Revolutionizing Gastrointestinal Disorder Management: Cutting-Edge Advances and Future Prospects
Abstract
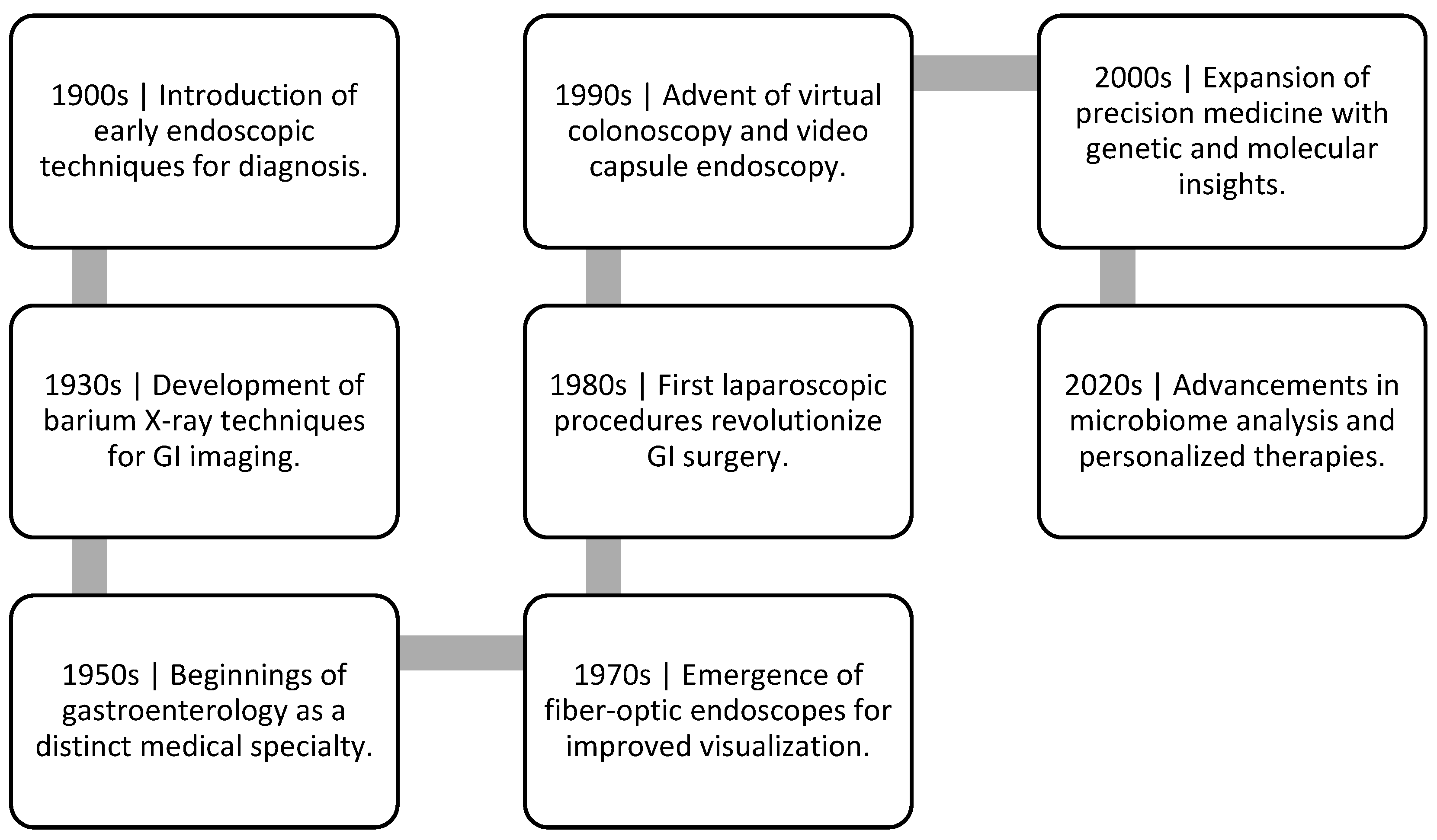
1. Introduction
2. Diagnostic Innovations: From Traditional to Precision Medicine
| Diagnostic Method | Advantages | Applications | Associated Disease Types | Reference |
|---|---|---|---|---|
| Advanced Endoscopy | Early detection of lesions and abnormalities | Gastrointestinal cancer screening, Precise localization of lesions | Gastrointestinal cancers | [36] |
| Biomarker Analysis | Non-invasive disease detection | Monitoring disease progression, Treatment response assessment | IBD, Cancer | [51] |
| Molecular Imaging | High-resolution visualization of tissues | Visualizing inflammation, tissue changes, Disease staging | Gastrointestinal cancers, Inflammation | [52] |
| Serological Assays | Identification of disease-specific antibodies | Autoimmune gastrointestinal disorder, Infection diagnosis | Celiac disease, Infections | [53] |
| Capsule Endoscopy | Minimally invasive visualization of the GI tract | Small intestine exploration, Diagnosing obscure bleeding | Small intestinal disorders, Bleeding disorders | [54] |
| Virtual Colonoscopy | CT scan-based colon imaging without invasive procedure | Colorectal cancer screening, Polyp detection | Colorectal cancer, Polyps | [55] |
| Breath Tests | Analysis of gases for detecting specific gastrointestinal conditions | H. pylori infection, Carbohydrate malabsorption | H. pylori infection, Malabsorption | [56] |
| Stool DNA Testing | DNA analysis from stool samples for colorectal cancer screening | Early detection of colorectal cancer, Adenoma identification | Colorectal cancer, Precancerous lesions | [57] |
3. Artificial Intelligence in Gastrointestinal Care
4. Therapeutic Breakthroughs: Precision Treatment Approaches
5. Biomarkers in Precision Medicine for IBD
6. Advancements in Pharmacotherapy for Gastrointestinal Disorders
7. Gene Therapies and Gene Editing for Inherited Gastrointestinal Disorders
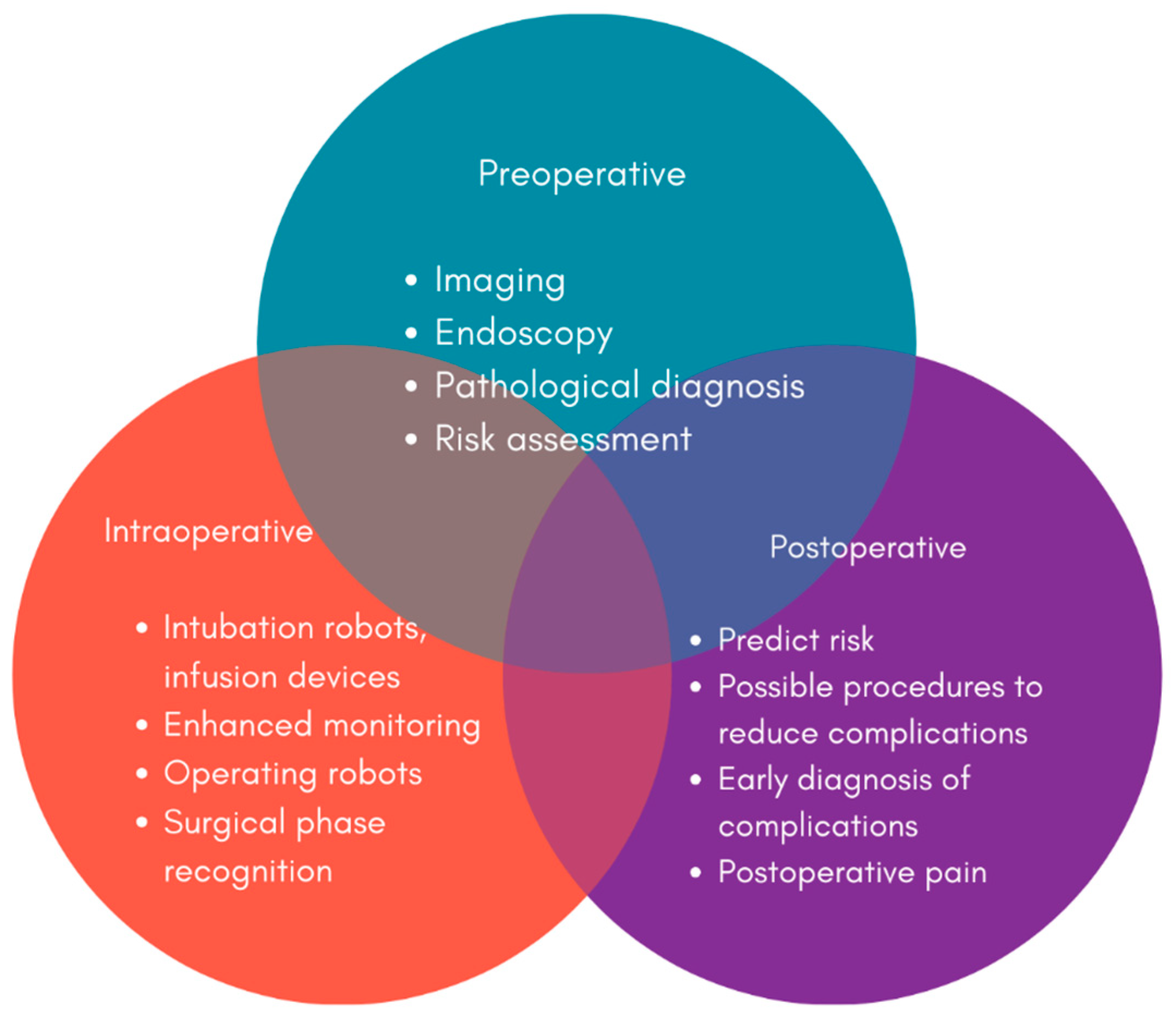
8. Minimally Invasive Interventions: Endoscopic and Surgical Advances
9. Robotic and Laparoscopic Surgery for Improved Outcomes and Reduced Invasiveness
10. Personalized Medicine in Gastrointestinal Disorders
11. Future Prospects: Transforming Gastrointestinal Disorder Management
12. Emerging Technologies Such as Nanomedicine and Wearable Devices
13. Utilization of Telemedicine and Remote Patient Monitoring
14. Potential of 3D Printing in Customizing Treatment Solutions
15. Future Prospects and Challenges
16. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mukhtar, K.; Nawaz, H.; Abid, S. Functional gastrointestinal disorders and gut-brain axis: What does the future hold? World J. Gastroenterol. 2019, 25, 552–566. [Google Scholar] [CrossRef] [PubMed]
- Onwuzo, S.; Boustany, A.; Khaled Abou Zeid, H.; Hitawala, A.; Almomani, A.; Onwuzo, C.; Lawrence, F.; Monteiro, J.M.; Ndubueze, C.; Asaad, I.; et al. Prevalence and Risk Factors Associated with Inflammatory Bowel Disease in Patients Using Proton-Pump Inhibitors: A Population-Based Study. Cureus 2023, 15, e34088. [Google Scholar] [CrossRef] [PubMed]
- Seyedian, S.S.; Nokhostin, F.; Malamir, M.D. A review of the diagnosis, prevention, and treatment methods of inflammatory bowel disease. J. Med. Life 2019, 12, 113–122. [Google Scholar] [CrossRef]
- Mitropoulou, M.-A.; Fradelos, E.C.; Lee, K.Y.; Malli, F.; Tsaras, K.; Christodoulou, N.G.; Papathanasiou, I.V. Quality of Life in Patients with Inflammatory Bowel Disease: Importance of Psychological Symptoms. Cureus 2022, 14, e28502. [Google Scholar] [CrossRef]
- Lee, H.H.; Gweon, T.-G.; Kang, S.-G.; Jung, S.H.; Lee, K.-M.; Kang, S.-B. Assessment of Fatigue and Associated Factors in Patients with Inflammatory Bowel Disease: A Questionnaire-Based Study. J. Clin. Med. 2023, 12, 3116. [Google Scholar] [CrossRef] [PubMed]
- Bokemeyer, A.; Buskermolen, J.; Ketelhut, S.; Tepasse, P.-R.; Vollenberg, R.; Trebicka, J.; Schmidt, H.H.; Vieth, M.; Bettenworth, D.; Kemper, B. Quantitative Phase Imaging Using Digital Holographic Microscopy to Assess the Degree of Intestinal Inflammation in Patients with Ulcerative Colitis. J. Clin. Med. 2023, 12, 4067. [Google Scholar] [CrossRef] [PubMed]
- Gajendran, M.; Loganathan, P.; Catinella, A.P.; Hashash, J.G. A comprehensive review and update on Crohn’s disease. Disease-a-Month 2018, 64, 20–57. [Google Scholar] [CrossRef] [PubMed]
- Meima-van Praag, E.M.; Buskens, C.J.; Hompes, R.; Bemelman, W.A. Surgical management of Crohn’s disease: A state of the art review. Int. J. Color. Dis. 2021, 36, 1133–1145. [Google Scholar] [CrossRef] [PubMed]
