Idiopathic Ventricular Arrhythmias Originating from the Left Ventricular Summit: A Diagnostic and Therapeutic Challenge
Abstract
1. Introduction
2. The Aims and the Methods of the Review
3. Anatomy of LVS
4. Incidence
5. Clinical Presentation
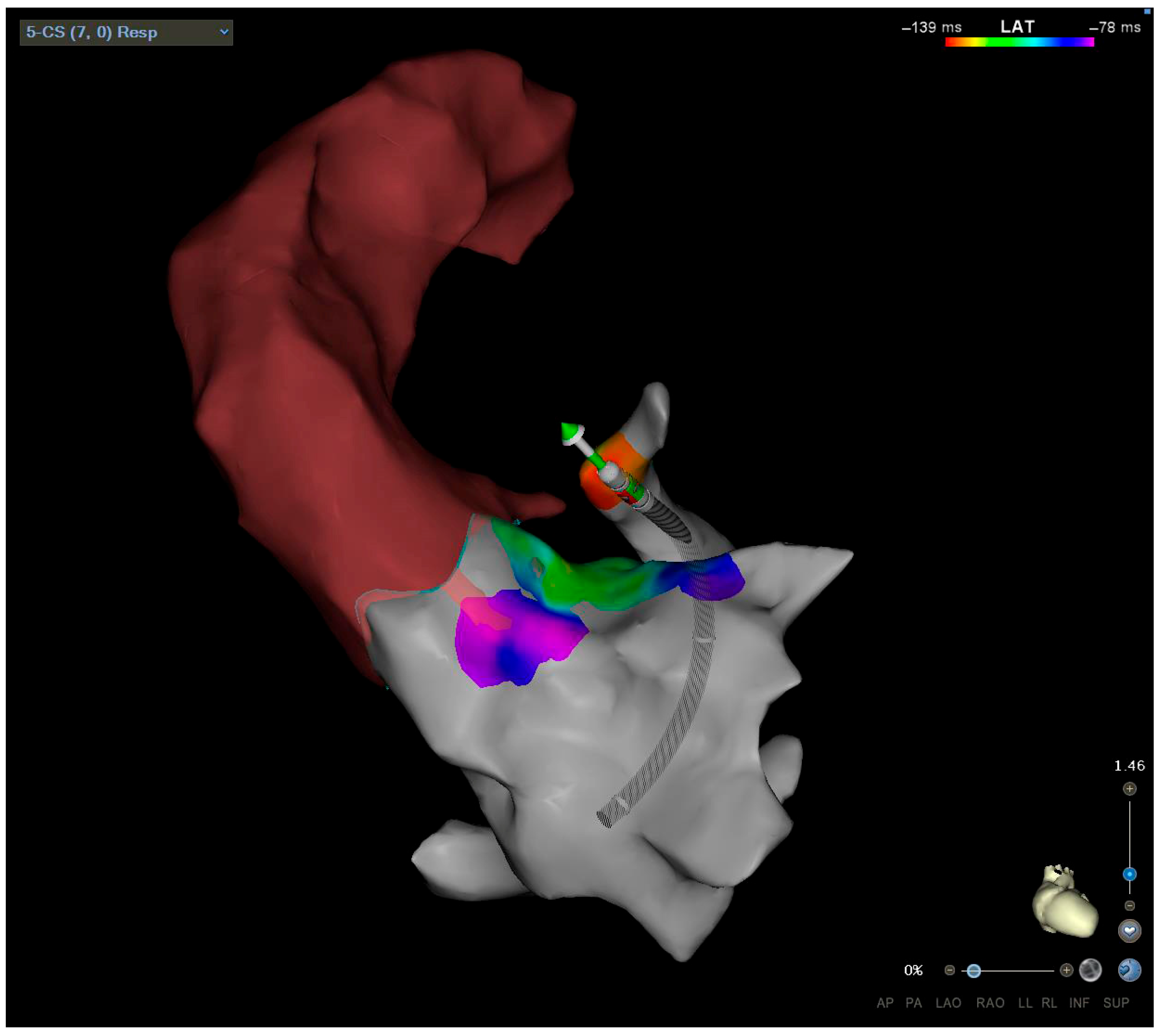
6. Diagnostic Evaluation
6.1. 12-Lead ECG
6.2. Imaging
- Cardiac MRI
- Intracardiac Echocardiography (ICE)
7. Pharmacological Treatment
- Beta-blockers
- Sodium channel blockers
- Potassium channel blockers
- Calcium Channel Blockers
8. Interventional Treatment
| Drug Class | Mechanism | Efficacy | Limitations |
|---|---|---|---|
| Beta-blockers | Sympathetic activity | PVC burden; symptoms; useful in structural heart disease | Fatigue, bradycardia, and hypotension |
| Sodium Channel Blockers | Block sodium channels | PVC burden: symptoms and quality of life | Proarrhythmic risk in structural heart disease |
| Potassium Channel Blockers | Prolonged action potential and refractory period | PVC burden; exercise capacity in PVC-induced cardiomyopathy | Thyroid and pulmonary toxicity; QT prolongation |
| Calcium Channel Blockers | Inhibit calcium influx | Effective in some cases of idiopathic PVCs | Limited evidence base |
| Combination Therapy | Combines mechanisms to achieve better symptom control | Useful when monotherapy is insufficient | Increased risk of side effects; requires close monitoring |
- Endocardial Ablation
- Coronary Sinus Ablation
- Ethanol Ablation
- Left Atrial Appendage
- Epicardial Ablation
- Surgical Ablation
