Palliative Luminal Treatment of Colorectal Cancer Using Endoscopic Calcium-Electroporation: First Case Series from United Kingdom
Abstract
1. Introduction
2. Materials and Methods
2.1. Setting
2.2. Eligibility
2.3. Pre-Procedure Evaluation
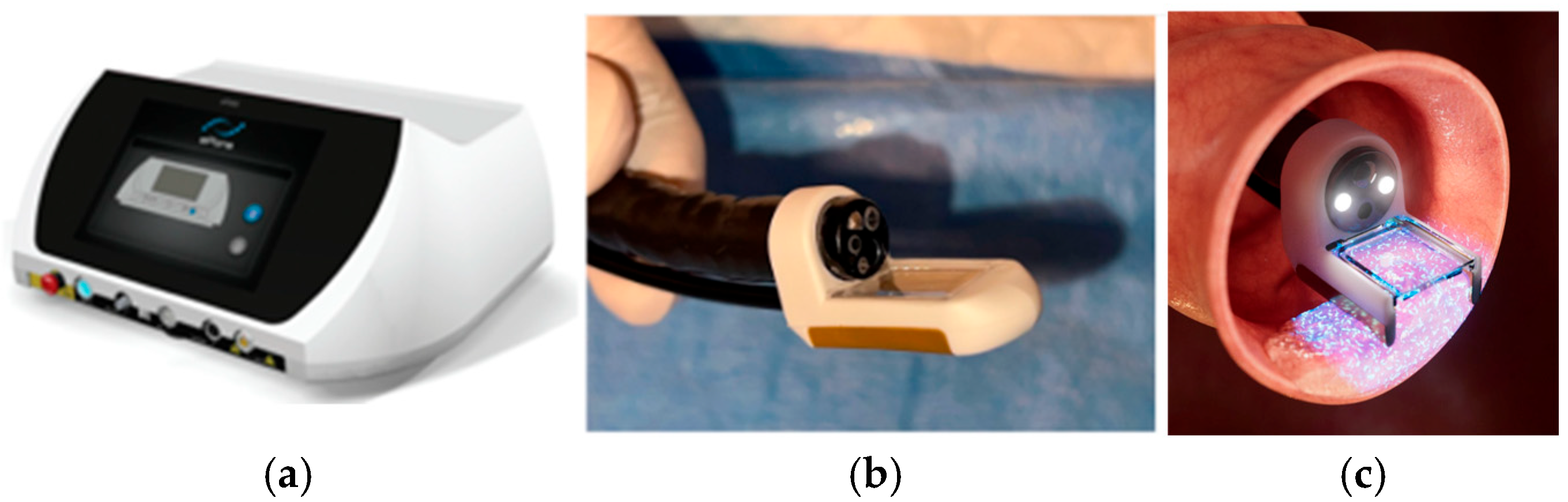
2.4. Endoscopic Calcium Electroporation Protocol
3. Results
3.1. Patient Demographics and Clinical Characteristics
3.2. Primary Endpoints
3.2.1. Safety Assessment
3.2.2. Symptomatic Response
3.2.3. Quality of Life Assessment Response
3.2.4. Tumor Response
3.3. Secondary Endpoints
3.3.1. Overall Survival (OS)
3.3.2. Reduction in Blood Transfusion and Iron Requirements
4. Discussion
4.1. Limitations of This Study
4.2. Future Studies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AE | Adverse Event |
| APC | Argon Plasma Coagulation |
| ASA | American Society of Anesthesiologists |
| BSG | British Society of Gastroenterology |
| Ca-EP | Calcium Electroporation |
| CCI | Charlson Comorbidity Index |
| CE | Conformité Europénne |
| CRC | Complex Colorectal Cancer |
| ECOG | Eastern Cooperative Oncology Group |
| GI | Gastrointestinal |
| IV | Intravenous |
| MDD | Medical Device Directive |
| MDT | Multidisciplinary Team |
| NHS | National Health Service |
| OS | Overall survival |
| PFS | Performance Status |
| QOL | Quality of Life |
| SAE | Serious Adverse Event |
| SELCA | South East London Cancer Alliance |
| UK | United Kingdom |
References
- Morgan, E.; Arnold, M.; Gini, A.; Lorenzoni, V.; Cabasag, C.J.; Laversanne, M.; Vignat, J.; Ferlay, J.; Murphy, N.; Bray, F. Global burden of colorectal cancer in 2020 and 2040: Incidence and mortality estimates from GLOBOCAN. Gut 2023, 72, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/bowel-cancer (accessed on 1 April 2025).
