expander (RPE): An Observational Pilot Study with Comparison with Non-Treated Patients. Journal of Clinical Medicine, 14(12), 4357. https://doi.org/10.3390/jcm14124357