- Desai, J.; Elnaggar, M.; Hanfy, A.A.; Doshi, R. Toxic Megacolon: Background, Pathophysiology, Management Challenges and Solutions. Clin. Exp. Gastroenterol. 2020, 13, 203–210. [Google Scholar] [CrossRef]
- Luciano, E.; Macek, S.; Pacheco, F.; Solh, W. Synchronous colon cancer presenting as toxic megacolon in a patient with ulcerative colitis: A case report. Int. J. Surg. Case Rep. 2023, 112, 108984. [Google Scholar] [CrossRef]
- Bull, M.J.; Plummer, N.T. Part 1: The Human Gut Microbiome in Health and Disease. Integr. Med. 2014, 13, 17–22. [Google Scholar]
- Park, S.C.; Jeen, Y.T. Genetic Studies of Inflammatory Bowel Disease-Focusing on Asian Patients. Cells 2019, 8, 404. [Google Scholar] [CrossRef] [PubMed]
- Rizzello, F.; Spisni, E.; Giovanardi, E.; Imbesi, V.; Salice, M.; Alvisi, P.; Valerii, M.C.; Gionchetti, P. Implications of the Westernized Diet in the Onset Progression of IBD. Nutrients 2019, 11, 1033. [Google Scholar] [CrossRef] [PubMed]
- Lakatos, P.L.; Szamosi, T.; Lakatos, L. Smoking in inflammatory bowel diseases: Good, bad or ugly? World J. Gastroenterol. 2007, 13, 6134–6139. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, M.D.; Remedios, K.A.; Abbas, A.K. Mechanisms of human autoimmunity. J. Clin. Investig. 2015, 125, 2228–2233. [Google Scholar] [CrossRef] [PubMed]
- Muzammil, M.A.; Fariha, F.; Patel, T.; Sohail, R.; Kumar, M.; Khan, E.; Khanam, B.; Kumar, S.; Khatri, M.; Varrassi, G.; et al. Advancements in Inflammatory Bowel Disease: A Narrative Review of Diagnostics, Management, Epidemiology, Prevalence, Patient Outcomes, Quality of Life, and Clinical Presentation. Cureus 2023, 15, e41120. [Google Scholar] [CrossRef]
- Secretariat, M.A. Screening methods for early detection of colorectal cancers and polyps: Summary of evidence-based analyses. Ont. Health Technol. Assess. Ser. 2009, 9, 1–65. [Google Scholar]
- Van Oudenhove, L.; Levy, R.L.; Crowell, M.D.; Drossman, D.A.; Halpert, A.D.; Keefer, L.; Lackner, J.M.; Murphy, T.B.; Naliboff, B.D. Biopsychosocial Aspects of Functional Gastrointestinal Disorders: How Central and Environmental Processes Contribute to the Development and Expression of Functional Gastrointestinal Disorders. Gastroenterology 2016, 150, 1355–1367.e2. [Google Scholar] [CrossRef]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015, 28, 203–209. [Google Scholar]
- Khreefa, Z.; Barbier, M.T.; Koksal, A.R.; Love, G.; Del Valle, L. Pathogenesis and Mechanisms of SARS-CoV-2 Infection in the Intestine, Liver, and Pancreas. Cells 2023, 12, 262. [Google Scholar] [CrossRef]
- Wang, L.; Xiang, Y. Spike Glycoprotein-Mediated Entry of SARS Coronaviruses. Viruses 2020, 12, 1289. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Liu, Z.-J.; Zhang, Y.-K.; Sun, H.-J. Mechanism and potential treatments for gastrointestinal dysfunction in patients with COVID-19. World J. Gastroenterol. 2022, 28, 6811–6826. [Google Scholar] [CrossRef]
- Colombel, J.-F.; Shin, A.; Gibson, P.R. AGA Clinical Practice Update on Functional Gastrointestinal Symptoms in Patients with Inflammatory Bowel Disease: Expert Review. Clin. Gastroenterol. Hepatol. 2019, 17, 380–390.e1. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Wang, S.; Li, J. Treatment of Inflammatory Bowel Disease: A Comprehensive Review. Front. Med. 2021, 8, 765474. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.; Panaccione, R.; Ghosh, S.; Rioux, K. Optimizing clinical use of mesalazine [5-aminosalicylic acid] in inflammatory bowel disease. Therap. Adv. Gastroenterol. 2011, 4, 237–248. [Google Scholar] [CrossRef]
- Tripathi, K.; Dong, J.; Mishkin, B.F.; Feuerstein, J.D. Patient Preference and Adherence to Aminosalicylates for the Treatment of Ulcerative Colitis. Clin. Exp. Gastroenterol. 2021, 14, 343–351. [Google Scholar] [CrossRef]
- Ardizzone, S.; Cassinotti, A.; Manes, G.; Porro, G.B. Immunomodulators for all patients with inflammatory bowel disease? Therap. Adv. Gastroenterol. 2010, 3, 31–42. [Google Scholar] [CrossRef]
- D’Haens, G. Risks and benefits of biologic therapy for inflammatory bowel diseases. Gut 2007, 56, 725–732. [Google Scholar] [CrossRef]
- Ledder, O. Antibiotics in inflammatory bowel diseases: Do we know what we’re doing? Transl. Pediatr. 2019, 8, 42–55. [Google Scholar] [CrossRef]
- Stanley, A.J. Update on risk scoring systems for patients with upper gastrointestinal haemorrhage. World J. Gastroenterol. 2012, 18, 2739. [Google Scholar] [CrossRef]
- Saha, L. Irritable bowel syndrome: Pathogenesis, diagnosis, treatment, and evidence-based medicine. World J. Gastroenterol. 2014, 20, 6759–6773. [Google Scholar] [CrossRef] [PubMed]
- Holtmann, G.; Shah, A.; Morrison, M. Pathophysiology of Functional Gastrointestinal Disorders: A Holistic Overview. Dig. Dis. 2017, 35 (Suppl. S1), 5–13. [Google Scholar] [CrossRef]