- Biller, L.H.; Schrag, D. Diagnosis and Treatment of Metastatic Colorectal Cancer: A Review. JAMA 2021, 325, 669–685. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.H.; Liu, Z.Z.; Yuan, Z.X.; Ma, T.H.; Huang, X.Y.; Wang, H.M.; Chen, D.C.; Wang, J.P.; Wang, L. Efficacy and complications of argon plasma coagulation for hemorrhagic chronic radiation proctitis. World J. Gastroenterol. 2019, 25, 1618–1627. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Seo, S.Y.; Kim, S.W. Endoscopic Management of Malignant Colonic Obstruction. Clin. Endosc. 2020, 53, 9–17. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Frandsen, S.K.; Vissing, M.; Gehl, J. A Comprehensive Review of Calcium Electroporation—A Novel Cancer Treatment Modality. Cancers 2020, 12, 290. [Google Scholar] [CrossRef]
- Batista Napotnik, T.; Polajžer, T.; Miklavčič, D. Cell death due to electroporation—A review. Bioelectrochemistry 2021, 141, 107871. [Google Scholar] [CrossRef] [PubMed]
- Szewczyk, A.; Baczyńska, D.; Choromańska, A.; Łapińska, Z.; Chwiłkowska, A.; Saczko, J.; Kulbacka, J. Advancing cancer therapy: Mechanisms, efficacy, and limitations of calcium electroporation. Biochim. Et Biophys. Acta (BBA)—Rev. Cancer 2025, 1880, 189319. [Google Scholar] [CrossRef]
- Ágoston, D.; Baltás, E.; Ócsai, H.; Rátkai, S.; Lázár, P.G.; Korom, I.; Varga, E.; Németh, I.B.; Dósa-Rácz Viharosné, É.; Gehl, J.; et al. Evaluation of Calcium Electroporation for the Treatment of Cutaneous Metastases: A Double-Blinded Randomised Controlled Phase II Trial. Cancers 2020, 12, 179. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rudno-Rudzińska, J.; Kielan, W.; Guziński, M.; Płochocki, M.; Antończyk, A.; Kulbacka, J. New therapeutic strategy: Personalization of pancreatic cancer treatment-irreversible electroporation (IRE), electrochemotherapy (ECT) and calcium electroporation (Ca-EP)—A pilot preclinical study. Surg. Oncol. 2021, 38, 101634. [Google Scholar] [CrossRef]
- Plaschke, C.C.; Gehl, J.; Johannesen, H.H.; Fischer, B.M.; Kjaer, A.; Lomholt, A.F.; Wessel, I. Calcium electroporation for recurrent head and neck cancer: A clinical phase I study. Laryngoscope Investig. Otolaryngol. 2019, 4, 49–56. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Broholm, M.; Vogelsang, R.; Bulut, M.; Stigaard, T.; Falk, H.; Frandsen, S.; Pedersen, D.L.; Perner, T.; Fiehn, A.K.; Mølholm, I.; et al. Endoscopic calcium electroporation for colorectal cancer: A phase I study. Endosc. Int. Open 2023, 11, E451–E459. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Falk Hansen, H.; Bourke, M.; Stigaard, T.; Clover, J.; Buckley, M.; O’Riordain, M.; Winter, D.C.; Hjorth Johannesen, H.; Hansen, R.H.; Heebøll, H.; et al. Electrochemotherapy for colorectal cancer using endoscopic electroporation: A phase 1 clinical study. Endosc. Int. Open 2020, 8, E124–E132. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Broholm, M.; Vogelsang, R.; Bulut, M.; Gögenur, M.; Stigaard, T.; Orhan, A.; Schefte, X.; Fiehn, A.M.K.; Gehl, J.; Gögenur, I. Neoadjuvant calcium electroporation for potentially curable colorectal cancer. Surg. Endosc. 2024, 38, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Egeland, C.; Baeksgaard, L.; Johannesen, H.H.; Löfgren, J.; Plaschke, C.C.; Svendsen, L.B.; Gehl, J.; Achiam, M.J. Endoscopic electrochemotherapy for esophageal cancer: A phase I clinical study. Endosc. Int. Open 2018, 6, E727–E734. [Google Scholar] [CrossRef]
- Egeland, C.; Baeksgaard, L.; Gehl, J.; Gögenur, I.; Achiam, M.P. Palliative treatment of esophageal cancer using calcium electroporation. Cancers 2022, 14, 5283. [Google Scholar] [CrossRef]
