Infective Endocarditis During Pregnancy: Challenges and Future Directions
Abstract
1. Introduction
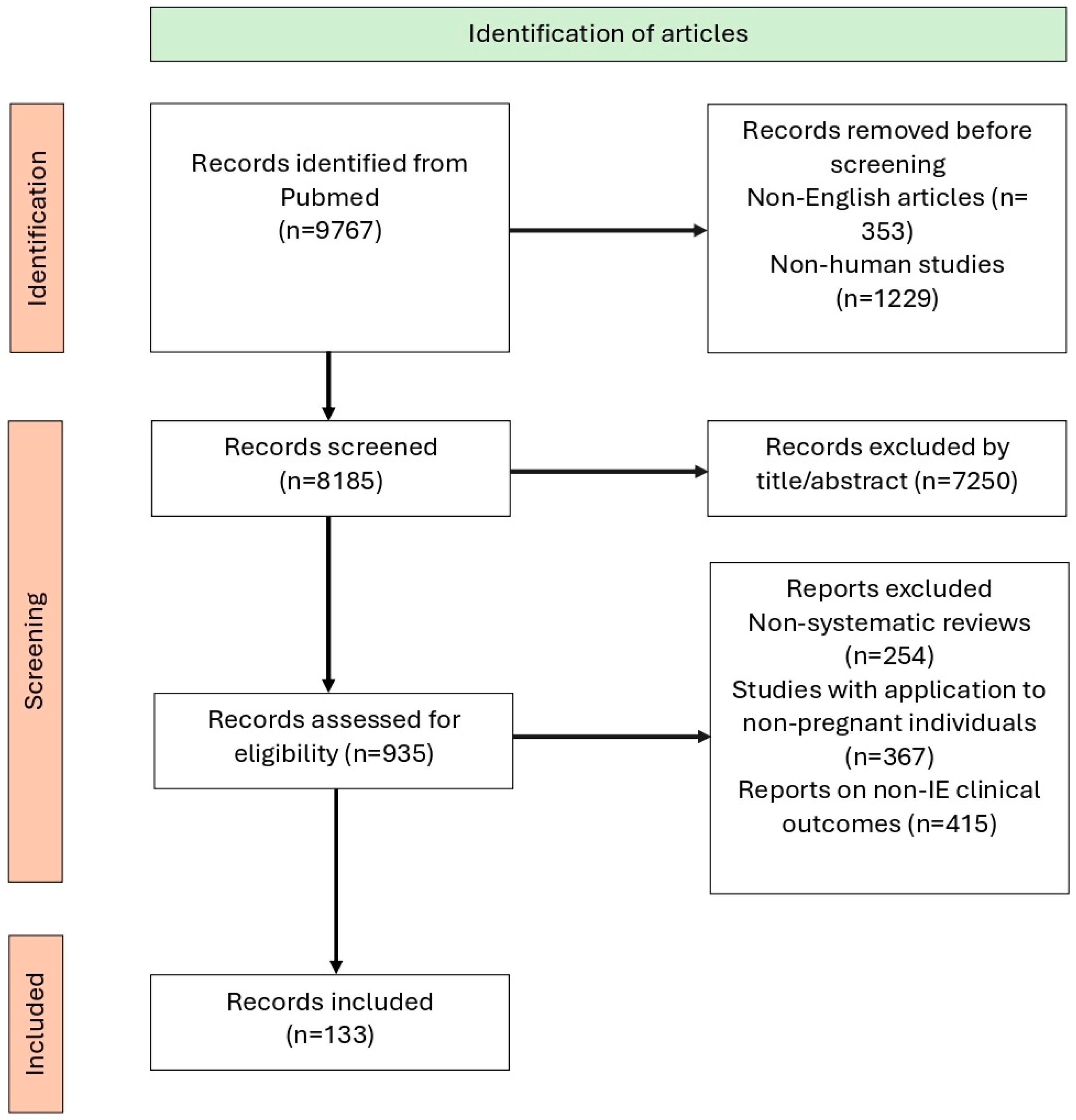
2. Methodology
3. Epidemiology and Risk Factors
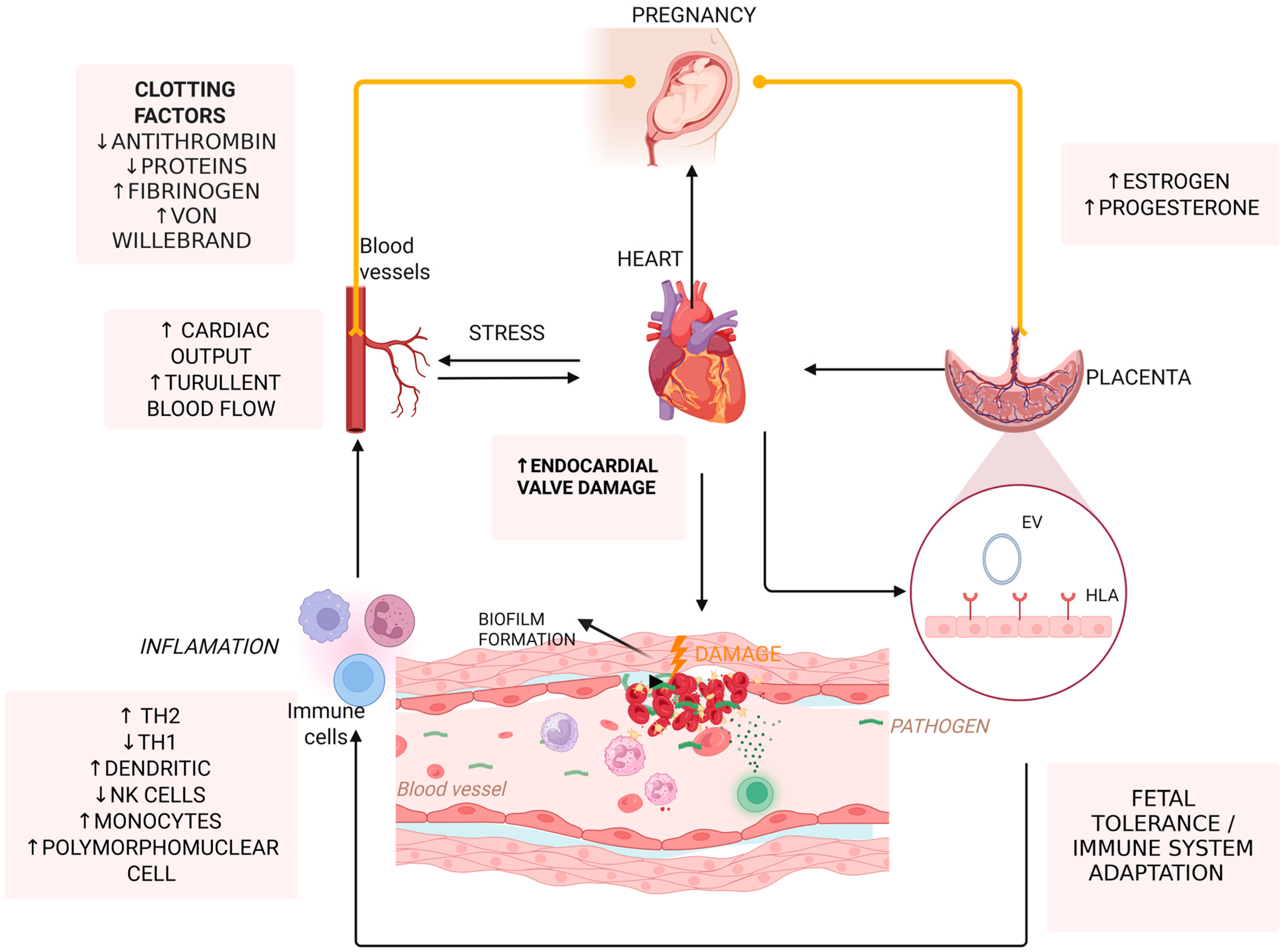
4. Pathophysiology of IE in Pregnancy
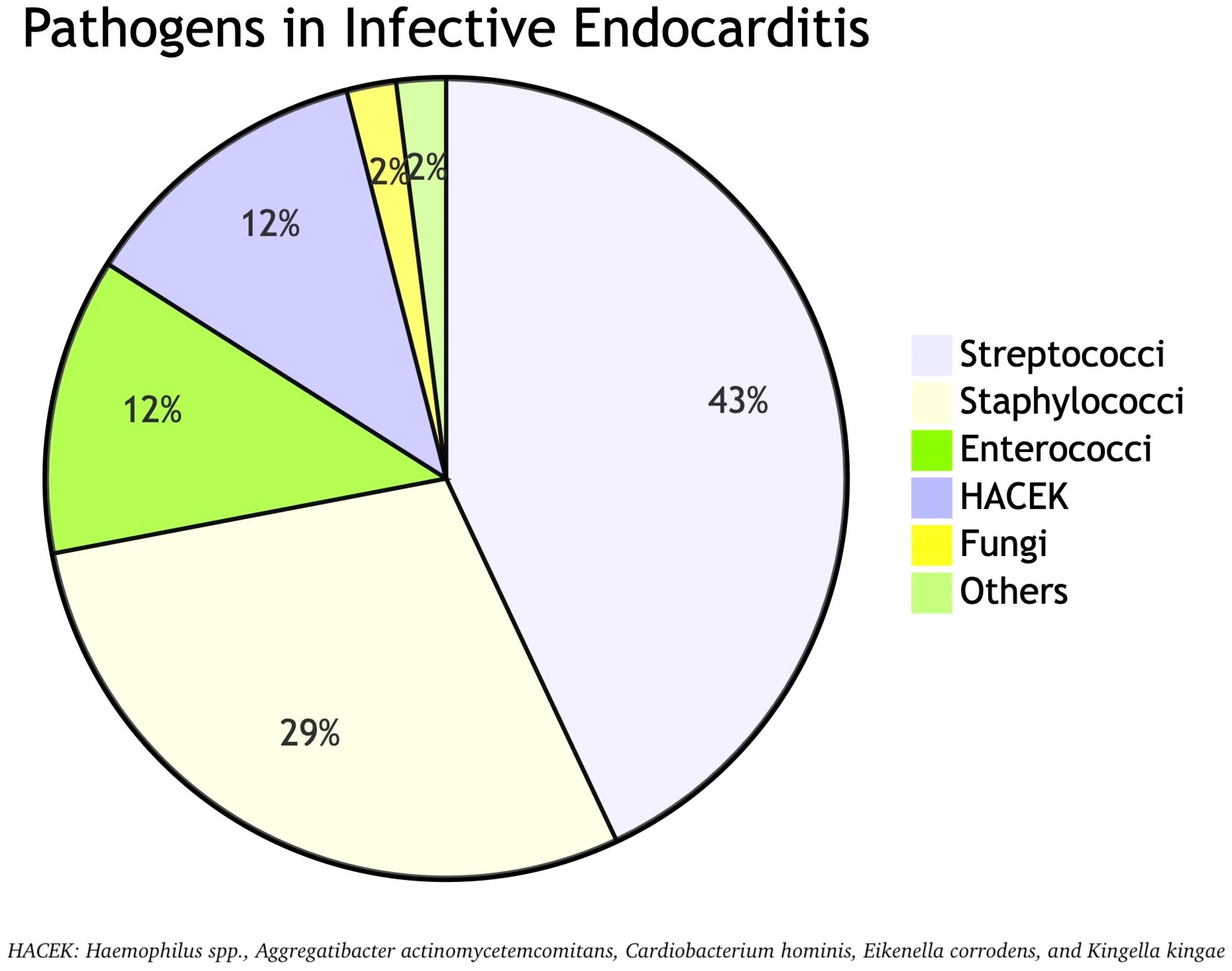
5. Microbiology of Pregnancy-Associated IE
6. Screening and Risk Assessment for High-Risk Pregnant Patients—Is There a Role?
7. Clinical Presentation and Diagnostic Challenges
8. Maternal and Fetal Complications
9. Management Strategies
9.1. Antimicrobial Therapy
9.1.1. General Principles
9.1.2. Empirical Therapy
9.1.3. S. viridans and Streptococcus gallolyticus Group
9.1.4. S. aureus and Coagulase-Negative Staphylococci
9.1.5. Enterococci spp.
9.1.6. HACEK and Non-HACEK Gram-Negative Bacteria
9.1.7. Fungi
9.1.8. Follow-Up and Duration of Therapy
9.2. Anticoagulant Therapy
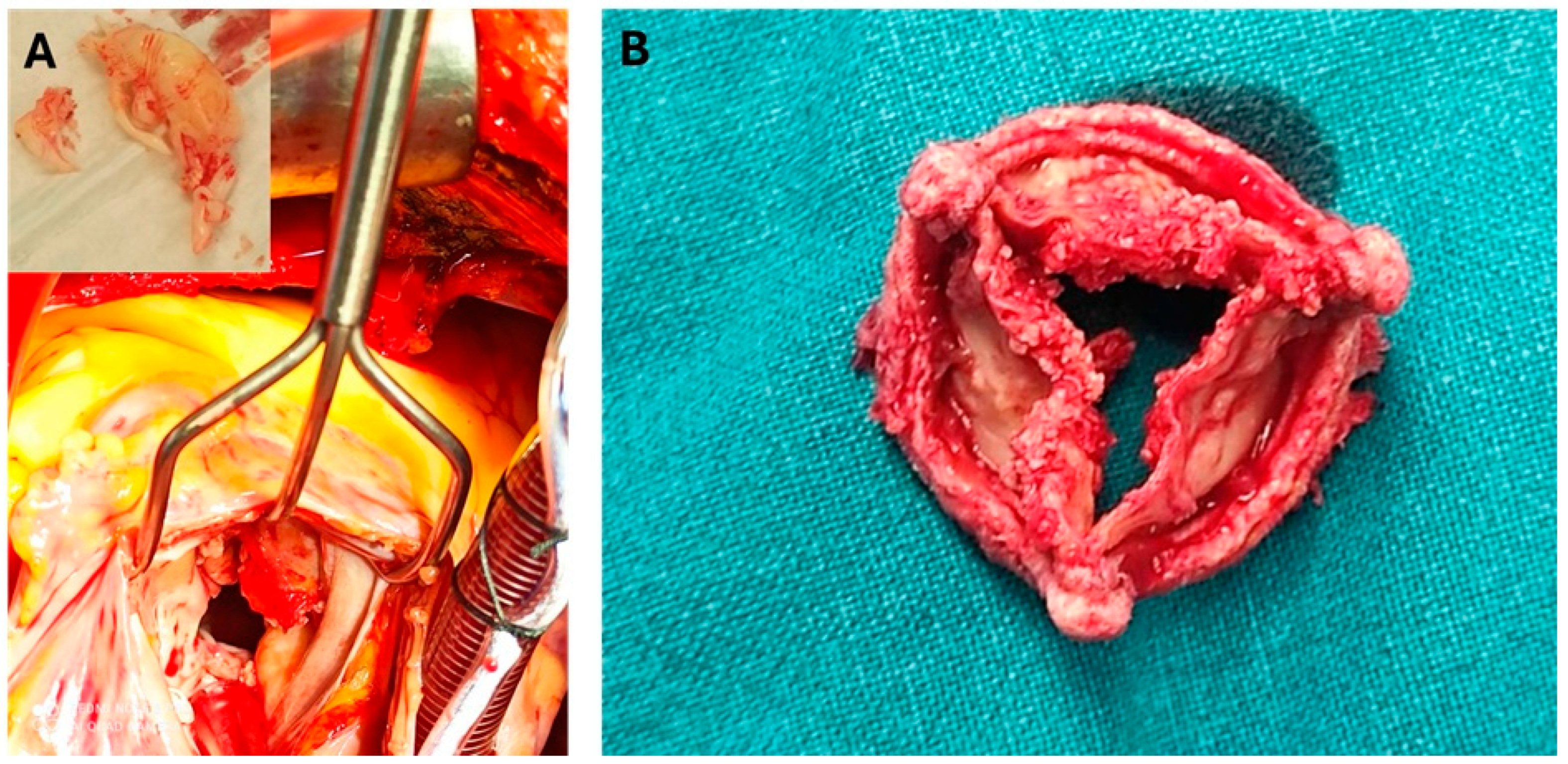
9.3. Surgical Management
9.4. Management of Device-Related Infections
10. Antimicrobial Prophylaxis
11. Future Perspectives
12. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| IE | Infective Endocarditis |
| S. aureus | Staphylococcus aureus |
| IUGR | Intrauterine Growth Restriction |
| IVDU | Intravenous Drug Use |
| EVs | Extracellular Vesicles |
| HLA-G | Human Leukocyte Antigen G |
| NK | Natural Killer (cells) |
| miRNAs | MicroRNAs |
| Treg | Regulatory T cell |
| Th1 | T helper type 1 |
| Th2 | T helper type 2 |
| IL-10 | Interleukin 10 |
| S. viridans | Streptococcus viridans |
| E. faecalis | Enterococcus faecalis |
| HACEK | Haemophilus spp., Aggregatibacter actinomycetemcomitans, Cardiobacterium hominis, Eikenella corrodens, Kingella kingae |
| ESC | European Society of Cardiology |
| AHA | American Heart Association |
| ACOG | American College of Obstetricians and Gynecologists |
| TTE | Transthoracic Echocardiography |
| TEE | Transesophageal Echocardiography |
| MRI | Magnetic Resonance Imaging |
| CT | Computed Tomography |
| FDA | Food and Drug Administration |
| PK | Pharmacokinetics |
| CL | Clearance |
| Vd | Volume of Distribution |
| NVE | Native Valve Endocarditis |
| PVE | Prosthetic Valve Endocarditis |
| CoNS | Coagulase-Negative Staphylococci |
| HLAR | High-Level Aminoglycoside Resistance |
| MSSA | Methicillin-Sensitive Staphylococcus aureus |
| MIC | Minimum Inhibitory Concentration |
| NICE | National Institute for Health and Care Excellence |
| OPAT | Outpatient Parenteral Antibiotic Therapy |
| CS | Cesarean Section |
| E. faecium | Enterococcus faecium |
| cfDNA | Cell-Free DNA |
| mNGS | Metagenomic Next-Generation Sequencing |
| PCR | Polymerase Chain Reaction |
| LMWH | Low Molecular Weight Heparin |
| i.v. | Intravenous |
| i.m. | Intramuscular |
| min | Minutes |
| LVEF | Left Ventricular Ejection Fraction |
| PH | Pulmonary Hypertension |
| PLLR | Pregnancy and Lactation Labeling Rule |
| TLE | Transvenous Lead Extraction |
| FIRS | Fetal Inflammatory Response Syndrome |
References
- Yallowitz, A.W.; Decker, L.C. Infectious Endocarditis. In StatPearls; StatPearls Publishing: Tampa, FL, USA, 2025. [Google Scholar]
- Friedman, N.D.; Kaye, K.S.; Stout, J.E.; McGarry, S.A.; Trivette, S.L.; Briggs, J.P.; Lamm, W.; Clark, C.; MacFarquhar, J.; Walton, A.L.; et al. Health care–associated bloodstream infections in adults: A reason to change the accepted definition of community-acquired infections. Ann. Intern. Med. 2002, 137, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.L.; Vos, T.; Lozano, R.; Naghavi, M.; Flaxman, A.D.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2197–2223. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, F.; Paradossi, U.; Trimarchi, G.; Benedetti, G.; Marchi, F.; Chiappino, S.; Conti, M.; Di Bella, G.; Murzi, M.; Di Sibio, S.; et al. Clinical Features and Patient Outcomes in Infective Endocarditis with Surgical Indication: A Single-Centre Experience. J. Cardiovasc. Dev. Dis. 2024, 11, 138. [Google Scholar] [CrossRef]
- Dajani, A.S.; Taubert, K.A.; Wilson, W.; Bolger, A.F.; Bayer, A.; Ferrieri, P.; Gewitz, M.H.; Shulman, S.T.; Nouri, S.; Newburger, J.W.; et al. Prevention of bacterial endocarditis. Recommendations by the American Heart Association. Circulation 1997, 96, 358–366. [Google Scholar] [CrossRef]