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kuniewicz, M.; Baszko, A.; Ali, D.; Karkowski, G.; Loukas, M.; Walocha, J.A.; Hołda, M.K. Left Ventricular Summit—Concept, Anatomical Structure and Clinical Significance. Diagnostics 2021, 11, 1423. [Google Scholar] [CrossRef] [PubMed]
- Kuniewicz, M.; Krupiński, M.; Gosnell, M.; Budnicka, K.; Jakob, N.; Karkowski, G.; Urbańczyk-Zawadzka, M.; Lelakowski, J.; Walocha, J. Applicability of computed tomography preoperative assessment of the LAA in LV summit ablations. J. Interv. Card. Electrophysiol. 2021, 61, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; McElderry, H.T.; Doppalapudi, H.; Okada, T.; Murakami, Y.; Yoshida, Y.; Yoshida, N.; Inden, Y.; Murohara, T.; Plumb, V.J.; et al. Idiopathic Ventricular Arrhythmias Originating From the Left Ventricular Summit. Circ. Arrhythm. Electrophysiol. 2010, 3, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Słodowska, K.; Szczepanek, E.; Dudkiewicz, D.; Hołda, J.; Bolechała, F.; Strona, M.; Lis, M.; Batko, J.; Koziej, M.; Hołda, M.K. Morphology of the Left Atrial Appendage: Introduction of a New Simplified Shape-Based Classification System. Heart Lung Circ. 2021, 30, 1014–1022. [Google Scholar] [CrossRef]
- Moreira De Andrade, F.; Andrade, F.M.; Ribeiro, D.C.; Babinski, M.A.; Góes, M.L. Triangle of Brocq and Mouchet: Anatomical Study in Brazilian Cadavers and Clinical Implications. J. Morphol. Sci. 2010, 27, 3–4. Available online: https://www.researchgate.net/publication/230561315 (accessed on 1 January 2010).