- Tang, Y.; Anandasabapathy, S.; Richards-Kortum, R. Advances in optical gastrointestinal endoscopy: A technical review. Mol. Oncol. 2021, 15, 2580–2599. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Kong, X.; Zhong, W.; Liu, W. Fecal biomarkers: Non-invasive diagnosis of colorectal cancer. Front. Oncol. 2022, 12, 971930. [Google Scholar] [CrossRef] [PubMed]
- Satam, H.; Joshi, K.; Mangrolia, U.; Waghoo, S.; Zaidi, G.; Rawool, S.; Thakare, R.P.; Banday, S.; Mishra, A.K.; Das, G.; et al. Next-Generation Sequencing Technology: Current Trends and Advancements. Biology 2023, 12, 997. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Jiang, Y.; Abboud, Y.; Gaddam, S. Role of Endoscopy in Management of Upper Gastrointestinal Cancers. Diseases 2022, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Fikree, A.; Byrne, P. Management of functional gastrointestinal disorders. Clin. Med. 2021, 21, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.A.; Brunnhuber, S. Gastrointestinal disorders. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2012; pp. 607–631. [Google Scholar]
- Logiudice, F.P.; Bernardo, W.M.; Galetti, F.; Sagae, V.M.; Matsubayashi, C.O.; Neto, A.C.M.; Brunaldi, V.O.; de Moura, D.T.H.; Franzini, T.; Cheng, S.; et al. Endoscopic ultrasound-guided vs. endoscopic retrograde cholangiopancreatography biliary drainage for obstructed distal malignant biliary strictures: A systematic review and meta-analysis. World J. Gastrointest. Endosc. 2019, 11, 281–291. [Google Scholar] [CrossRef]
- Njoku, K.; Sutton, C.J.; Whetton, A.D.; Crosbie, E.J. Metabolomic Biomarkers for Detection, Prognosis and Identifying Recurrence in Endometrial Cancer. Metabolites 2020, 10, 314. [Google Scholar] [CrossRef]
- Yen, S.; Johnson, J.S. Metagenomics: A path to understanding the gut microbiome. Mamm. Genome 2021, 32, 282–296. [Google Scholar] [CrossRef]
- Cheema, H.I.; Tharian, B.; Inamdar, S.; Garcia-Saenz-de-Sicilia, M.; Cengiz, C. Recent advances in endoscopic management of gastric neoplasms. World J. Gastrointest. Endosc. 2023, 15, 319–337. [Google Scholar] [CrossRef] [PubMed]
- Akarsu, M.; Akarsu, C. Evaluation of New Technologies in Gastrointestinal Endoscopy. JSLS J. Soc. Laparoendosc. Surg. 2018, 22, e2017.00053. [Google Scholar] [CrossRef] [PubMed]
- Mathews, A.A.; Draganov, P.V.; Yang, D. Endoscopic management of colorectal polyps: From benign to malignant polyps. World J. Gastrointest. Endosc. 2021, 13, 356–370. [Google Scholar] [CrossRef] [PubMed]
- Gono, K. Narrow Band Imaging: Technology Basis and Research and Development History. Clin. Endosc. 2015, 48, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, S.; Kaul, V. Endoscopic Ultrasound Stagingof Esophageal Cancer. Gastroenterol Hepatol. 2020, 16, 14–20. [Google Scholar]
- Alghoul, Z.; Yang, C.; Merlin, D. The Current Status of Molecular Biomarkers for Inflammatory Bowel Disease. Biomedicines 2022, 10, 1492. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Que, S.; Xu, J.; Peng, T. Alanine aminotransferase-old biomarker and new concept: A review. Int. J. Med. Sci. 2014, 11, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Sekiguchi, M.; Matsuda, T. Limited usefulness of serum carcinoembryonic antigen and carbohydrate antigen 19-9 levels for gastrointestinal and whole-body cancer screening. Sci. Rep. 2020, 10, 18202. [Google Scholar] [CrossRef] [PubMed]
- Mao, R.; Krautscheid, P.; Graham, R.P.; Ganguly, A.; Shankar, S.; Ferber, M.; Hegde, M.; ACMG Laboratory Quality Assurance Committee. Genetic testing for inherited colorectal cancer and polyposis, 2021 revision: A technical standard of the American College of Medical Genetics and Genomics [ACMG]. Genet. Med. 2021, 23, 1807–1817. [Google Scholar] [CrossRef]
- Arasaradnam, R.P.; McFarlane, M.; Daulton, E.; Skinner, J.; O’Connell, N.; Wurie, S.; Chambers, S.; Nwokolo, C.; Bardhan, K.; Savage, R.; et al. Non-invasive exhaled volatile organic biomarker analysis to detect inflammatory bowel disease [IBD]. Dig. Liver Dis. 2016, 48, 148–153. [Google Scholar] [CrossRef]
- Harold, K.M.; MacCuaig, W.M.; Holter-Charkabarty, J.; Williams, K.; Hill, K.; Arreola, A.X.; Sekhri, M.; Carter, S.; Gomez-Gutierrez, J.; Salem, G.; et al. Advances in Imaging of Inflammation, Fibrosis, and Cancer in the Gastrointestinal Tract. Int. J. Mol. Sci. 2022, 23, 16109. [Google Scholar] [CrossRef] [PubMed]
- Majsiak, E.; Cukrowska, B.; Choina, M.; Bielawski, K.; Cielecka-Kuszyk, J.; Konopka, E.; Wysokiński, M.; Bierła, J.B. Evaluation of the Usefulness of a Serological Test for Diagnosis of Celiac Disease Simultaneously Detecting Specific Antibodies Total IgA. Nutrients 2022, 15, 202. [Google Scholar] [CrossRef] [PubMed]