- Sinner, G.J.; Annabathula, R.; Viquez, K.; Alnabelsi, T.S.; Leung, S.W. Infective endocarditis in pregnancy from 2009 to 2019: The consequences of injection drug use. Infect Dis. 2021, 53, 633–639. [Google Scholar] [CrossRef]
- Shapero, K.S.; Nauriyal, V.; Megli, C.; Berlacher, K.; El-Dalati, S. Management of infective endocarditis in pregnancy by a multidisciplinary team: A case series. Ther. Adv. Infect. Dis. 2022, 9, 20499361221080644. [Google Scholar] [PubMed]
- Kebed, K.Y.; Bishu, K.; Al Adham, R.I.; Baddour, L.M.; Connolly, H.M.; Sohail, M.R.; Steckelberg, J.M.; Wilson, W.R.; Murad, M.H.; Anavekar, N.S. Pregnancy and postpartum infective endocarditis: A systematic review. Mayo Clin. Proc. 2014, 89, 1143–1152. [Google Scholar] [CrossRef]
- Wahbah Makhoul, G.; Lahoud, C.; Asogwa, N.; Ling, J.; Matar, M. Infective Endocarditis in Pregnancy: Unveiling the Challenges, Outcomes, and Strategies for Management. SN Compr. Clin. Med. 2024, 6, 66. [Google Scholar] [CrossRef]
- Chen, H.; Zhan, Y.; Zhang, K.; Gao, Y.; Chen, L.; Zhan, J.; Chen, Z.; Zeng, Z. The Global, Regional, and National Burden and Trends of Infective Endocarditis From 1990 to 2019: Results from the Global Burden of Disease Study 2019. Front. Med. 2022, 9, 774224. [Google Scholar] [CrossRef]
- Hoen, B.; Duval, X. Infective endocarditis. N. Engl. J. Med. 2013, 369, 785. [Google Scholar] [CrossRef]
- Baddour, L.M.; Wilson, W.R.; Bayer, A.S.; Fowler, V.G., Jr.; Tleyjeh, I.M.; Rybak, M.J.; Barsic, B.; Lockhart, P.B.; Gewitz, M.H.; Levison, M.E.; et al. Infective Endocarditis in Adults: Diagnosis, Antimicrobial Therapy, and Management of Complications: A Scientific Statement for Healthcare Professionals From the American Heart Association. Circulation 2015, 132, 1435–1486. [Google Scholar] [CrossRef] [PubMed]
- Adesomo, A.; Gonzalez-Brown, V.; Rood, K.M. Infective Endocarditis as a Complication of Intravenous Drug Use in Pregnancy: A Retrospective Case Series and Literature Review. AJP Rep. 2020, 10, e288–e293. [Google Scholar] [CrossRef] [PubMed]
- Onofrei, V.A.; Adam, C.A.; Marcu, D.T.M.; Crisan Dabija, R.; Ceasovschih, A.; Constantin, M.; Grigorescu, E.D.; Petroaie, A.D.; Mitu, F. Infective Endocarditis during Pregnancy-Keep It Safe and Simple! Medicina 2023, 59, 939. [Google Scholar] [CrossRef]
- Sengupta, S.P.; Prendergast, B.; Laroche, C.; Furnaz, S.; Ronderos, R.; Almaghraby, A.; Asch, F.M.; Blechova, K.; Zaky, H.; Strahilevitz, J.; et al. Socioeconomic variations determine the clinical presentation, aetiology, and outcome of infective endocarditis: A prospective cohort study from the ESC-EORP EURO-ENDO (European Infective Endocarditis) registry. Eur. Heart J. Qual. Care Clin. Outcomes 2022, 9, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Adeyeye, V.O.; Balogun, M.O.; Adebayo, R.A.; Makinde, O.N.; Akinwusi, P.O.; Ajayi, E.A.; Ogunyemi, S.A.; Akintomide, A.O.; Ajayi, E.O.; Adeyeye, A.G.; et al. Echocardiographic Assessment of Cardiac Changes During Normal Pregnancy Among Nigerians. Clin. Med. Insights Cardiol. 2016, 10, 157–162. [Google Scholar] [CrossRef]
- Slipczuk, L.; Codolosa, J.N.; Davila, C.D.; Romero-Corral, A.; Yun, J.; Pressman, G.S.; Figueredo, V.M. Infective endocarditis epidemiology over five decades: A systematic review. PLoS ONE 2013, 8, e82665. [Google Scholar] [CrossRef]
- Abu-Raya, B.; Michalski, C.; Sadarangani, M.; Lavoie, P.M. Maternal Immunological Adaptation During Normal Pregnancy. Front. Immunol. 2020, 11, 575197. [Google Scholar] [CrossRef]
- Oliveira, F.M.; Fraife, M.T.; Barbosa, G.I.F.; Monteiro, T.S.; Lamas, C.C. Endocarditis in pregnancy and postpartum: Cases in a prospective adult cohort and literature review. Heart Vessel. Transplant 2022, 6, 178–189. [Google Scholar] [CrossRef]
- Windram, J.D.; Colman, J.M.; Wald, R.M.; Udell, J.A.; Siu, S.C.; Silversides, C.K. Valvular heart disease in pregnancy. Best. Pract. Res. Clin. Obstet. Gynaecol. 2014, 28, 507–518. [Google Scholar] [CrossRef]
- Morton, A. Physiological Changes and Cardiovascular Investigations in Pregnancy. Heart Lung Circ. 2021, 30, e6–e15. [Google Scholar] [CrossRef]
- Sharma, S.; Rodrigues, P.R.S.; Zaher, S.; Davies, L.C.; Ghazal, P. Immune-metabolic adaptations in pregnancy: A potential stepping-stone to sepsis. EBioMedicine 2022, 86, 104337. [Google Scholar] [CrossRef] [PubMed]
- Nichols, K.; Henkin, S.; Creager, M. Venous Thromboembolism Associated With Pregnancy. J. Am. Coll. Cardiol. 2020, 76, 2128–2141. [Google Scholar] [CrossRef] [PubMed]