- Muser, D.; Lavalle, C.; Guarracini, F.; Sassone, B.; Conte, E.; Magnani, S.; Notarstefano, P.; Barbato, G.; Sgarito, G.; Grandinetti, G.; et al. Role of cardiac imaging in patients undergoing catheter ablation of ventricular tachycardia. J. Cardiovasc. Med. 2021, 22, 727–737. [Google Scholar] [CrossRef]
- Yamada, T.; Litovsky, S.; Neal Kay, G. Prevalence and electrocardiographic and electrophysiological characteristics of idiopathic ventricular arrhythmias originating from the septal left ventricular summit. J. Cardiovasc. Electrophysiol. 2024, 35, 1174–1184. [Google Scholar] [CrossRef]
- Lerman, B.B.; Stein, K.M.; Markowitz, S.M. Mechanisms of Idiopathic Left Ventricular Tachycardia. J. Cardiovasc. Electrophysiol. 1997, 8, 571–583. [Google Scholar] [CrossRef]
- Baman, T.S.; Lange, D.C.; Ilg, K.J.; Gupta, S.K.; Liu, T.-Y.; Alguire, C.; Armstrong, W.; Good, E.; Chugh, A.; Jongnarangsin, K.; et al. Relationship between burden of premature ventricular complexes and left ventricular function. Heart Rhythm 2010, 7, 865–869. [Google Scholar] [CrossRef]
- Dragasis, S.; Vlachos, K.; Frontera, A.; Mililis, P.; Saplaouras, A.; Zygouri, A.; Zymatoura, M.E.; Kontonika, M.; Kafkas, N.; Efremidis, M.; et al. Modern mapping and ablation of idiopathic outflow tract ventricular arrhythmias. Rev. Cardiovasc. Med. 2022, 23, 103. [Google Scholar] [CrossRef]
- Okubo, K.; Gigli, L.; Della Bella, P. Catheter ablation of ventricular tachycardia in nonischemic cardiomyopathy. J. Arrhythm. 2018, 34, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Muser, D.; Tritto, M.; Mariani, M.V.; Di Monaco, A.; Compagnucci, P.; Accogli, M.; De Ponti, R.; Guarracini, F. Diagnosis and Treatment of Idiopathic Premature Ventricular Contractions: A Stepwise Approach Based on the Site of Origin. Diagnostics 2021, 11, 1840. [Google Scholar] [CrossRef] [PubMed]
- Bogun, F.; Crawford, T.; Reich, S.; Koelling, T.M.; Armstrong, W.; Good, E.; Jongnarangsin, K.; Marine, J.E.; Chugh, A.; Pelosi, F.; et al. Radiofrequency ablation of frequent, idiopathic premature ventricular complexes: Comparison with a control group without intervention. Heart Rhythm 2007, 4, 863–867. [Google Scholar] [CrossRef] [PubMed]
- Cronin, E.M.; Bogun, F.M.; Maury, P.; Peichl, P.; Chen, M.; Namboodiri, N.; Aguinaga, L.; Leite, L.R.; Al-Khatib, S.M.; Anter, E.; et al. 2019 HRS/EHRA/APHRS/LAHRS expert consensus statement on catheter ablation of ventricular arrhythmias. Heart Rhythm 2020, 17, e2–e154. [Google Scholar] [CrossRef]
- Bazan, V.; Gerstenfeld, E.P.; Garcia, F.C.; Bala, R.; Rivas, N.; Dixit, S.; Zado, E.; Callans, D.J.; Marchlinski, F.E. Site-specific twelve-lead ECG features to identify an epicardial origin for left ventricular tachycardia in the absence of myocardial infarction. Heart Rhythm 2007, 4, 1403–1410. [Google Scholar] [CrossRef]
- Kamali, F.; Haghjoo, M.; Pasebani, Y.; Alizadehdiz, A.; Emkanjoo, Z.; Fazelifar, A.; Masoudkabir, F.; Keykhavani, A.; Madadi, S. A Comprehensive Review of Left Ventricular Summit Ventricular Arrhythmias. J. Tehran Univ. Heart Cent. 2022, 17, 91. [Google Scholar] [CrossRef]