- Koffas, A.; Laskaratos, F.-M.; Epstein, O. Non-small bowel lesion detection at small bowel capsule endoscopy: A comprehensive literature review. World J. Clin. Cases 2018, 6, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Cotton, P.B.; Durkalski, V.L.; Pineau, B.C.; Palesch, Y.Y.; Mauldin, P.D.; Hoffman, B.; Vining, D.J.; Small, W.C.; Affronti, J.; Rex, D.; et al. Computed tomographic colonography [virtual colonoscopy]: A multicenter comparison with standard colonoscopy for detection of colorectal neoplasia. JAMA 2004, 291, 1713–1719. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.V.; Malik, A. Hydrogen breath tests in gastrointestinal diseases. Indian J. Clin. Biochem. 2014, 29, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Imperiale, T.F.; Porter, K.; Zella, J.; Gagrat, Z.D.; Olson, M.C.; Statz, S.; Garces, J.; Lavin, P.T.; Aguilar, H.; Brinberg, D.; et al. Next-Generation Multitarget Stool DNA Test for Colorectal Cancer Screening. N. Engl. J. Med. 2024, 390, 984–993. [Google Scholar] [CrossRef] [PubMed]
- Quazi, S. Artificial intelligence and machine learning in precision and genomic medicine. Med. Oncol. 2022, 39, 120. [Google Scholar] [CrossRef]
- Viscaino, M.; Torres Bustos, J.; Muñoz, P.; Auat Cheein, C.; Cheein, F.A. Artificial intelligence for the early detection of colorectal cancer: A comprehensive review of its advantages and misconceptions. World J. Gastroenterol. 2021, 27, 6399–6414. [Google Scholar] [CrossRef]
- Koh, F.H.; Ladlad, J.; Teo, E.-K.; Lin, C.-L.; Foo, F.-J. Real-time artificial intelligence [AI]-aided endoscopy improves adenoma detection rates even in experienced endoscopists: A cohort study in Singapore. Surg. Endosc. 2023, 37, 165–171. [Google Scholar] [CrossRef]
- Najjar, R. Redefining Radiology: A Review of Artificial Intelligence Integration in Medical Imaging. Diagnostics 2023, 13, 2760. [Google Scholar] [CrossRef]
- van der Zander, Q.E.W.; van der Ende-van Loon, M.C.M.; Janssen, J.M.M.; Winkens, B.; van der Sommen, F.; Masclee, A.A.M.; Schoon, E.J. Artificial intelligence in [gastrointestinal] healthcare: Patients’ and physicians’ perspectives. Sci. Rep. 2022, 12, 16779. [Google Scholar] [CrossRef]
- Yang, C.C. Explainable Artificial Intelligence for Predictive Modeling in Healthcare. J. Healthc. Inform. Res. 2022, 6, 228–239. [Google Scholar] [CrossRef]
- El Hajjar, A.; Rey, J.-F. Artificial intelligence in gastrointestinal endoscopy: General overview. Chin. Med. J. 2020, 133, 326–334. [Google Scholar] [CrossRef]
- Giordano, C.; Brennan, M.; Mohamed, B.; Rashidi, P.; Modave, F.; Tighe, P. Accessing Artificial Intelligence for Clinical Decision-Making. Front. Digit. Health 2021, 3, 645232. [Google Scholar] [CrossRef] [PubMed]
- Bajwa, J.; Munir, U.; Nori, A.; Williams, B. Artificial intelligence in healthcare: Transforming the practice of medicine. Future Healthc. J. 2021, 8, e188–e194. [Google Scholar] [CrossRef]
- Paul, D.; Sanap, G.; Shenoy, S.; Kalyane, D.; Kalia, K.; Tekade, R.K. Artificial intelligence in drug discovery and development. Drug Discov. Today 2021, 26, 80–93. [Google Scholar] [CrossRef]
- Al-Antari, M.A. Artificial Intelligence for Medical Diagnostics—Existing and Future AI Technology! Diagnostics 2023, 13, 688. [Google Scholar] [CrossRef]
- Simon, P.; Vijayasundaram, U. Deep Learning based Feature Extraction for Texture Classification. Procedia Comput. Sci. 2020, 171, 1680–1687. [Google Scholar] [CrossRef]
- Hmoud Al-Adhaileh, M.; Mohammed Senan, E.; Alsaade, F.W.; Aldhyani, T.H.H.; Alsharif, N.; Abdullah Alqarni, A.; Uddin, M.I.; Alzahrani, M.Y.; Alzain, E.D.; Jadhav, M.E. Deep Learning Algorithms for Detection and Classification of Gastrointestinal Diseases. Complexity 2021, 2021, 6170416. [Google Scholar] [CrossRef]
- Johnson, K.B.; Wei, W.-Q.; Weeraratne, D.; Frisse, M.E.; Misulis, K.; Rhee, K.; Zhao, J.; Snowdon, J.L. Precision Medicine, AI, and the Future of Personalized Health Care. Clin. Transl. Sci. 2021, 14, 86–93. [Google Scholar] [CrossRef]
- Herrera-deGuise, C.; Serra-Ruiz, X.; Lastiri, E.; Borruel, N. JAK inhibitors: A new dawn for oral therapies in inflammatory bowel diseases. Front. Med. 2023, 10, 1089099. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.; Aslam, N.; Nigam, G.; Limdi, J.K. Tofacitinib and newer JAK inhibitors in inflammatory bowel disease—Where we are and where we are going. Drugs Context 2022, 11, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, M.; Sabino, J. Efficacy of JAK inhibitors in Ulcerative Colitis. J. Crohn’s Colitis. 2020, 14 (Suppl. S2), S737–S745. [Google Scholar]