- Parunov, L.A.; Soshitova, N.P.; Ovanesov, M.V.; Panteleev, M.A.; Serebriyskiy, I.I. Epidemiology of venous thromboembolism (VTE) associated with pregnancy. Birth Defects Res. C Embryo Today 2015, 105, 167–184. [Google Scholar] [CrossRef]
- Almeida, F.A.; Pavan, M.V.; Rodrigues, C.I. The haemodynamic, renal excretory and hormonal changes induced by resting in the left lateral position in normal pregnant women during late gestation. BJOG 2009, 116, 1749–1754. [Google Scholar] [CrossRef]
- Kourtis, A.P.; Read, J.S.; Jamieson, D.J. Pregnancy and infection. N. Engl. J. Med. 2014, 370, 2211–2218. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Suano, A.; Hamilton, A.B.; Betz, A.G. Gimme shelter: The immune system during pregnancy. Immunol. Rev. 2011, 241, 20–38. [Google Scholar] [CrossRef]
- Kumar, R.K.; Antunes, M.J.; Beaton, A.; Mirabel, M.; Nkomo, V.T.; Okello, E.; Regmi, P.R.; Reményi, B.; Sliwa-Hähnle, K.; Zühlke, L.J.; et al. Contemporary Diagnosis and Management of Rheumatic Heart Disease: Implications for Closing the Gap: A Scientific Statement From the American Heart Association. Circulation 2020, 142, e337–e357. [Google Scholar] [CrossRef]
- Ander, S.E.; Diamond, M.S.; Coyne, C.B. Immune responses at the maternal-fetal interface. Sci. Immunol. 2019, 4, eaat6114. [Google Scholar] [CrossRef]
- Kumar, M.; Saadaoui, M.; Al Khodor, S. Infections and Pregnancy: Effects on Maternal and Child Health. Front. Cell. Infect. Microbiol. 2022, 12, 873253. [Google Scholar] [CrossRef]
- Degner, K.; Magness, R.R.; Shah, D.M. Establishment of the Human Uteroplacental Circulation: A Historical Perspective. Reprod. Sci. 2017, 24, 753–761. [Google Scholar] [CrossRef]
- Lockwood, C.J.; Huang, S.J.; Chen, C.P.; Huang, Y.; Xu, J.; Faramarzi, S.; Kayisli, O.; Kayisli, U.; Koopman, L.; Smedts, D.; et al. Decidual cell regulation of natural killer cell-recruiting chemokines: Implications for the pathogenesis and prediction of preeclampsia. Am. J. Pathol. 2013, 183, 841–856. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.M. Infective Endocarditis during pregnancy. J. Coll. Physicians Surg. Pak. 2015, 25, 134–139. [Google Scholar]
- Garrison, P.K.; Freedman, L.R. Experimental endocarditis I. Staphylococcal endocarditis in rabbits resulting from placement of a polyethylene catheter in the right side of the heart. Yale J. Biol. Med. 1970, 42, 394–410. [Google Scholar] [PubMed]
- Durack, D.T.; Beeson, P.B. Experimental bacterial endocarditis. I. Colonization of a sterile vegetation. Br. J. Exp. Pathol. 1972, 53, 44–49. [Google Scholar] [PubMed]
- Werdan, K.; Dietz, S.; Löffler, B.; Niemann, S.; Bushnaq, H.; Silber, R.E.; Peters, G.; Müller-Werdan, U. Mechanisms of infective endocarditis: Pathogen-host interaction and risk states. Nat. Rev. Cardiol. 2014, 11, 35–50. [Google Scholar] [CrossRef]
- Kornacki, J.; Gutaj, P.; Kalantarova, A.; Sibiak, R.; Jankowski, M.; Wender-Ozegowska, E. Endothelial Dysfunction in Pregnancy Complications. Biomedicines 2021, 9, 1756. [Google Scholar] [CrossRef]
- Escolà-Vergé, L.; Rello, P.; Declerck, C.; Dubée, V.; Rouleau, F.; Duval, X.; Habib, G.; Lavie-Badie, Y.; Martin-Blondel, G.; Porte, L.; et al. Infective endocarditis in pregnant women without intravenous drug use: A multicentre retrospective case series. J. Antimicrob. Chemother. 2022, 77, 2701–2705. [Google Scholar] [CrossRef]
- Li, M.; Kim, J.B.; Sastry, B.K.S.; Chen, M. Infective endocarditis. Lancet 2024, 404, 377–392. [Google Scholar] [CrossRef]
- Bumm, C.V.; Folwaczny, M. Infective endocarditis and oral health-a Narrative Review. Cardiovasc. Diagn. Ther. 2021, 11, 1403–1415. [Google Scholar] [CrossRef]
- Chirouze, C.; Athan, E.; Alla, F.; Chu, V.H.; Ralph Corey, G.; Selton-Suty, C.; Erpelding, M.L.; Miro, J.M.; Olaison, L.; Hoen, B. Enterococcal endocarditis in the beginning of the 21st century: Analysis from the International Collaboration on Endocarditis-Prospective Cohort Study. Clin. Microbiol. Infect. 2013, 19, 1140–1147. [Google Scholar] [CrossRef]
- Khaledi, M.; Sameni, F.; Afkhami, H.; Hemmati, J.; Asareh Zadegan Dezfuli, A.; Sanae, M.J.; Validi, M. Infective endocarditis by HACEK: A review. J. Cardiothorac. Surg. 2022, 17, 185. [Google Scholar] [CrossRef]