- Lin, C.Y.; Chung, F.P.; Lin, Y.J.; Chong, E.; Chang, S.-L.; Lo, L.-W.; Hu, Y.-F.; Tuan, T.-C.; Chao, T.-F.; Liao, J.-N.; et al. Radiofrequency catheter ablation of ventricular arrhythmias originating from the continuum between the aortic sinus of Valsalva and the left ventricular summit: Electrocardiographic characteristics and correlative anatomy. Heart Rhythm 2016, 13, 111–121. [Google Scholar] [CrossRef]
- Santangeli, P.; Marchlinski, F.E.; Zado, E.S.; Benhayon, D.; Hutchinson, M.D.; Lin, D.; Frankel, D.S.; Riley, M.P.; Supple, G.E.; Garcia, F.C.; et al. Percutaneous Epicardial Ablation of Ventricular Arrhythmias Arising From the Left Ventricular Summit. Circ. Arrhythm. Electrophysiol. 2015, 8, 337–343. [Google Scholar] [CrossRef]
- Chang, D.; Gabriels, J.; Vaishnav, A.; Kim, B.S.; Coleman, K.; Khan, M.; Maisel, K.; Ismail, H.; Goldner, B.; Willner, J.; et al. Electrocardiographic localization of ventricular arrhythmias successfully ablated from the distal great cardiac vein. J. Cardiovasc. Electrophysiol. 2020, 31, 2668–2676. [Google Scholar] [CrossRef]
- Gorenek, B.; Fisher, J.D.; Kudaiberdieva, G.; Baranchuk, A.; Burri, H.; Campbell, K.B.; Chung, M.K.; Enriquez, A.; Heidbuchel, H.; Kutyifa, V.; et al. Premature ventricular complexes: Diagnostic and therapeutic considerations in clinical practice. J. Interv. Card. Electrophysiol. 2020, 57, 5–26. [Google Scholar] [CrossRef]
- Nikolaidou, C.; Kotanidis, C.P.; Wijesurendra, R.; Leal-Pelado, J.; Kouskouras, K.; Vassilikos, V.P.; Karvounis, H.; Ntusi, N.; Antoniades, C.; Neubauer, S.; et al. Cardiac Magnetic Resonance to Detect the Underlying Substrate in Patients with Frequent Idiopathic Ventricular Arrhythmias. Diagnostics 2021, 11, 1109. [Google Scholar] [CrossRef] [PubMed]
- Bennett, R.G.; Campbell, T.; Sood, A.; Bhaskaran, A.; De Silva, K.; Davis, L.; Qian, P.; Sivagangabalan, G.; Cooper, M.J.; Chow, C.K.; et al. Remote magnetic navigation compared to contemporary manual techniques for the catheter ablation of ventricular arrhythmias in structural heart disease. Heliyon 2021, 7, e08538. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, F.; Thibert, M.J.; Gulsin, G.S.; Murphy, D.; Alexander, G.; Andrade, J.G.; Hawkins, N.M.; Laksman, Z.W.; Yeung-Lai-Wah, J.A.; Chakrabarti, S.; et al. Cardiac Magnetic Resonance in the Evaluation of Patients With Frequent Premature Ventricular Complexes. JACC Clin. Electrophysiol. 2022, 8, 1122–1132. [Google Scholar] [CrossRef] [PubMed]
- Enriquez, A.; Saenz, L.C.; Rosso, R.; Silvestry, F.E.; Callans, D.; Marchlinski, F.E.; Garcia, F. Use of Intracardiac Echocardiography in Interventional Cardiology. Circulation 2018, 137, 2278–2294. [Google Scholar] [CrossRef]
- Romero, J.; Diaz, J.C.; Gamero, M.; Alviz, I.; Lorente, M.; Gabr, M.; Toquica, C.C.; Krishnan, S.; Velasco, A.; Lin, A.; et al. Fluoroless Catheter Ablation of Left Ventricular Summit Arrhythmias. Card. Electrophysiol. Clin. 2023, 15, 75–83. [Google Scholar] [CrossRef]