- Ananthakrishnan, A.N. Precision medicine in inflammatory bowel diseases. Intestg. Res. 2024, 22, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Marafini, I.; Monteleone, G. Precision Medicine in Inflammatory Bowel Diseases. Front. Pharmacol. 2021, 12, 653924. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, J.E.; Warner, N.; Staples, J.; Crowley, E.; Gosalia, N.; Murchie, R.; Van Hout, C.; Fiedler, K.; Welch, G.; King, A.K.; et al. Mutation spectrum of NOD2 reveals recessive inheritance as a main driver of Early Onset Crohn’s Disease. Sci. Rep. 2021, 11, 5595. [Google Scholar] [CrossRef] [PubMed]
- Michail, S.; Bultron, G.; Depaolo, R.W. Genetic variants associated with Crohn’s disease. Appl. Clin. Genet. 2013, 6, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Zittan, E.; Gralnek, I.M.; Berns, M.S. The New Proactive Approach and Precision Medicine in Crohn’s Disease. Biomedicines 2020, 8, 193. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Tang, H.; Zhou, Q.Y.; Zeng, Y.L.; Chen, D.; Xu, H.; Li, Y.; Tan, B.; Qian, J.-M. Advancing the precision management of inflammatory bowel disease in the era of omics approaches and new technology. World J. Gastroenterol. 2023, 29, 272–285. [Google Scholar] [CrossRef]
- Pagnini, C.; Pizarro, T.T.; Cominelli, F. Novel pharmacological therapy in inflammatory bowel diseases: Beyond anti-tumor necrosis factor. Front. Pharmacol. 2019, 10, 671. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, K.; Ma, W.; Li, D.; Mo, T.; Liu, Q. The gut microbiome in human health and disease-Where are we and where are we going? A bibliometric analysis. Front. Microbiol. 2022, 13, 1018594. [Google Scholar] [CrossRef]
- Newman, K.M.; Rank, K.M.; Vaughn, B.P.; Khoruts, A. Treatment of recurrent Clostridium difficile infection using fecal microbiota transplantation in patients with inflammatory bowel disease. Gut Microbes 2017, 8, 303–309. [Google Scholar] [CrossRef]
- Hitch, T.C.A.; Hall, L.J.; Walsh, S.K.; Leventhal, G.E.; Slack, E.; de Wouters, T.; Walter, J.; Clavel, T. Microbiome-based interventions to modulate gut ecology and the immune system. Mucosal Immunol. 2022, 15, 1095–1113. [Google Scholar] [CrossRef]
- Gulliver, E.L.; Young, R.B.; Chonwerawong, M.; D’Adamo, G.L.; Thomason, T.; Widdop, J.T.; Rutten, E.L.; Marcelino, V.R.; Bryant, R.V.; Costello, S.P.; et al. Review article: The future of microbiome-based therapeutics. Aliment. Pharmacol. Ther. 2022, 56, 192–208. [Google Scholar] [CrossRef] [PubMed]
- Weingarden, A.R.; Vaughn, B.P. Intestinal microbiota, fecal microbiota transplantation, and inflammatory bowel disease. Gut Microbes 2017, 8, 238–252. [Google Scholar] [CrossRef] [PubMed]
- Boicean, A.; Birlutiu, V.; Ichim, C.; Anderco, P.; Birsan, S. Fecal Microbiota Transplantation in Inflammatory Bowel Disease. Biomedicines 2023, 11, 1016. [Google Scholar] [CrossRef]
- Yang, R.; Chen, Z.; Cai, J. Fecal microbiota transplantation: Emerging applications in autoimmune diseases. J. Autoimmun. 2023, 141, 103038. [Google Scholar] [CrossRef]
- Tan, X.Y.; Xie, Y.J.; Liu, X.L.; Li, X.Y.; Jia, B. A Systematic Review and Meta-Analysis of Randomized Controlled Trials of Fecal Microbiota Transplantation for the Treatment of Inflammatory Bowel Disease. Evid.-Based Complement. Altern. Med. 2022, 2022, 8266793. [Google Scholar] [CrossRef]
- Wortelboer, K.; Nieuwdorp, M.; Herrema, H. Fecal microbiota transplantation beyond Clostridioides difficile infections. EBioMedicine 2019, 44, 716–729. [Google Scholar] [CrossRef]
- Khan, I.; Ullah, N.; Zha, L.; Bai, Y.; Khan, A.; Zhao, T.; Che, T.; Zhang, C. Alteration of gut microbiota in inflammatory bowel disease [IBD]: Cause or consequence? IBD treatment targeting the gut microbiome. Pathogens 2019, 8, 126. [Google Scholar] [CrossRef]
- Kingsley, M.J.; Abreu, M.T. A Personalized Approach to Managing Inflammatory Bowel Disease. Gastroenterol. Hepatol. 2016, 12, 308–315. [Google Scholar]
- Saeed, S.; Ekhator, C.; Abdelaziz, A.M.; Naveed, H.; Karski, A.; Cook, D.E.; Reddy, S.M.; Affaf, M.; Khan, S.J.; Bellegarde, S.B.; et al. Revolutionizing Inflammatory Bowel Disease Management: A Comprehensive Narrative Review of Innovative Dietary Strategies and Future Directions. Cureus 2023, 15, e44304. [Google Scholar] [CrossRef]
- Hazel, K.; O’Connor, A. Emerging treatments for inflammatory bowel disease. Ther. Adv. Chronic Dis. 2020, 11, 2040622319899297. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Zogg, H.; Ghoshal, U.C.; Ro, S. Current Treatment Options and Therapeutic Insights for Gastrointestinal Dysmotility and Functional Gastrointestinal Disorders. Front. Pharmacol. 2022, 13, 808195. [Google Scholar] [CrossRef]