- Thompson, G.R., 3rd; Jenks, J.D.; Baddley, J.W.; Lewis, J.S., 2nd; Egger, M.; Schwartz, I.S.; Boyer, J.; Patterson, T.F.; Chen, S.C.; Pappas, P.G.; et al. Fungal Endocarditis: Pathophysiology, Epidemiology, Clinical Presentation, Diagnosis, and Management. Clin. Microbiol. Rev. 2023, 36, e0001923. [Google Scholar] [CrossRef] [PubMed]
- Pollock, A.; Kiernan, T.J. Contemporary management of infective endocarditis in pregnancy. Expert. Rev. Cardiovasc. Ther. 2023, 21, 839–854. [Google Scholar] [CrossRef] [PubMed]
- Nappi, F.; Martuscelli, G.; Bellomo, F.; Avtaar Singh, S.S.; Moon, M.R. Infective Endocarditis in High-Income Countries. Metabolites 2022, 12, 682. [Google Scholar] [CrossRef] [PubMed]
- Nappi, F.; Spadaccio, C.; Mihos, C. Infective endocarditis in the 21st century. Ann. Transl. Med. 2020, 8, 1620. [Google Scholar] [CrossRef]
- Murdoch, D.R.; Corey, G.R.; Hoen, B.; Miró, J.M.; Fowler, V.G., Jr.; Bayer, A.S.; Karchmer, A.W.; Olaison, L.; Pappas, P.A.; Moreillon, P.; et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: The International Collaboration on Endocarditis-Prospective Cohort Study. Arch. Intern. Med. 2009, 169, 463–473. [Google Scholar] [CrossRef]
- Delgado, V.; Ajmone Marsan, N.; de Waha, S.; Bonaros, N.; Brida, M.; Burri, H.; Caselli, S.; Doenst, T.; Ederhy, S.; Erba, P.A.; et al. 2023 ESC Guidelines for the management of endocarditis. Eur. Heart J. 2023, 44, 3948–4042. [Google Scholar]
- Ali, R.M.; Hassan, B.E.-D.E.; Mahmoud, N.M.Y. Pregnancy-Related Infective Endocarditis. In Obstetric Catastrophes: A Clinical Guide; Montufar, C., Hidalgo, J., Gei, A.F., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2021; pp. 479–492. [Google Scholar]
- Montoya, M.E.; Karnath, B.M.; Ahmad, M. Endocarditis during pregnancy. South. Med. J. 2003, 96, 1156–1157. [Google Scholar] [CrossRef]
- Bello Natalie, A.; Bairey Merz, C.N.; Brown, H.; Davis Melinda, B.; Dickert Neal, W.; El Hajj Stephanie, C.; Giullian, C.; Quesada, O.; Park, K.; Sanghani, R.M.; et al. Diagnostic Cardiovascular Imaging and Therapeutic Strategies in Pregnancy. JACC 2021, 77, 1813–1822. [Google Scholar] [CrossRef]
- Snygg-Martin, U.; Gustafsson, L.; Rosengren, L.; Alsiö, A.; Ackerholm, P.; Andersson, R.; Olaison, L. Cerebrovascular complications in patients with left-sided infective endocarditis are common: A prospective study using magnetic resonance imaging and neurochemical brain damage markers. Clin. Infect. Dis. 2008, 47, 23–30. [Google Scholar] [CrossRef]
- Borhart, J.; Palmer, J. Cardiovascular Emergencies in Pregnancy. Emerg. Med. Clin. N. Am. 2019, 37, 339–350. [Google Scholar] [CrossRef]
- Dagher, M.M.; Eichenberger, E.M.; Addae-Konadu, K.L.; Dotters-Katz, S.K.; Kohler, C.L.; Fowler, V.G.; Federspiel, J.J. Maternal and Fetal Outcomes Associated With Infective Endocarditis in Pregnancy. Clin. Infect. Dis. 2021, 73, 1571–1579. [Google Scholar] [CrossRef]
- Habib, G.; Lancellotti, P.; Antunes, M.J.; Bongiorni, M.G.; Casalta, J.P.; Del Zotti, F.; Dulgheru, R.; El Khoury, G.; Erba, P.A.; Iung, B.; et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur. Heart J. 2015, 36, 3075–3128. [Google Scholar]
- Wang, J.; Wang, A.; Cui, Y.; Wang, C.; Zhang, J. Diagnosis and treatment of infective endocarditis in pregnancy: A case report. J. Cardiothorac. Surg. 2020, 15, 109. [Google Scholar] [CrossRef]
- Thuny, F.; Avierinos, J.F.; Tribouilloy, C.; Giorgi, R.; Casalta, J.P.; Milandre, L.; Brahim, A.; Nadji, G.; Riberi, A.; Collart, F.; et al. Impact of cerebrovascular complications on mortality and neurologic outcome during infective endocarditis: A prospective multicentre study. Eur. Heart J. 2007, 28, 1155–1161. [Google Scholar] [CrossRef] [PubMed]
- Bayer, A.S.; Bolger, A.F.; Taubert, K.A.; Wilson, W.; Steckelberg, J.; Karchmer, A.W.; Levison, M.; Chambers, H.F.; Dajani, A.S.; Gewitz, M.H.; et al. Diagnosis and management of infective endocarditis and its complications. Circulation 1998, 98, 2936–2948. [Google Scholar] [CrossRef] [PubMed]
- Siu, S.C.; Colman, J.M.; Sorensen, S.; Smallhorn, J.F.; Farine, D.; Amankwah, K.S.; Spears, J.C.; Sermer, M. Adverse neonatal and cardiac outcomes are more common in pregnant women with cardiac disease. Circulation 2002, 105, 2179–2184. [Google Scholar] [CrossRef] [PubMed]
- Knowles, S.J.; O’Sullivan, N.P.; Meenan, A.M.; Hanniffy, R.; Robson, M. Maternal sepsis incidence, aetiology and outcome for mother and fetus: A prospective study. BJOG 2015, 122, 663–671. [Google Scholar] [CrossRef]