- Egeland, C.; Baeksgaard, L.; Gehl, J.; Gögenur, I.; Achiam, M.P. Endoscopic-assisted electrochemotherapy versus argon plasma coagulation in non-curable esophageal cancer—A randomized clinical trial. Med. Res. Arch. 2023, 11, 1–11. [Google Scholar] [CrossRef]
- Beintaris, I.; Abdulhannan, P.; Etherson, K.; Jacob, J. Calcium electroporation for palliation of colorectal cancer. Endoscopy 2025, 57, eP205. [Google Scholar] [CrossRef]
- Gómez, A.G.; Martínez, M.R.; Nievas, R.F.; Roncero, F.S.; Romero, C.B.; Bayonas, L.M.; Vidal, G.C.; Valenzuela, J.E. Successful management of recurrent gastrointestinal bleeding in metastatic cholangiocarcinoma using calcium electroporation. Endoscopy 2025, 57, eP181. [Google Scholar] [CrossRef]
- Rodriguez, H.N.; Martinez, E.V.; Cuevas, C.M.; Crespo, A.S.J.; Redondo, P.D. Endoscopic Electroporation for controlling bleeding and stenosis secondary to digestive tract cancer: Experience in a tertiary hospital. Endoscopy 2025, 57, eP820. [Google Scholar] [CrossRef]
- Agazzi, S.; Cappellini, A.; Rovedatti, L.; Mazza, S.; Mauro, A.; Pozzi, L.; Delogu, C.; Scalvini, D.; Ravetta, V.; Anderloni, A. Calcium electroporation as palliative treatment in esophageal cancer: A case report. Endoscopy 2025, 57, eP557V. [Google Scholar]
- Taher, A.; Rey, J. Endoscopic electroporation, first experience in a German endoscopy center. Endoscopy 2024, 56, eP553. [Google Scholar]
- Carlisle, J.B. Pre-operative co-morbidity and postoperative survival in the elderly: Beyond one lunar orbit. Anaesthesia 2014, 69, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Soler-González, G.; Sastre-Valera, J.; Viana-Alonso, A.; Aparicio-Urtasun, J.; García-Escobar, I.; Gómez-España, M.A.; Guillén-Ponce, C.; Molina-Garrido, M.J.; Gironés-Sarrió, R. Update on the management of elderly patients with colorectal cancer. Clin. Transl. Oncol. 2024, 26, 69–84, Erratum in Clin. Transl. Oncol. 2024, 26, 308–309. https://doi.org/10.1007/s12094-023-03351-x. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Adeyeye, A.; Saini, A.; Gao, H.; Haji, A. Colorectal Cancer Survival in Patients Without Curative Measures: A Retrospective Cohort Review. 2025; unpublished. [Google Scholar]
- Franklyn, J.; Poole, A.; Lindsey, I. Colon cancer survival in the elderly without curative surgery. In The Annals of the Royal College of Surgeons of England; Royal College of Surgeons of England: London, UK, 2024; Volume 106, pp. 592–595. [Google Scholar] [CrossRef]
- He, C.; Wang, J.; Sun, S.; Zhang, Y.; Li, S. Immunomodulatory effect after irreversible electroporation in patients with locally advanced pancreatic cancer. J. Oncol. 2019, 2019, 9346017. [Google Scholar] [CrossRef]
- He, C.; Huang, X.; Zhang, Y.; Lin, X.; Li, S. T-cell activation and immune memory enhancement induced by irreversible electroporation in pancreatic cancer. Clin. Transl. Med. 2020, 10, e39. [Google Scholar] [CrossRef]
- Zhao, J.; Wen, X.; Tian, L.; Li, T.; Xu, C.; Wen, X.; Melancon, M.P.; Gupta, S.; Shen, B.; Peng, W.; et al. Irreversible electroporation reverses resistance to immune checkpoint blockade in pancreatic cancer. Nat. Commun. 2019, 10, 889. [Google Scholar] [CrossRef]
- Geboers, B.; Scheltema, M.J.; Jun, J.; Baker, K.; Timmer, F.E.F.; Cerutti, X.; Katelaris, A.; Doan, P.; Gondoputro, W.; Blazevski, A.; et al. Irreversible electroporation of localized prostate cancer downregulates immune suppression and induces systemic anti-tumour T-cell activation—IRE-IMMUNO study. BJU Int. 2024, 135, 319–328. [Google Scholar] [CrossRef]