- Chan, A.K.; Dohrmann, M.L. Management of premature ventricular complexes. Mo. Med. 2010, 107, 39–43. [Google Scholar]
- Bayacan, O.F.; Fidan, S.; Celik, F.B.; Tatlisu, M.A.; Ozyildrim, S.; Caliskan, M. Comparison of Medical Treatments According to the Characteristics of Idiopathic Premature Ventricular Contractions: Beta-blockers or Calcium Channel Blockers? Medeni. Med. J. 2023, 38, 32–38. [Google Scholar] [CrossRef]
- Krittayaphong, R.; Bhuripanyo, K.; Punlee, K.; Kangkagate, C.; Chaithiraphan, S. Effect of atenolol on symptomatic ventricular arrhythmia without structural heart disease: A randomized placebo-controlled study. Am. Heart J. 2002, 144, 1–5. [Google Scholar] [CrossRef]
- Kojić, D.; Radunović, A.; Bukumirić, Z.; Rajsic, S.; Sušić, M.; Marić, M.; Žugić, V.; Jurčević, R.; Tomović, M. Idiopathic premature ventricular complexes treatment: Comparison of flecainide, propafenone, and sotalol. Clin. Cardiol. 2023, 46, 1220–1226. [Google Scholar] [CrossRef]
- Bertels, R.A.; Kammeraad, J.A.E.; van Geloven, N.; Filippini, L.H.; van der Palen, R.L.; Tak, R.O.; Frerich, S.; Vanagt, W.; Rehbock, J.J.; Knobbe, I.; et al. ECTOPIC trial: The efficacy of flEcainide Compared To metOprolol in reducing Premature ventrIcular Contractions: A randomized open-label crossover study in pediatric patients. Heart Rhythm 2025, 22, 536–543. [Google Scholar] [CrossRef]
- Hyman, M.C.; Mustin, D.; Supple, G.; Schaller, R.D.; Santangeli, P.; Arkles, J.; Lin, D.; Muser, D.; Dixit, S.; Nazarian, S.; et al. Class IC antiarrhythmic drugs for suspected premature ventricular contraction–induced cardiomyopathy. Heart Rhythm 2018, 15, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Echt, D.S.; Liebson, P.R.; Mitchell, L.B.; Peters, R.W.; Obias-Manno, D.; Barker, A.H.; Arensberg, D.; Baker, A.; Friedman, L.; Greene, H.L.; et al. Mortality and Morbidity in Patients Receiving Encainide, Flecainide, or Placebo. N. Engl. J. Med. 1991, 324, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Fletcher, R.D.; Fisher, S.; Deedwania, P.; Lewis, D.; Massie, B.; Singh, B.; Colling, C.L. Congestive heart failure: Survival trial of antiarrhythmic therapy (CHF STAT). Control. Clin. Trials 1992, 13, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Latchamsetty, R.; Bogun, F. Premature Ventricular Complex–Induced Cardiomyopathy. JACC Clin. Electrophysiol. 2019, 5, 537–550. [Google Scholar] [CrossRef]
- Anderson, J.L.; Askins, J.C.; Gilbert, E.M.; Miller, R.H.; Keefe, D.L.; Somberg, J.C.; Freedman, R.A.; Haft, L.R.; Mason, J.W.; Lessem, J.N. Multicenter trial of sotalol for suppression of frequent, complex ventricular arrhythmias: A double-blind, randomized, placebo-controlled evaluation of two doses. J. Am. Coll. Cardiol. 1986, 8, 752–762. [Google Scholar] [CrossRef]
- Sharma, N.; Coleman, K.; Cunn, G.; Kleiman, J.; Mountantonakis, S. Anatomically Based Ablation of Left Ventricular Summit Premature Ventricular Complexes Guided by Intracardiac Echocardiography. J. Innov. Card. Rhythm. Manag. 2024, 15, 5774–5776. [Google Scholar] [CrossRef]