- Regitz-Zagrosek, V.; Blomstrom Lundqvist, C.; Borghi, C.; Cifkova, R.; Ferreira, R.; Foidart, J.M.; Gibbs, J.S.; Gohlke-Baerwolf, C.; Gorenek, B.; Iung, B.; et al. ESC Guidelines on the management of cardiovascular diseases during pregnancy: The Task Force on the Management of Cardiovascular Diseases during Pregnancy of the European Society of Cardiology (ESC). Eur. Heart J. 2011, 32, 3147–3197. [Google Scholar]
- Campuzano, K.; Roqué, H.; Bolnick, A.; Leo, M.V.; Campbell, W.A. Bacterial endocarditis complicating pregnancy: Case report and systematic review of the literature. Arch. Gynecol. Obstet. 2003, 268, 251–255. [Google Scholar] [CrossRef]
- Ezveci, H.; Doğru, Ş.; Akkuş, F.; Metin, Ü.S.; Gezginc, K. Maternal Cardiac Disease and Perinatal Outcomes in a Single Tertiary Care Center. Z. Geburtshilfe Neonatol. 2024, 228, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Wald, R.M.; Silversides, C.K.; Kingdom, J.; Toi, A.; Lau, C.S.; Mason, J.; Colman, J.M.; Sermer, M.; Siu, S.C. Maternal Cardiac Output and Fetal Doppler Predict Adverse Neonatal Outcomes in Pregnant Women With Heart Disease. J. Am. Heart Assoc. 2015, 4, e002414. [Google Scholar] [CrossRef] [PubMed]
- Eggleton, E.J.; Bhagra, C.J.; Patient, C.J.; Belham, M.; Pickett, J.; Aiken, C.E. Maternal left ventricular function and adverse neonatal outcomes in women with cardiac disease. Arch. Gynecol. Obstet. 2023, 307, 1431–1439. [Google Scholar] [CrossRef] [PubMed]
- Bamfo, J.; Kametas, N.A.; Turan, O.; Khaw, A.; Nicolaides, K.H. Maternal cardiac function in fetal growth restriction. BJOG Int. J. Obstet. Gynaecol. 2006, 113, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Melchiorre, K.; Sutherland, G.R.; Liberati, M.; Thilaganathan, B. Maternal cardiovascular impairment in pregnancies complicated by severe fetal growth restriction. Hypertension 2012, 60, 437–443. [Google Scholar] [CrossRef]
- Bucciarelli, V.; Moscucci, F.; Dei Cas, A.; Coppi, F.; Angeli, F.; Pizzi, C.; Renda, G.; Nodari, S.; Maffei, S.; Montisci, R.; et al. Maternal-fetal dyad beyond the phenomenology of pregnancy: From primordial cardiovascular prevention on out, do not miss this boat! Curr. Probl. Cardiol. 2024, 49, 102695. [Google Scholar] [CrossRef]
- Giovannini, E.; Bonasoni, M.P.; Pascali, J.P.; Giorgetti, A.; Pelletti, G.; Gargano, G.; Pelotti, S.; Fais, P. Infection Induced Fetal Inflammatory Response Syndrome (FIRS): State-of-the-Art and Medico-Legal Implications—A Narrative Review. Microorganisms 2023, 11, 1010. [Google Scholar] [CrossRef]
- Bhagirath, A.Y.; Medapati, M.R.; de Jesus, V.C.; Yadav, S.; Hinton, M.; Dakshinamurti, S.; Atukorallaya, D. Role of Maternal Infections and Inflammatory Responses on Craniofacial Development. Front. Oral Health 2021, 2, 735634. [Google Scholar] [CrossRef]
- Schwartz, J.; Czuzoj-Shulman, N.; Moss, E.; Spence, A.; Abenhaim, H. Incidence, risk factors and mortality associated with infective endocarditis in pregnancy. J. Obstet. Gynaecol. Can. 2020, 42, 691. [Google Scholar] [CrossRef]
- Adams, J.A.; Spence, C.; Shojaei, E.; Thandrasisla, P.; Gupta, A.; Choi, Y.H.; Skinner, S.; Silverman, M. Infective Endocarditis Among Women Who Inject Drugs. JAMA Netw. Open 2024, 7, e2437861. [Google Scholar] [CrossRef]
- Shapero, K.; El-Dalati, S.; Berlacher, K.; Megli, C. Outcomes of Endocarditis in Pregnancy: A Single-Center Experience. Open Forum Infect. Dis. 2023, 10, ofad470. [Google Scholar] [CrossRef] [PubMed]
- Countouris, M.E.; Marino, A.L.; Raymond, M.; Hauspurg, A.; Berlacher, K.L. Infective Endocarditis in Pregnancy: A Contemporary Cohort. Am. J. Perinatol. 2024, 41 (Suppl. S1), e230–e235. [Google Scholar] [CrossRef]
- Morelli, M.K.; Veve, M.P.; Shorman, M.A. Maternal Bacteremia Caused by Staphylococcus Aureus With a Focus on Infective Endocarditis. Open Forum Infect. Dis. 2020, 7, ofaa239. [Google Scholar] [CrossRef]
- Regitz-Zagrosek, V.; Roos-Hesselink, J.W.; Bauersachs, J.; Blomström-Lundqvist, C.; Cífková, R.; De Bonis, M.; Iung, B.; Johnson, M.R.; Kintscher, U.; Kranke, P.; et al. 2018 ESC Guidelines for the management of cardiovascular diseases during pregnancy. Eur. Heart J. 2018, 39, 3165–3241. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Pregnancy and Lactation Labeling Resources. Available online: https://www.fda.gov/drugs/labeling-information-drug-products/pregnancy-and-lactation-labeling-resources (accessed on 30 April 2025).