- Campana, L.G.; Daud, A.; Lancellotti, F.; Arroyo, J.P.; Davalos, R.V.; Di Prata, C.; Gehl, J. Pulsed Electric Fields in Oncology: A Snapshot of Current Clinical Practices and Research Directions from the 4th World Congress of Electroporation. Cancers 2023, 15, 3340. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

| Patient | Sex | Age | Tumor Location | Stage of Disease | ASA Score | ECOG Performance Score | Comorbidities | Previous Treatment |
|---|---|---|---|---|---|---|---|---|
| Patient 1 | F | 92 | Sigmoid | Nonmetastatic | 5 | 3 | Chronic kidney disease | None |
| Patient 2 | F | 88 | Sigmoid | Metastatic | 5 | 4 | Heart disease, hypercholesteremia, asthma, cataracts | Neoadjuvant CRT, loop colostomy |
| Patient 3 | M | 89 | Sigmoid | Metastatic | 4 | 4 | Osteoporosis, pathological fractures, bed sores, squamous cell carcinoma, actinic keratosis, cardiac arrhythmias on pacemaker and anticoagulants | None |
| Patient 4 | F | 86 | Rectal | Nonmetastatic | 5 | 4 | Alzheimer’s, hypertension, heart disease, stroke (on anticoagulants), asthma, hyperlipidemia | None |
| Patient 5 | M | 79 | Sigmoid | Nonmetastatic | 5 | 3 | Atrial fibrillation, ischemic heart disease, systemic hypertension, pulmonary hypertension, liver cirrhosis, Type 2 diabetes, chronic kidney disease, basal cell carcinoma of the skin, prostate cancer | None |
| Patient 6 | F | 86 | Rectal | Metastatic | 5 | 3 | Ischemic heart disease (on anticoagulants), hyperlipidemia, depression | Radiotherapy |
| Patient 7 | M | 92 | Descending colon | Metastatic | 5 | 4 | Osteoporosis, pathological fractures, postural hypotension (on long-term steroids), pre-diabetes, hiatal hernia, glaucoma | None |
| Patient 8 | M | 80 | Sigmoid | Metastatic | 5 | 5 | Hypertension, deep vein thrombosis, atrial fibrillation (on anticoagulants), pulmonary embolism, heart failure | None |
| Patient 9 | M | 84 | Sigmoid | Metastatic | 4 | 3 | Hypertension, chronic kidney, chronic obstructive lung disease, benign prostate hyperplasia, asthma | None |
| Patient 10 | M | 89 | Sigmoid | Nonmetastatic | 4 | 3 | Type 2 diabetes, hypertension, heart failure, chronic kidney disease | None |
| Patient 11 | M | 88 | Rectal | Metastatic | 4 | 3 | Hypertension, chronic kidney disease, benign prostatic hyperplasia, hyperlipidemia | None |
| Patient 12 | M | 87 | Rectal | Metastatic | 4 | 3 | Hypertension, hyperlipidemia | Chemo- radiotherapy |
| Patient 13 | F | 63 | Rectal | Metastatic | 4 | 3 | Stroke | Surgery, chemotherapy, immunotherapy, radiotherapy |
| Patient 14 | F | 85 | Rectal | Nonmetastatic | 4 | 3 | Pre-diabetes, osteopenia, hypertension, chronic obstructive lung disease, chronic kidney disease | Chemo-radiotherapy |
| Patient 15 | M | 81 | Sigmoid | Nonmetastatic | 4 | 3 | Stroke, hypertension, deep vein thrombosis, atrial fibrillation (on anticoagulants), chronic kidney disease | None |
| Patient 16 | M | 86 | Recto Sigmoid | Nonmetastatic | 4 | Multiple Sclerosis with pulmonary embolism and on anticoagulants | None |
| Patient | Presenting Symptoms | Symptomatic Response | Duration of Response at Time of Reporting |
|---|---|---|---|
| Patient 1 | Pain, Bleeding, Constipation | Yes | 30 months at last follow-up for pain and bleeding. Occasional use of laxatives. |
| Patient 2 | Bleeding, Constipation | Yes | The patient died 11 months after treatment. Asymptomatic for bleeding and constipation for duration. |
