Diagnostic Challenges and Management Strategies of Pelvic Inflammatory Disease in Sexually Inactive Pediatric and Adolescent Patients: A Systematic Review of Case Reports
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Study Selection
2.2. Eligibility Criteria
2.3. Exclusion Criteria
2.4. Risk of Bias Evaluation
2.5. Data Collection
2.6. Data Analysis
2.7. Limitations of Included Evidence
3. Results
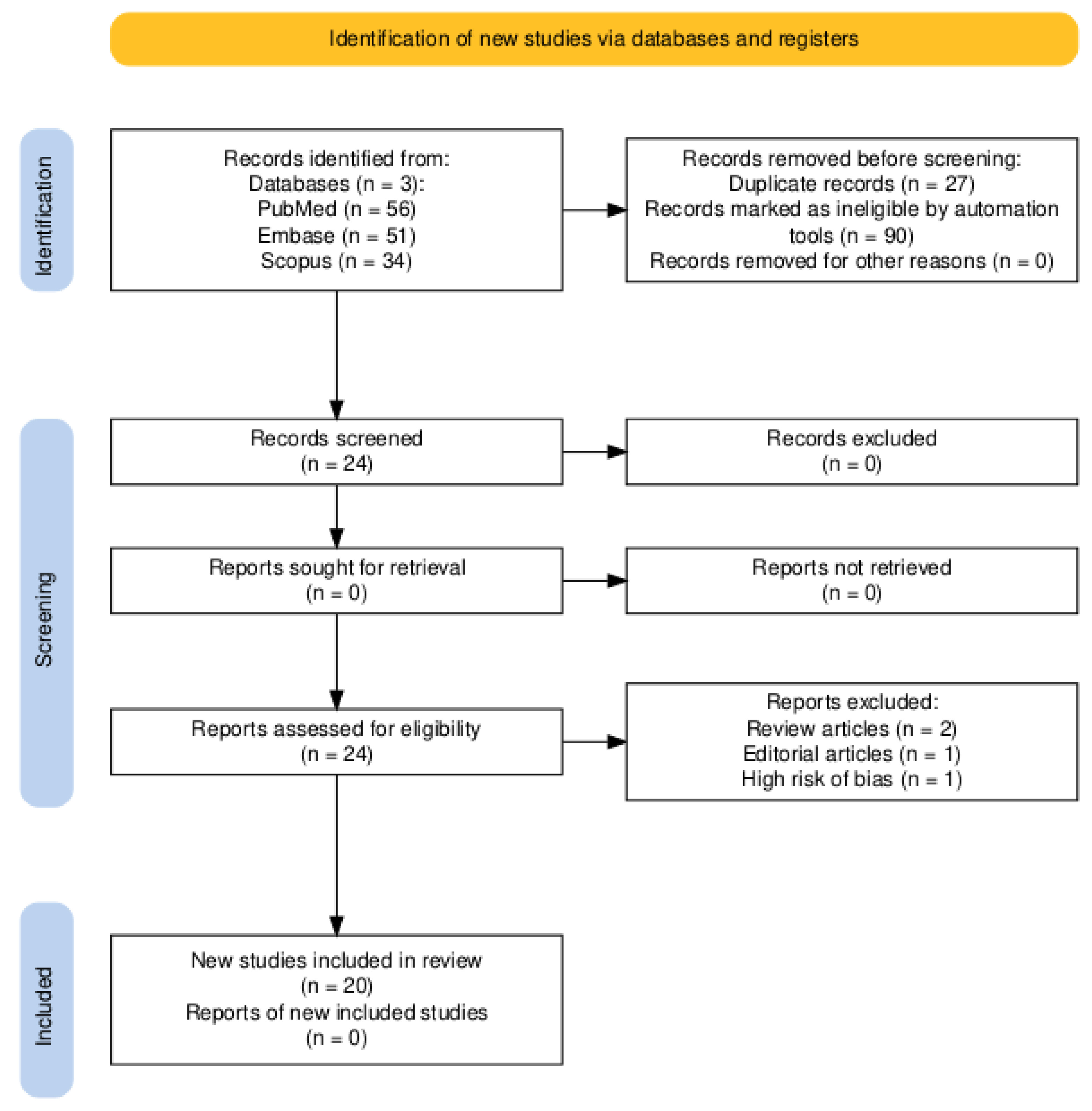
3.1. Literature Search
3.2. Bias Risk Evaluation
3.3. Studies Characteristics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PDI | Pelvic inflammatory disease |
| TOA | Tubo-ovarian abscess |
| US | Ultrasound |
| CT | Computed tomography |
| MRI | Magnetic resonance imaging |
| IV | Intravenously |
| PO | Orally |
References
- Chernick, L.S. Tubo-Ovarian Abscesses in Nonsexually Active Adolescents: A Rare but Consequential Miss. J. Adolesc. Health 2019, 65, 175–176. [Google Scholar] [CrossRef]
- Greydanus, D.E.; Dodich, C. Pelvic inflammatory disease in the adolescent: A poignant, perplexing, potentially preventable problem for patients and physicians. Curr. Opin. Pediatr. 2015, 27, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Mollen, C.J.; Pletcher, J.R.; Bellah, R.D.; Lavelle, J.M. Prevalence of tubo-ovarian abscess in adolescents diagnosed with pelvic inflammatory disease in a pediatric emergency department. Pediatr. Emerg. Care 2006, 22, 621–625. [Google Scholar] [CrossRef] [PubMed]
- Hakim, J.; Childress, K.J.; Hernandez, A.M.; Bercaw-Pratt, J.L. Tubo-Ovarian Abscesses in Nonsexually Active Adolescent Females: A Large Case Series. J. Adolesc. Health 2019, 65, 303–305. [Google Scholar] [CrossRef] [PubMed]
- Fink, D.; Lim, P.; Desai, A.; Stephans, A.B.; Wien, M.A.; Fink, D.; Lim, P.; Desai, A.; Stephans, A.; Wien, M. Recurrent tubo-ovarian abscess in a nonsexually active adolescent. Consultant 2022, 62, e26–e28. [Google Scholar]
- McKinnon, A.; Black, A.Y.; Lortie, K.; Fleming, N.A. A case of adolescent pelvic inflammatory disease caused by a rare bacterium: Fusobacterium nucleatum. J. Pediatr. Adolesc. Gynecol. 2013, 26, e113–e115. [Google Scholar] [CrossRef]