- Garg, L.; Daubert, T.; Lin, A.; Dhakal, B.; Santangeli, P.; Schaller, R.; Hyman, M.C.; Kumareswaran, R.; Arkles, J.; Nazarian, S.; et al. Utility of Prolonged Duration Endocardial Ablation for Ventricular Arrhythmias Originating From the Left Ventricular Summit. JACC Clin. Electrophysiol. 2022, 8, 465–476. [Google Scholar] [CrossRef]
- Efremidis, M.; Vlachos, K.; Bazoukis, G.; Frontera, A.; Martin, C.A.; Dragasis, S.; Valkanas, K.; Letsas, K.P. Novel technique targeting left ventricular summit premature ventricular contractions using radiofrequency ablation through a guidewire. Hear. Case Rep. 2021, 7, 134–138. [Google Scholar] [CrossRef]
- Cheung, J.W.; Anderson, R.H.; Markowitz, S.M.; Lerman, B.B. Catheter Ablation of Arrhythmias Originating From the Left Ventricular Outflow Tract. JACC Clin. Electrophysiol. 2019, 5, 1–12. [Google Scholar] [CrossRef]
- Sosa, E.; Scanavacca, M.; D’Avila, A.; Pilleggi, E. A New Technique to Perform Epicardial Mapping in the Electrophysiology Laboratory. J. Cardiovasc. Electrophysiol. 1996, 7, 531–536. [Google Scholar] [CrossRef]
- Flautt, T.; Valderrábano, M. Retrograde Coronary Venous Ethanol Infusion for Ablation of Refractory Left Ventricular Summit Arrhythmias. Card. Electrophysiol. Clin. 2023, 15, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Barbhaiya, C.R.; Sobieszczyk, P.; Eisenhauer, A.C.; Couper, G.S.; Nagashima, K.; Mahida, S.; Baldinger, S.H.; Choi, E.-K.; Epstein, L.M.; et al. Role of Alternative Interventional Procedures When Endo- and Epicardial Catheter Ablation Attempts for Ventricular Arrhythmias Fail. Circ. Arrhythm. Electrophysiol. 2015, 8, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Nsahlai, M.; Flautt, T.; Da-Warikobo, A.; Lador, A.; Tapias, C.; Rodríguez, D.; Sáenz, L.C.; Schurmann, P.A.; Dave, A.; et al. Advanced Techniques for Ethanol Ablation of Left Ventricular Summit Region Arrhythmias. Circ. Arrhythm. Electrophysiol. 2022, 15, e011017. [Google Scholar] [CrossRef] [PubMed]
- Kreidieh, B.; Rodríguez-Mañero, M.; Schurmann, P.A.; Ibarra-Cortez, S.H.; Dave, A.S.; Valderrábano, M. Retrograde Coronary Venous Ethanol Infusion for Ablation of Refractory Ventricular Tachycardia. Circ. Arrhythm. Electrophysiol. 2016, 9, e004352. [Google Scholar] [CrossRef]
- Yakubov, A.; Salayev, O.; Hamrayev, R.; Sultankhonov, S. A case of successful ablation of ventricular tachycardia focus in the left ventricular summit through the left atrial appendage: A case report. Eur. Heart J. Case Rep. 2018, 2, yty110. [Google Scholar] [CrossRef]
- Benhayon, D.; Cogan, J.; Young, M. Left atrial appendage as a vantage point for mapping and ablating premature ventricular contractions originating in the epicardial left ventricular summit. Clin. Case Rep. 2018, 6, 1124–1127. [Google Scholar] [CrossRef]
- Enriquez, A.; Malavassi, F.; Saenz, L.C.; Supple, G.; Santangeli, P.; Marchlinski, F.E.; Garcia, F.C. How to map and ablate left ventricular summit arrhythmias. Heart Rhythm 2017, 14, 141–148. [Google Scholar] [CrossRef]