- Singh, O.; Agrawal, P.; Garg, R.; Agarwal, A. Drugs In Pregnancy: An Update. J. South Asian Fed. Obstet. Gynecol. 2014, 6, 7–11. [Google Scholar]
- Scheuerle, A.E.; Aylsworth, A.S. Birth defects and neonatal morbidity caused by teratogen exposure after the embryonic period. Birth Defects Res. A Clin. Mol. Teratol. 2016, 106, 935–939. [Google Scholar] [CrossRef]
- Nguyen, J.; Madonia, V.; Bland, C.M.; Stover, K.R.; Eiland, L.S.; Keating, J.; Lemmon, M.; Bookstaver, P.B. A review of antibiotic safety in pregnancy—2025 update. Pharmacother. J. Human. Pharmacol. Drug Ther. 2025, 45, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Stojanova, J.; Arancibia, M.; Ghimire, S.; Sandaradura, I. Understanding the Pharmacokinetics of Antibiotics in Pregnancy: Is There a Role for Therapeutic Drug Monitoring? A Narrative Review. Ther. Drug Monit. 2022, 44, 50–64. [Google Scholar] [CrossRef]
- Hesse, M.R.; Prins, J.R.; Hooge, M.N.L.; Winter, H.L.J.; Kosterink, J.G.W.; Touw, D.J.; Mian, P. Pharmacokinetics and Target Attainment of Antimicrobial Drugs Throughout Pregnancy: Part I-Penicillins. Clin. Pharmacokinet. 2023, 62, 221–247. [Google Scholar] [CrossRef]
- McDonald, E.G.; Aggrey, G.; Aslan, A.T.; Casias, M.; Cortes-Penfield, N.; Dong, M.Q.; Egbert, S.; Footer, B.; Isler, B.; King, M.; et al. Guidelines for Diagnosis and Management of Infective Endocarditis in Adults: A WikiGuidelines Group Consensus Statement. JAMA Network Open. 2023, 6, e2326366. [Google Scholar] [CrossRef]
- Yim, J.; Smith, J.R.; Singh, N.B.; Rice, S.; Stamper, K.; Garcia de la Maria, C.; Bayer, A.S.; Mishra, N.N.; Miró, J.M.; Tran, T.T.; et al. Evaluation of daptomycin combinations with cephalosporins or gentamicin against Streptococcus mitis group strains in an in vitro model of simulated endocardial vegetations (SEVs). J. Antimicrob. Chemother. 2017, 72, 2290–2296. [Google Scholar] [CrossRef] [PubMed]
- Mishra, N.; Abdelhady, W.; Elsayed, A.; Lapitan, C.; Proctor, R.; Rybak, M.; Miro, J.M.; Bayer, A.S. Combinations of Daptomycin plus Ceftriaxone, but Not Ascending Daptomycin Dose-Regimens, Are Effective in Experimental Endocarditis Caused by Streptococcus mitis-oralis Strains: Target Tissue Clearances and Prevention of Emergence of Daptomycin-Resistance. Antimicrob. Agents Chemother. 2023, 67, e0147222. [Google Scholar] [CrossRef] [PubMed]
- del Río, A.; Gasch, O.; Moreno, A.; Peña, C.; Cuquet, J.; Soy, D.; Mestres, C.A.; Suárez, C.; Pare, J.C.; Tubau, F.; et al. Efficacy and safety of fosfomycin plus imipenem as rescue therapy for complicated bacteremia and endocarditis due to methicillin-resistant Staphylococcus aureus: A multicenter clinical trial. Clin. Infect. Dis. 2014, 59, 1105–1112. [Google Scholar] [CrossRef]
- Tattevin, P.; Boutoille, D.; Vitrat, V.; Van Grunderbeeck, N.; Revest, M.; Dupont, M.; Alfandari, S.; Stahl, J.P. Salvage treatment of methicillin-resistant staphylococcal endocarditis with ceftaroline: A multicentre observational study. J. Antimicrob. Chemother. 2014, 69, 2010–2013. [Google Scholar] [CrossRef] [PubMed]
- Batard, E.; Jacqueline, C.; Boutoille, D.; Hamel, A.; Drugeon, H.B.; Asseray, N.; Leclercq, R.; Caillon, J.; Potel, G.; Bugnon, D. Combination of quinupristin-dalfopristin and gentamicin against methicillin-resistant Staphylococcus aureus: Experimental rabbit endocarditis study. Antimicrob. Agents Chemother. 2002, 46, 2174–2178. [Google Scholar] [CrossRef]
- Chu, V.H.; Miro, J.M.; Hoen, B.; Cabell, C.H.; Pappas, P.A.; Jones, P.; Stryjewski, M.E.; Anguera, I.; Braun, S.; Muñoz, P.; et al. Coagulase-negative staphylococcal prosthetic valve endocarditis--a contemporary update based on the International Collaboration on Endocarditis: Prospective cohort study. Heart 2009, 95, 570–576. [Google Scholar] [CrossRef]
- Almeida-Santos, A.C.; Novais, C.; Peixe, L.; Freitas, A.R. Vancomycin-resistant Enterococcus faecium: A current perspective on resilience, adaptation, and the urgent need for novel strategies. J. Glob. Antimicrob. Resist. 2025, 41, 233–252. [Google Scholar] [CrossRef]
- Pericàs, J.M.; García-de-la-Mària, C.; Brunet, M.; Armero, Y.; García-González, J.; Casals, G.; Almela, M.; Quintana, E.; Falces, C.; Ninot, S.; et al. Early in vitro development of daptomycin non-susceptibility in high-level aminoglycoside-resistant Enterococcus faecalis predicts the efficacy of the combination of high-dose daptomycin plus ampicillin in an in vivo model of experimental endocarditis. J. Antimicrob. Chemother. 2017, 72, 1714–1722. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Loubet, P.; Lescure, F.X.; Lepage, L.; Kirsch, M.; Armand-Lefevre, L.; Bouadma, L.; Lariven, S.; Duval, X.; Yazdanpanah, Y.; Joly, V. Endocarditis due to gram-negative bacilli at a French teaching hospital over a 6-year period: Clinical characteristics and outcome. Infect. Dis. 2015, 47, 889–895. [Google Scholar] [CrossRef]
- Rajaratnam, D.; Rajaratnam, R. Outpatient Antimicrobial Therapy for Infective Endocarditis is Safe. Heart Lung Circ. 2021, 30, 207–215. [Google Scholar] [CrossRef]
- Tackett, M.S.; Ahmed, T.; El-Dalati, S.A.; Ahmed, T. Paradoxical embolisation to the brain in right-sided infective endocarditis and patent foramen ovale in a pregnant woman. BMJ Case Rep. 2023, 16, e254403. [Google Scholar] [CrossRef] [PubMed]
- Dahshan, D.; Suliman, M.; Rahman, E.U.; Curtis, Z.; Thompson, E. Intravenous Drug Use-Associated Infective Endocarditis in Pregnant Patients at a Hospital in West Virginia. Cureus 2021, 13, e17218. [Google Scholar] [CrossRef] [PubMed]