- Bonelli, M.; Kerschbaumer, A.; Kastrati, K.; Ghoreschi, K.; Gadina, M.; Heinz, L.X.; Smolen, J.S.; Aletaha, D.; O’Shea, J.; Laurence, A. Selectivity, efficacy and safety of JAKinibs: New evidence for a still evolving story. Ann. Rheum. Dis. 2024, 83, 139–160. [Google Scholar] [CrossRef]
- Campa, M.; Mansouri, B.; Warren, R.; Menter, A. A Review of Biologic Therapies Targeting IL-23 and IL-17 for Use in Moderate-to-Severe Plaque Psoriasis. Dermatol. Ther. 2016, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Saha, S.; Khanna, S. Therapies to modulate gut microbiota: Past, present and future. World J. Gastroenterol. 2020, 26, 777–788. [Google Scholar] [CrossRef]
- Song, E.-J.; Shin, J.-H. Personalized Diets based on the Gut Microbiome as a Target for Health Maintenance: From Current Evidence to Future Possibilities. J. Microbiol. Biotechnol. 2022, 32, 1497–1505. [Google Scholar] [CrossRef]
- Horton, R.H.; Lucassen, A.M. Recent developments in genetic/genomic medicine. Clin Sci. 2019, 133, 697–708. [Google Scholar] [CrossRef]
- Lee, J.-A.; Cho, A.; Huang, E.N.; Xu, Y.; Quach, H.; Hu, J.; Wong, A.P. Gene therapy for cystic fibrosis: New tools for precision medicine. J. Transl. Med. 2021, 19, 452. [Google Scholar] [CrossRef]
- Lomunova, M.A.; Gershovich, P.M. Gene Therapy for Cystic Fibrosis: Recent Advances and Future Prospects. Acta Naturae 2023, 15, 20–31. [Google Scholar] [CrossRef]
- Abdelnour, S.A.; Xie, L.; Hassanin, A.A.; Zuo, E.; Lu, Y. The Potential of CRISPR/Cas9 Gene Editing as a Treatment Strategy for Inherited Diseases. Front. Cell Dev. Biol. 2021, 9, 699597. [Google Scholar] [CrossRef]
- Li, T.; Yang, Y.; Qi, H.; Cui, W.; Zhang, L.; Fu, X.; He, X.; Liu, M.; Li, P.-F.; Yu, T. CRISPR/Cas9 therapeutics: Progress and prospects. Signal Transduct. Target. Ther. 2023, 8, 36. [Google Scholar] [CrossRef]
- Yue, N.; Xu, H.; Xu, J.; Zhu, M.; Zhang, Y.; Tian, C.-M.; Nie, Y.-Q.; Yao, J.; Liang, Y.-J.; Li, D.-F.; et al. Therapeutic potential of gene therapy for gastrointestinal diseases: Advancements and future perspectives. Mol. Ther.-Oncolytics 2023, 30, 193–215. [Google Scholar] [CrossRef]
- Janik, E.; Niemcewicz, M.; Ceremuga, M.; Krzowski, L.; Saluk-Bijak, J.; Bijak, M. Various Aspects of a Gene Editing System—CRISPR–Cas9. Int. J. Mol. Sci. 2020, 21, 9604. [Google Scholar] [CrossRef]
- Hills, R.; Pontefract, B.; Mishcon, H.; Black, C.; Sutton, S.; Theberge, C. Gut Microbiome: Profound Implications for Diet and Disease. Nutrients 2019, 11, 1613. [Google Scholar] [CrossRef]
- Napolitano, M.; Fasulo, E.; Ungaro, F.; Massimino, L.; Sinagra, E.; Danese, S.; Mandarino, F.V. Gut Dysbiosis in Irritable Bowel Syndrome: A Narrative Review on Correlation with Disease Subtypes and Novel Therapeutic Implications. Microorganisms 2023, 11, 2369. [Google Scholar] [CrossRef]
- Ratiner, K.; Ciocan, D.; Abdeen, S.K.; Elinav, E. Utilization of the microbiome in personalized medicine. Nat. Rev. Microbiol. 2023, 22, 291–308. [Google Scholar] [CrossRef]
- Park, E.; Nishimura, M.; Simoes, P. Endoscopic advances in the management of gastric cancer and premalignant gastric conditions. World J. Gastrointest. Endosc. 2023, 15, 114–121. [Google Scholar] [CrossRef]
- Li, H.; Hou, X.; Lin, R.; Fan, M.; Pang, S.; Jiang, L.; Liu, Q.; Fu, L. Advanced endoscopic methods in gastrointestinal diseases: A systematic review. Quant. Imaging Med. Surg. 2019, 9, 905–920. [Google Scholar] [CrossRef]
- Bramhe, S.; Pathak, S.S. Robotic Surgery: A Narrative Review. Cureus 2022, 14, e29179. [Google Scholar] [CrossRef]
- Nguyen, V.X.; Le Nguyen, V.T.; Nguyen, C.C. Appropriate use of endoscopy in the diagnosis and treatment of gastrointestinal diseases: Up-to-date indications for primary care providers. Int. J. Gen. Med. 2010, 3, 345. [Google Scholar] [CrossRef]
- Teh, J.-L.; Shabbir, A.; Yuen, S.; So, J.B.-Y. Recent advances in diagnostic upper endoscopy. World J. Gastroenterol. 2020, 26, 433–447. [Google Scholar] [CrossRef]
- Rudiman, R. Advances in gastrointestinal surgical endoscopy. Ann. Med. Surg. 2021, 72, 103041. [Google Scholar] [CrossRef]
- Shah, R.M.; Tarnasky, P.; Kedia, P. A review of endoscopic ultrasound guided endoscopic retrograde cholangiopancreatography techniques in patients with surgically altered anatomy. Transl. Gastroenterol. Hepatol. 2018, 3, 90. [Google Scholar] [CrossRef]
- Sanders, D.J.; Bomman, S.; Krishnamoorthi, R.; Kozarek, R.A. Endoscopic retrograde cholangiopancreatography: Current practice and future research. World J. Gastrointest. Endosc. 2021, 13, 260–274. [Google Scholar] [CrossRef]
- Glass, J.; Chaudhry, A.; Zeeshan, M.S.; Ramzan, Z. New Era: Endoscopic treatment options in obesity—A paradigm shift. World J. Gastroenterol. 2019, 25, 4567–4579. [Google Scholar] [CrossRef] [PubMed]