- Della Bella, P.; Brugada, J.; Zeppenfeld, K.; Merino, J.; Neuzil, P.; Maury, P.; Maccabelli, G.; Vergara, P.; Baratto, F.; Berruezo, A.; et al. Epicardial Ablation for Ventricular Tachycardia. Circ. Arrhythm. Electrophysiol. 2011, 4, 653–659. [Google Scholar] [CrossRef]
- Di Biase, L.; Al-Ahamad, A.; Santangeli, P.; Hsia, H.H.; Sanchez, J.; Bai, R.; Bailey, S.; Horton, R.; Gallinghouse, G.J.; Burkhardt, D.J.; et al. Safety and outcomes of cryoablation for ventricular tachyarrhythmias: Results from a multicenter experience. Heart Rhythm 2011, 8, 968–974. [Google Scholar] [CrossRef]
- Okabe, T.; Rushing, G.; Kalbfleisch, S. Surgical Mapping and Ablation in the Left Ventricular Summit Guided by Presurgery Pericardial Mapping. J. Innov. Card. Rhythm. Manag. 2019, 10, 3582–3587. [Google Scholar] [CrossRef][Green Version]

| Symptom | Notes |
|---|---|
| Palpitations | Common with high PVC burden, often linked to anxiety and exercise activity. |
| Fatigue | Caused by ventricular dyssynchrony and reduced cardiac output during activity. |
| Exercise Intolerance | Common in active individuals, linked to reduced cardiac output during exertion. |
| Chest Pain (Atypical) | Rare, sharp, non-ischemic pain from mechanical effects of PVCs. |
| PVC-Induced Cardiomyopathy | Risk increases with high PVC burden; untreated cases show progressive LV dysfunction. |
| Malignant Arrhythmias | Rare, but includes VT or VF, especially with structural heart disease or critical anatomy. |
| Asymptomatic | Common; a high PVC burden may still lead to cardiomyopathy or subclinical LV dysfunction. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falco, R.; Tognola, C.; Gigli, L.; Baroni, M.; Frontera, A.; Varrenti, M.; Preda, A.; Carbonaro, M.; Menè, R.; Milillo, L.F.; et al. Idiopathic Ventricular Arrhythmias Originating from the Left Ventricular Summit: A Diagnostic and Therapeutic Challenge. J. Clin. Med. 2025, 14, 4261. https://doi.org/10.3390/jcm14124261
Falco R, Tognola C, Gigli L, Baroni M, Frontera A, Varrenti M, Preda A, Carbonaro M, Menè R, Milillo LF, et al. Idiopathic Ventricular Arrhythmias Originating from the Left Ventricular Summit: A Diagnostic and Therapeutic Challenge. Journal of Clinical Medicine. 2025; 14(12):4261. https://doi.org/10.3390/jcm14124261
Chicago/Turabian StyleFalco, Raffaele, Chiara Tognola, Lorenzo Gigli, Matteo Baroni, Antonio Frontera, Marisa Varrenti, Alberto Preda, Marco Carbonaro, Roberto Menè, Leandro Fabrizio Milillo, and et al. 2025. "Idiopathic Ventricular Arrhythmias Originating from the Left Ventricular Summit: A Diagnostic and Therapeutic Challenge" Journal of Clinical Medicine 14, no. 12: 4261. https://doi.org/10.3390/jcm14124261
APA StyleFalco, R., Tognola, C., Gigli, L., Baroni, M., Frontera, A., Varrenti, M., Preda, A., Carbonaro, M., Menè, R., Milillo, L. F., Sultana, A., Vargiu, S., Colombo, G., Giordano, F., Giannattasio, C., Mazzone, P., & Guarracini, F. (2025). Idiopathic Ventricular Arrhythmias Originating from the Left Ventricular Summit: A Diagnostic and Therapeutic Challenge. Journal of Clinical Medicine, 14(12), 4261. https://doi.org/10.3390/jcm14124261