- McLintock, C. Thromboembolism in pregnancy: Challenges and controversies in the prevention of pregnancy-associated venous thromboembolism and management of anticoagulation in women with mechanical prosthetic heart valves. Best. Pract. Res. Clin. Obstet. Gynaecol. 2014, 28, 519–536. [Google Scholar] [CrossRef] [PubMed]
- Ayad, S.W.; Hassanein, M.M.; Mohamed, E.A.; Gohar, A.M. Maternal and Fetal Outcomes in Pregnant Women with a Prosthetic Mechanical Heart Valve. Clin. Med. Insights Cardiol. 2016, 10, 11–17. [Google Scholar] [CrossRef]
- Jha, N.; Jha, A.K.; Chand Chauhan, R.; Chauhan, N.S. Maternal and Fetal Outcome After Cardiac Operations During Pregnancy: A Meta-Analysis. Ann. Thorac. Surg. 2018, 106, 618–626. [Google Scholar] [CrossRef]
- Jing, H.; Wei, M.; Lu, J.; Zhou, L.; Huang, J.; Cheng, W.; Zhang, Q.; Qiao, Z.; Zhu, J.; Ye, Y.; et al. Outcomes and risk factors for cardiac surgery during pregnancy: A 13-year, two-centre, retrospective cohort study. J. Thorac. Dis. 2024, 16, 7561–7573. [Google Scholar] [CrossRef]
- van Steenbergen, G.J.; Tsang, Q.H.Y.; van der Heijden, O.W.H.; Vart, P.; Rodwell, L.; Roos-Hesselink, J.W.; van Kimmenade, R.R.J.; Li, W.W.L.; Verhagen, A.F.T.M. Timing of cardiac surgery during pregnancy: A patient-level meta-analysis. Eur. Heart J. 2022, 43, 2801–2811. [Google Scholar] [CrossRef]
- Vizzardi, E.; De Cicco, G.; Zanini, G.; D’Aloia, A.; Faggiano, P.; Lo Russo, R.; Chiari, E.; Dei Cas, L. Infectious endocarditis during pregnancy, problems in the decision-making process: A case report. Cases J. 2009, 2, 6537. [Google Scholar] [CrossRef]
- Mahli, A.; Izdes, S.; Coskun, D. Cardiac operations during pregnancy: Review of factors influencing fetal outcome. Ann. Thorac. Surg. 2000, 69, 1622–1626. [Google Scholar] [CrossRef]
- Coolen, J.; Turnelp, R.; Vonder Muhll, I.; Chandra, S. Permanent pacemakers in pregnancy. Clin. Exp. Obstet. Gynecol. 2011, 38, 297–298. [Google Scholar]
- Vinh, D.C.; Embil, J.M. Device-related infections: A review. J. Long Term Eff. Med. Implants. 2005, 15, 467–488. [Google Scholar] [CrossRef] [PubMed]
- Baddour, L.M.; Esquer Garrigos, Z.; Rizwan Sohail, M.; Havers-Borgersen, E.; Krahn, A.D.; Chu, V.H.; Radke, C.S.; Avari-Silva, J.; El-Chami, M.F.; Miro, J.M.; et al. Update on Cardiovascular Implantable Electronic Device Infections and Their Prevention, Diagnosis, and Management: A Scientific Statement From the American Heart Association. Circulation 2024, 149, e201–e216. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, Z.; Sohal, M.; Sheppard, M.N.; Gallagher, M.M. Transvenous Lead Extraction: Work in Progress. Eur. Cardiol. Rev. 2023, 18, e44. [Google Scholar] [CrossRef]
- Thornhill, M.; Prendergast, B.; Dayer, M.; Frisby, A.; Baddour, L.M. Endocarditis prevention: Time for a review of NICE guidance. Lancet Reg. Health Eur. 2024, 39, 100876. [Google Scholar] [CrossRef] [PubMed]
- Imazio, M. The 2023 new European guidelines on infective endocarditis: Main novelties and implications for clinical practice. J. Cardiovasc. Med. 2024, 25, 718–726. [Google Scholar] [CrossRef]
- Giouleka, S.; Tsakiridis, I.; Chalkia-Prapa, E.M.; Katzi, F.; Liberis, A.; Michos, G.; Kalogiannidis, I.; Mamopoulos, A.; Dagklis, T. Antibiotic Prophylaxis in Obstetrics and Gynecology: A Comparative Review of Guidelines. Obstet. Gynecol. Surv. 2025, 80, 186–203. [Google Scholar] [CrossRef]
- Yang, M.; Yuan, F.; Guo, Y.; Wang, S. Efficacy of adding azithromycin to antibiotic prophylaxis in caesarean delivery: A meta-analysis and systematic review. Int. J. Antimicrob. Agents. 2022, 59, 106533. [Google Scholar] [CrossRef]
- Li, J.S.; Sexton, D.J.; Mick, N.; Nettles, R.; Fowler, V.G.; Ryan, T., Jr.; Bashore, T.; Corey, G.R. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin. Infect. Dis. 2000, 30, 633–638. [Google Scholar] [CrossRef]
- Bronkhorst, A.J.; Ungerer, V.; Oberhofer, A.; Gabriel, S.; Polatoglou, E.; Randeu, H.; Uhlig, C.; Pfister, H.; Mayer, Z.; Holdenrieder, S. New Perspectives on the Importance of Cell-Free DNA Biology. Diagnostics 2022, 12, 2147. [Google Scholar] [CrossRef]
- Ranucci, R. Cell-Free DNA: Applications in Different Diseases. Methods Mol. Biol. 2019, 1909, 3–12. [Google Scholar]
- Eichenberger, E.M.; Degner, N.; Scott, E.R.; Ruffin, F.; Franzone, J.; Sharma-Kuinkel, B.; Shah, P.; Hong, D.; Dalai, S.C.; Blair, L.; et al. Microbial Cell-Free DNA Identifies the Causative Pathogen in Infective Endocarditis and Remains Detectable Longer Than Conventional Blood Culture in Patients with Prior Antibiotic Therapy. Clin. Infect. Dis. 2023, 76, e1492–e1500. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Yang, Y.; Pan, J.; Miao, Q.; Jin, W.; Ma, Y.; Zhou, C.; Gao, X.; Wang, C.; Hu, B. The clinical value of valve metagenomic next-generation sequencing when applied to the etiological diagnosis of infective endocarditis. Ann. Transl. Med. 2021, 9, 1490. [Google Scholar] [CrossRef]
- Shishido, A.A.; Noe, M.; Saharia, K.; Luethy, P. Clinical impact of a metagenomic microbial plasma cell-free DNA next-generation sequencing assay on treatment decisions: A single-center retrospective study. BMC Infect. Dis. 2022, 22, 372. [Google Scholar] [CrossRef]
- Khehra, N.; Padda, I.S.; Swift, C.J. Polymerase Chain Reaction (PCR). In StatPearls; StatPearls Publishing: Tampa, FL, USA, 2025. [Google Scholar]
- Lee, R. Metagenomic Next Generation Sequencing: How Does It Work and Is It Coming to Your Clinical Microbiology Lab? American Society of Microbiology. Available online: https://asm.org/articles/2019/november/metagenomic-next-generation-sequencing-how-does-it (accessed on 30 April 2025).