- Buia, A.; Stockhausen, F.; Hanisch, E. Laparoscopic surgery: A qualified systematic review. World J. Methodol. 2015, 5, 238–254. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Y.; Chase, R.C.; Li, T.; Ramai, D.; Li, S.; Huang, X.; Antwi, S.O.; Keaveny, A.P.; Pang, M. Global Burden of Digestive Diseases: A Systematic Analysis of the Global Burden of Diseases Study, 1990 to 2019. Gastroenterology 2023, 165, 773–783.e15. [Google Scholar] [CrossRef]
- Bustin, S.A.; Jellinger, K.A. Advances in Molecular Medicine: Unravelling Disease Complexity and Pioneering Precision Healthcare. Int. J. Mol. Sci. 2023, 24, 14168. [Google Scholar] [CrossRef]
- Borg-Bartolo, S.P.; Boyapati, R.K.; Satsangi, J.; Kalla, R. Precision medicine in inflammatory bowel disease: Concept, progress and challenges. F1000Research 2020, 9, 54. [Google Scholar] [CrossRef]
- Arosa, L.; Camba-Gómez, M.; Golubnitschaja, O.; Conde-Aranda, J. Predictive, preventive and personalised approach as a conceptual and technological innovation in primary and secondary care of inflammatory bowel disease benefiting affected individuals and populations. EPMA J. 2024, 15, 111–123. [Google Scholar] [CrossRef]
- Singh, S.; Sarma, D.K.; Verma, V.; Nagpal, R.; Kumar, M. Unveiling the future of metabolic medicine: Omics technologies driving personalized solutions for precision treatment of metabolic disorders. Biochem. Biophys. Res. Commun. 2023, 682, 1–20. [Google Scholar] [CrossRef]
- Mukherjee, S.; Vagha, S.; Gadkari, P. Navigating the Future: A Comprehensive Review of Artificial Intelligence Applications in Gastrointestinal Cancer. Cureus 2024, 16, e54467. [Google Scholar] [CrossRef]
- Wang, X.; Hu, X.; Xu, Y.; Yong, J.; Li, X.; Zhang, K.; Gan, T.; Yang, J.; Rao, N. A systematic review on diagnosis and treatment of gastrointestinal diseases by magnetically controlled capsule endoscopy and artificial intelligence. Therap. Adv. Gastroenterol. 2023, 16, 17562848231206991. [Google Scholar] [CrossRef]
- Junaid, S.B.; Imam, A.A.; Balogun, A.O.; De Silva, L.C.; Surakat, Y.A.; Kumar, G.; Abdulkarim, M.; Shuaibu, A.N.; Garba, A.; Sahalu, Y.; et al. Recent Advancements in Emerging Technologies for Healthcare Management Systems: A Survey. Healthcare 2022, 10, 1940. [Google Scholar] [CrossRef]
- Pelaz, B.; Alexiou, C.; Alvarez-Puebla, R.A.; Alves, F.; Andrews, A.M.; Ashraf, S.; Balogh, L.P.; Ballerini, L.; Bestetti, A.; Brendel, C.; et al. Diverse Applications of Nanomedicine. ACS Nano 2017, 11, 2313–2381. [Google Scholar] [CrossRef]
- Yue, N.; Xu, H.; Xu, J.; Zhu, M.; Zhang, Y.; Tian, C.-M.; Nie, Y.-Q.; Yao, J.; Liang, Y.-J.; Li, D.-F.; et al. Application of Nanoparticles in the Diagnosis of Gastrointestinal Diseases: A Complete Future Perspective. Int. J. Nanomed. 2023, 18, 4143–4170. [Google Scholar] [CrossRef]
- Yusuf, A.; Almotairy, A.R.Z.; Henidi, H.; Alshehri, O.Y.; Aldughaim, M.S. Nanoparticles as Drug Delivery Systems: A Review of the Implication of Nanoparticles’ Physicochemical Properties on Responses in Biological Systems. Polymers 2023, 15, 1596. [Google Scholar] [CrossRef]
- Chong, K.P.; Woo, B.K. Emerging wearable technology applications in gastroenterology: A review of the literature. World J. Gastroenterol. 2021, 27, 1149–1160. [Google Scholar] [CrossRef]
- Haleem, A.; Javaid, M.; Singh, R.P.; Suman, R. Telemedicine for healthcare: Capabilities, features, barriers, and applications. Sens. Int. 2021, 2, 100117. [Google Scholar] [CrossRef]
- Gao, J.; Fan, C.; Chen, B.; Fan, Z.; Li, L.; Wang, L.; Ma, Q.; He, X.; Zhai, Y.; Zhao, J. Telemedicine Is Becoming an Increasingly Popular Way to Resolve the Unequal Distribution of Healthcare Resources: Evidence From China. Front. Public Health 2022, 10, 916303. [Google Scholar] [CrossRef]
- Villarin, L.A.; Patrick, J.R. How a digital patient experience can lead to future outcome driven healthcare: Thoughts for executive teams. mHealth 2023, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Aimar, A.; Palermo, A.; Innocenti, B. The Role of 3D Printing in Medical Applications: A State of the Art. J. Healthc. Eng. 2019, 2019, 5340616. [Google Scholar] [CrossRef]
- Mamo, H.B.; Adamiak, M.; Kunwar, A. 3D printed biomedical devices and their applications: A review on state-of-the-art technologies, existing challenges, and future perspectives. J. Mech. Behav. Biomed. Mater. 2023, 143, 105930. [Google Scholar] [CrossRef]
- Alzoubi, L.; Aljabali, A.A.A.; Tambuwala, M.M. Empowering Precision Medicine: The Impact of 3D Printing on Personalized Therapeutic. AAPS PharmSciTech 2023, 24, 228. [Google Scholar] [CrossRef]
- Manero, A.; Smith, P.; Sparkman, J.; Dombrowski, M.; Courbin, D.; Kester, A.; Womack, I.; Chi, A. Implementation of 3D Printing Technology in the Field of Prosthetics: Past, Present, and Future. Int. J. Environ. Res. Public. Health 2019, 16, 1641. [Google Scholar] [CrossRef]