| Patient 3 | Change in Movements, pain, Bleeding, Anemia, Fatigue, | Yes | Durable response for change in movement, pain, and fatigue for 8 months. Cessation of bleeding following first treatment for approx. 3 months. Cessation in bleeding following third treatment for 6 months. The patient died 12 months after the first treatment; the cause of death was not device-related. |
| Patient 4 | Constipation, Pain, Bleeding | Yes | Durable response for pain and constipation following first treatment (approx. 1.5 years). Transient response for bleeding between treatments (median interval between visits = 16.75 weeks). |
| Patient 5 | Anemia Requiring Multiple Blood Transfusions and Iron Infusions | Yes | Durable response for anemia to 12 months. The patient has not required a blood transfusion since initial treatment (12 months). The patient required one iron infusion 8 weeks after initial treatment. Likely that this is a result of underlying kidney disease. |
| Patient 6 | Anemia, Pain | Yes | 12 months for anemia and pain. |
| Patient 7 | Pain, Bleeding | Yes | 12 months for bleeding and pain. Complete response to treatment. |
| Patient 8 | Change in bowels, Loose Stools, Bloating, Flatulence | Yes | Symptomatic response for 2 months. The patient died two months after treatment; unrelated causality. |
| Patient 9 | Bleeding | Yes | 6 months following the second treatment. The patient had a temporary bleeding response of 4 weeks following the first treatment. |
| Patient 10 | Bleeding | Yes | 7 months. |
| Patient 11 | Pain, Bleeding, Mucus/Diarrhea | Yes | 3-month lasting response for pain and bleeding. Significant reduction in diarrhea and mucus. The patient is no longer taking medication for this. |
| Patient 12 | Pain | Unk | The patient reported improvement in pain. |
| Patient 13 | Pain, Bleeding, Discharge | Yes | 2-month response for all symptoms. |
| Patient 14 | Pain, Tenesmus, Constipation | Yes | 2-month response for all symptoms. |
| Patient 15 | Bleeding, Pain | Yes | 2-month response for all symptoms. |
| Patient 16 | Bleeding, Pain, Incontinence | Yes | 2-month response for bleeding and pain. Still experiencing incontinence, likely a symptom of underlying Multiple Sclerosis. |
| Patient # | Treatment # | Weeks Since Previous Treatment | Presenting Symptoms | Tumor Size | Number of EndoVE Applications | Tumor Response |
|---|---|---|---|---|---|---|
| Patient 1 | 1 | NA | Pain, Constipation | Not recorded | Not recorded | Reduction in tumor size |
| 2 | 3.5 | Asymptomatic | Not recorded | Not recorded | Reduction in tumor size | |
| 3 | 16 | Asymptomatic | Not recorded | Not recorded | Reduction in tumor size | |
| Patient 2 | 1 | NA | Pain, Bleeding | Not recorded | Not recorded | Did not return for an endoscopic assessment |
| Patient 3 | 1 | NA | Change in Movements, Pain, Bleeding, Anemia, Fatigue | 7 cm–8 cm | 12 | ~50% reduction in size and vascularization 2 months after initial treatment |
| 2 | 10 | Asymptomatic | ~4 cm | Not recorded | Unk—increase in tumor size at next visit | |
| 3 | 15.5 | Bleeding | 8 cm × 10 cm | Not recorded | Symptomatic response to bleeding | |
| 4 | 16 | Obstruction | 10 cm × 12 cm | 8 | Unk—the patient passed away 2 months later. Cause of death unrelated to disease | |
| Patient 4 | 1 | NA | Constipation, Pain, Bleeding | Not recorded | Not recorded | Symptomatic response for pain, bleeding, and constipation |
| 2 | 10 | Asymptomatic | Not recorded | 9 | Transient cessation of bleeding (6 weeks). Decrease in tumor size | |