- Solanky, D.; Ahmed, A.; Fierer, J.; Golts, E.; Jones, M.; Mehta, S. Utility of Plasma Microbial Cell-Free DNA Decay Kinetics After Aortic Valve Replacement for Bartonella Endocarditis: Case Report. Front. Trop. Dis. 2022, 3, 842100. [Google Scholar] [CrossRef]
- Ensom, M.H.H.; Davis, G.A.; Cropp, C.D.; Ensom, R.J. Clinical Pharmacokinetics in the 21st Century. Clin. Pharmacokinet. 1998, 34, 265–279. [Google Scholar] [CrossRef]
- Matsui, D.M. Therapeutic drug monitoring in pregnancy. Ther. Drug Monit. 2012, 34, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Vähäkangas, K.; Myllynen, P. Drug transporters in the human blood-placental barrier. Br. J. Pharmacol. 2009, 158, 665–678. [Google Scholar] [CrossRef]
- Nazir, F.; Tabish, T.A.; Tariq, F.; Iftikhar, S.; Wasim, R.; Shahnaz, G. Stimuli-sensitive drug delivery systems for site-specific antibiotic release. Drug Discov. Today 2022, 27, 1698–1705. [Google Scholar] [CrossRef]
- Lattwein, K.R.; Shekhar, H.; Kouijzer, J.J.P.; van Wamel, W.J.B.; Holland, C.K.; Kooiman, K. Sonobactericide: An Emerging Treatment Strategy for Bacterial Infections. Ultrasound Med. Biol. 2020, 46, 193–215. [Google Scholar] [CrossRef]
- Schuch, R.; Cassino, C.; Vila-Farres, X. Direct Lytic Agents: Novel, Rapidly Acting Potential Antimicrobial Treatment Modalities for Systemic Use in the Era of Rising Antibiotic Resistance. Front. Microbiol. 2022, 13, 841905. [Google Scholar] [CrossRef]
- Camou, F.; Dijos, M.; Barandon, L.; Cornolle, C.; Greib, C.; Laine, M.; Lecomte, R.; Boutoille, D.; Machelart, I.; Peuchant, O.; et al. Management of infective endocarditis and multidisciplinary approach. Méd. Mal. Infect. 2019, 49, 17–22. [Google Scholar] [CrossRef] [PubMed]
- El-Dalati, S.; Cronin, D.; Riddell, J.I.V.; Shea, M.; Weinberg, R.L.; Washer, L.; Stoneman, E.; Perry, D.A.; Bradley, S.; Burke, J.; et al. The Clinical Impact of Implementation of a Multidisciplinary Endocarditis Team. Ann. Thorac. Surg. 2022, 113, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Elad, B.; Perl, L.; Hamdan, A.; Yahav, D.; Atamna, A.; Shaked, H.; Rubchevsky, V.; Sharony, R.; Bernstine, H.; Shapira, Y.; et al. The clinical value of the endocarditis team: Insights from before and after guidelines implementation strategy. Infection 2022, 50, 57–64. [Google Scholar] [CrossRef]
- Gibbons, E.F.; Huang, G.; Aldea, G.; Koomalsingh, K.; Klein, J.W.; Dhanireddy, S.; Harrington, R. A Multidisciplinary Pathway for the Diagnosis and Treatment of Infectious Endocarditis. Crit. Pathw. Cardiol. 2020, 19, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Amit, K.; Jonathan, B.; Amanda, F.; Ranjit, D.; Max, B.; Margaret, G.; Whitaker, D.; Monaghan, M.; MacCarthy, P.A.; Wendler, O.; et al. Inception of the “endocarditis team’’ is associated with improved survival in patients with infective endocarditis who are managed medically: Findings from a before-and-after study. Open Heart 2017, 4, e000699. [Google Scholar]
- Ruch, Y.; Mazzucotelli, J.-P.; Lefebvre, F.; Martin, A.; Lefebvre, N.; Douiri, N.; Riegel, P.; Hoang Minh, T.; Petit-Eisenmann, H.; Hansmann, Y.; et al. Impact of Setting up an “Endocarditis Team” on the Management of Infective Endocarditis. Open Forum Infect. Dis. 2019, 6, ofz308. [Google Scholar] [CrossRef]
| Pathogen | Recommended Regimen | Consideration During Pregnancy |
|---|---|---|
| Empiric Treatment | NVE: Daptomycin (8–10 mg/kg/day IV) or (10–12 mg/kg/day) + Ceftriaxone (2 g/day IV) OR Cefazolin (6 g/day IV in 3 doses) PVE: Same regimens apply for NVE and PVE | |
| S. viridans and S. gallolyticus | NVE: Penicillin G (12–18 million U/day IV in 4–6 doses or continuous) OR Amoxicillin (12 g/day IV in 4–6 doses) OR Ceftriaxone (2 g/day IV in 1 dose) for 4 weeks PVE: Same regimens for 6 weeks | |
| Penicillin-resistant S. viridans and Streptococcus gallolyticus | NVE: Penicillin G (24 million U/day IV) OR Amoxicillin (12 g/day IV) OR Ceftriaxone (2 g/day IV) + *Gentamicin (3 mg/kg/day IV) for 2 weeks PVE: Same regimens as NVE but for 6 weeks | *Gentamicin and vancomycin generally avoided in pregnancy. Consider ceftriaxone + daptomycin OR ceftaroline, but efficacy data in pregnancy are limited. |
| MSSA | NVE: Flucloxacillin (12 g/day IV) OR Cefazolin (6 g/day IV) for 4–6 weeks | |
| PVE: Daptomycin (10 mg/kg/day IV) + Ceftaroline (1800 mg/day IV in 3 doses) OR Fosfomycin (8–12 g/day IV) | ||
| MRSA and CoNS | NVE: Daptomycin (10 mg/kg/day IV) + Cloxacillin (12 g/day IV) OR Ceftaroline (1800 mg/day IV in 3 doses) OR Fosfomycin (8–12 g/day IV) | |
| PVE: Daptomycin (10 mg/kg/day IV) + *Rifampicin + *Gentamicin (3 mg/kg/day IV) for 6 weeks | *Rifampicin and gentamicin carry fetal risks. Weigh maternal benefit versus fetal risk. Other potential safe alternatives include fosfomycin plus imipenem, ceftaroline, or quinupristin–dalfopristin with or without beta-lactams, although these data from reports do not exclusively include PVE MRSA. | |
| Enterococcus spp. | ||
| non-HLAR | Ampicillin (200 mg/kg/day) or Amoxicillin (12 g/day IV in 4–6 doses) + Ceftriaxone (4 g/day IV in 2 doses) for 6 weeks | |
| HLAR | Same regimen as non-HLAR Multiresistant: Daptomycin (10 mg/kg/day IV) + one of Ampicillin (200 mg/kg/day), Ertapenem (2 g/day IV), Ceftaroline (1800 mg/day IV in 3 doses), or Fosfomycin (8–12 g/day IV) | |
| HACEK Group | Ceftriaxone (2 g/day IV for 4 weeks—NVE, 6 weeks—PVE) If beta-lactamase-negative: Ampicillin monotherapy possible | |
| Non-HACEK Gram-negative | Beta-lactams + *Aminoglycosides for 6 weeks; sometimes + *Quinolones or *Cotrimoxazole | Many agents carry risk: aminoglycosides, quinolones, or cotrimoxazole. Requires early surgery and specialist consultation |
| Fungi (e.g., Candida, Aspergillus) | Liposomal Amphotericin B (3–5 mg/kg IV) | |
| Antibiotic | Dose | Route of Administration | Time Before Incision |
|---|---|---|---|
| Amoxicillin | 2 g | Orally | 30–60 min |
| Ampicillin | 2 g | i.m. or i.v. | 30–60 min |
| Cefazolin or Ceftriaxone | 1 g | i.m. or i.v. | 30–60 min |
| Cephaxelin | 2 g | Orally | 30–60 min |
| Azithromycin or Clarithromycin | 500 mg | Orally | 30–60 min |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polyzou, E.; Ntalaki, E.; Efthymiou, D.; Papageorgiou, D.; Gavatha, M.; Rigopoulos, E.A.; Skintzi, K.; Tsoupra, S.; Manios, K.; Baikoussis, N.G.; et al. Infective Endocarditis During Pregnancy: Challenges and Future Directions. J. Clin. Med. 2025, 14, 4262. https://doi.org/10.3390/jcm14124262
Polyzou E, Ntalaki E, Efthymiou D, Papageorgiou D, Gavatha M, Rigopoulos EA, Skintzi K, Tsoupra S, Manios K, Baikoussis NG, et al. Infective Endocarditis During Pregnancy: Challenges and Future Directions. Journal of Clinical Medicine. 2025; 14(12):4262. https://doi.org/10.3390/jcm14124262
Chicago/Turabian StylePolyzou, Eleni, Evangelia Ntalaki, Dimitrios Efthymiou, Despoina Papageorgiou, Maria Gavatha, Emmanouil Angelos Rigopoulos, Katerina Skintzi, Stamatia Tsoupra, Konstantinos Manios, Nikolaos G. Baikoussis, and et al. 2025. "Infective Endocarditis During Pregnancy: Challenges and Future Directions" Journal of Clinical Medicine 14, no. 12: 4262. https://doi.org/10.3390/jcm14124262
APA StylePolyzou, E., Ntalaki, E., Efthymiou, D., Papageorgiou, D., Gavatha, M., Rigopoulos, E. A., Skintzi, K., Tsoupra, S., Manios, K., Baikoussis, N. G., & Akinosoglou, K. (2025). Infective Endocarditis During Pregnancy: Challenges and Future Directions. Journal of Clinical Medicine, 14(12), 4262. https://doi.org/10.3390/jcm14124262