- Harrison, K.L.; Farrell, R.M.; Brinich, M.A.; Highland, J.; Mercer, M.; McCormick, J.B.; Tilburt, J.; Geller, G.; Marshall, P.; Sharp, R.R. ‘Someone should oversee it’: Patient perspectives on the ethical issues arising with the regulation of probiotics. Heal. Expect. 2015, 18, 250–261. [Google Scholar] [CrossRef]
- Plumeri, P.A. Informed consent and the gastrointestinal endoscopist. Gastrointest. Endosc. 1985, 31, 218–221. [Google Scholar] [CrossRef]
- Varkey, B. Principles of Clinical Ethics and Their Application to Practice. Med. Princ. Pract. 2021, 30, 17–28. [Google Scholar] [CrossRef]
- Sanger, G.J.; Chang, L.; Bountra, C.; Houghton, L.A. Challenges and prospects for pharmacotherapy in functional gastrointestinal disorders. Therap. Adv. Gastroenterol. 2010, 3, 291–305. [Google Scholar] [CrossRef]
- Fogel, D.B. Factors associated with clinical trials that fail and opportunities for improving the likelihood of success: A review. Contemp. Clin. Trials Commun. 2018, 11, 156–164. [Google Scholar] [CrossRef]
- Stoumpos, A.I.; Kitsios, F.; Talias, M.A. Digital Transformation in Healthcare: Technology Acceptance and Its Applications. Int. J. Environ. Res. Public. Health 2023, 20, 3407. [Google Scholar] [CrossRef]
- Dickman, R.; Maradey-Romero, C.; Gingold-Belfer, R.; Fass, R. Unmet Needs in the Treatment of Gastroesophageal Reflux Disease. J. Neurogastroenterol. Motil. 2015, 21, 309–319. [Google Scholar] [CrossRef]
- Sharma, N.; Bhatia, S.; Chunduri, V.; Kaur, S.; Sharma, S.; Kapoor, P.; Kumari, A.; Garg, M. Pathogenesis of Celiac Disease and Other Gluten Related Disorders in Wheat and Strategies for Mitigating Them. Front. Nutr. 2020, 7, 6. [Google Scholar] [CrossRef]
- Testa, U.; Pelosi, E.; Castelli, G. Colorectal cancer: Genetic abnormalities, tumor progression, tumor heterogeneity, clonal evolution and tumor-initiating cells. Med. Sci. 2018, 6, 31. [Google Scholar] [CrossRef]
- Narayanan, M.; Reddy, K.M.; Marsicano, E. Peptic Ulcer Disease and Helicobacter pylori infection. Mo. Med. 2018, 115, 219–224. [Google Scholar]


| Disease Type | Molecular Mechanism | Pathway Involved | Treatment Options | Target Drugs | Significance | Reference |
|---|---|---|---|---|---|---|
| IBD [IBD] | Dysregulated immune response to gut microbiota | TNF-alpha signaling, IL-23/Th17 | Biologics [anti-TNF, anti-IL-23] | Infliximab, Vedolizumab | Revolutionized IBD management, improved quality of life | [16,81] |
| Gastroesophageal Reflux Disease [GERD] | Weak lower esophageal sphincter, acid reflux | Esophageal motility dysfunction | Proton pump inhibitors [PPIs] | Omeprazole, Esomeprazole | Alleviates symptoms, prevents complications | [148] |
| Celiac Disease | Immune reaction to gluten in the small intestine | Immune-mediated pathways | Gluten-free diet | None [dietary management] | Avoids long-term complications, improves health | [146] |
| Colorectal Cancer | Genetic mutations, abnormal cell growth | Wnt signaling pathway | Surgery, chemotherapy, radiation | Oxaliplatin, Fluorouracil | Early detection and treatment improve survival | [147] |
| IBDs [IBS] | Altered gut-brain communication, motility issues | Serotonin signaling, gut-brain axis | Dietary modifications, medications | Antispasmodics, Linaclotide | Enhances quality of life, symptom management | [31] |
| Peptic Ulcer Disease | H. pylori infection, acid erosion of stomach lining | H. pylori infection, acid production | Antibiotics, proton pump inhibitors | Amoxicillin, Omeprazole | Prevents complications, promotes ulcer healing | [148] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suri, C.; Pande, B.; Sahu, T.; Sahithi, L.S.; Verma, H.K. Revolutionizing Gastrointestinal Disorder Management: Cutting-Edge Advances and Future Prospects. J. Clin. Med. 2024, 13, 3977. https://doi.org/10.3390/jcm13133977
Suri C, Pande B, Sahu T, Sahithi LS, Verma HK. Revolutionizing Gastrointestinal Disorder Management: Cutting-Edge Advances and Future Prospects. Journal of Clinical Medicine. 2024; 13(13):3977. https://doi.org/10.3390/jcm13133977
Chicago/Turabian StyleSuri, Chahat, Babita Pande, Tarun Sahu, Lakkakula Suhasini Sahithi, and Henu Kumar Verma. 2024. "Revolutionizing Gastrointestinal Disorder Management: Cutting-Edge Advances and Future Prospects" Journal of Clinical Medicine 13, no. 13: 3977. https://doi.org/10.3390/jcm13133977
APA StyleSuri, C., Pande, B., Sahu, T., Sahithi, L. S., & Verma, H. K. (2024). Revolutionizing Gastrointestinal Disorder Management: Cutting-Edge Advances and Future Prospects. Journal of Clinical Medicine, 13(13), 3977. https://doi.org/10.3390/jcm13133977