| 3 | 16 | Bleeding | 5 cm × 15 cm | 2 | Decrease in tumor size | |
| 4 | 23 | Bleeding | 5 cm × 5 cm | 2 | Plateau in response—stable disease | |
| 5 | 18 | Bleeding | 5 cm × 5 cm | 2 | Endoscopic assessment pending | |
| Patient 5 | 1 | NA | Anemia Requiring Multiple Blood Transfusions and Iron Infusions | 18 cm | Not recorded | Decrease in tumor size |
| 2 | 11 | Asymptomatic | 6 cm | 4 | Decrease in tumor size | |
| 3 | 6.5 | Asymptomatic | 4 cm × 4 cm | 7 | Plateau in response—stable disease | |
| 4 | 7.5 | Asymptomatic | 4 cm × 4 cm | 4 | Endoscopic assessment pending | |
| Patient 6 | 1 | NA | Anemia, Pain | 6 cm × 5 cm | 5 | Did not return for endoscopic assessment |
| Patient 7 | 1 | NA | Pain, Bleeding | 6 cm | 4 | Complete response |
| Patient 8 | 1 | NA | Change in Bowels, Loose Stools, Bloating, Flatulence | 8 cm | Not Recorded | ~50% reduction in tumor size |
| Patient 9 | 1 | NA | Bleeding | 8 cm | 4 | Increase in tumor size. Bulky and covering the circumference of the colon |
| 2 | 14 | Asymptomatic | 10 cm × 10 cm | 4 | >50% reduction in tumor size | |
| 3 | 7.5 | Asymptomatic | 4 cm × 4 cm | 4 | Endoscopic assessment pending | |
| Patient 10 | 1 | NA | Bleeding | 9 cm | 7 | ~20% tumor response |
| 2 | 5.5 | Asymptomatic | Not recorded | Not Recorded | Plateau in response—stable disease | |
| 3 | 8.5 | Asymptomatic | 6 cm | 4 | Endoscopic assessment pending | |
| Patient 11 | 1 | NA | Pain, Bleeding, Mucus/Diarrhea | 8 × 10 | 5 | 50% of the treated area responded |
| 2 | 5 | Mucus/Diarrhea | 5 cm | 8 | Endoscopic assessment pending | |
| Patient 12 | 1 | NA | Pain | 2 cm × 4 cm | 2 | Did not return for endoscopic assessment |
| Patient 13 | 1 | NA | Pain, Bleeding, Discharge | Unable to assess—extensive gastric disease extending to the lumen | 2 | Did not return for endoscopic assessment |
| Patient 14 | 1 | NA | Pain, Tenesmus, Constipation | 10 cm × 8 cm | 2 | Stable disease—the patient has opted for surgical intervention |
| Patient 15 | 1 | NA | Bleeding, Pain | 8 cm × 8 cm | 5 | Stable disease |
| 2 | 5 | Bleeding | 8 cm × 8 cm | 8 | Endoscopic assessment pending | |
| Patient 16 | 1 | NA | Bleeding, Pain, Incontinence | 10 cm × 8 cm | 7 | ~25% reduction in width of tumor |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adeyeye, A.; Olabintan, O.; Ayubi, H.; Gao, H.; Saini, A.; Emmanuel, A.; Hayee, B.; Haji, A. Palliative Luminal Treatment of Colorectal Cancer Using Endoscopic Calcium-Electroporation: First Case Series from United Kingdom. J. Clin. Med. 2025, 14, 4138. https://doi.org/10.3390/jcm14124138
Adeyeye A, Olabintan O, Ayubi H, Gao H, Saini A, Emmanuel A, Hayee B, Haji A. Palliative Luminal Treatment of Colorectal Cancer Using Endoscopic Calcium-Electroporation: First Case Series from United Kingdom. Journal of Clinical Medicine. 2025; 14(12):4138. https://doi.org/10.3390/jcm14124138
Chicago/Turabian StyleAdeyeye, Ademola, Olaolu Olabintan, Homira Ayubi, Hao Gao, Aman Saini, Andrew Emmanuel, Bu’Hussain Hayee, and Amyn Haji. 2025. "Palliative Luminal Treatment of Colorectal Cancer Using Endoscopic Calcium-Electroporation: First Case Series from United Kingdom" Journal of Clinical Medicine 14, no. 12: 4138. https://doi.org/10.3390/jcm14124138
APA StyleAdeyeye, A., Olabintan, O., Ayubi, H., Gao, H., Saini, A., Emmanuel, A., Hayee, B., & Haji, A. (2025). Palliative Luminal Treatment of Colorectal Cancer Using Endoscopic Calcium-Electroporation: First Case Series from United Kingdom. Journal of Clinical Medicine, 14(12), 4138. https://doi.org/10.3390/jcm14124138