- Moralioglu, S.; Ozen, İ.; Demirogullari, B.; Basaklar, A. Pyosalpinx and hydrosalpinx in virginal adolescents: Report of two cases. West. Indian. Med. J. 2013, 62, 257–259. [Google Scholar]
- Arda, İ.S.; Ergeneli, M.; Coskun, M.; Hicsonmez, A. Tubo-ovarian abscess in a sexually inactive adolescent patient. Eur. J. Pediatr. Surg. 2004, 14, 70–72. [Google Scholar]
- Niles, L.M.; Goyal, M.K.; Badolato, G.M.; Chamberlain, J.M.; Cohen, J.S. US Emergency Department Trends in Imaging for Pediatric Nontraumatic Abdominal Pain. Pediatrics 2017, 140, e20170615. [Google Scholar] [CrossRef]
- Lee, D.C.; Swaminathan, A.K. Sensitivity of ultrasound for the diagnosis of tubo-ovarian abscess: A case report and literature review. J. Emerg. Med. 2011, 40, 170–175. [Google Scholar] [CrossRef]
- Lee, S.W.; Rhim, C.C.; Kim, J.H.; Lee, S.J.; Yoo, S.H.; Kim, S.Y.; Hwang, Y.B.; Shin, S.Y.; Yoon, J.H. Predictive Markers of Tubo-Ovarian Abscess in Pelvic Inflammatory Disease. Gynecol. Obstet. Investig. 2015, 81, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Goje, O.; Markwei, M.; Kollikonda, S.; Chavan, M.; Soper, D.E. Outcomes of Minimally Invasive Management of Tubo-ovarian Abscess: A Systematic Review. J. Minim. Invasive Gynecol. 2021, 28, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Trent, M. Pelvic inflammatory disease. Pediatr. Rev. 2013, 34, 163–172. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Greydanus, D.E.; Cabral, M.D.; Patel, D.R. Pelvic inflammatory disease in the adolescent and young adult: An update. Dis. Mon. 2022, 68, 101287. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Moola, S.; Munn, Z.; Tufanaru, C.; Aromataris, E.; Sears, K.; Sfetcu, R.; Currie, M.; Qureshi, R.; Mattis, P.; Lisy, K. Systematic reviews of etiology and risk. Joanna Briggs Inst. Rev. Man. 2017, 5, 217–269. [Google Scholar]
- Simpson-Camp, L.; Richardson, E.J.; Alaish, S.M. Streptococcus viridans tubo-ovarian abscess in an adolescent virgin. Pediatr. Int. 2012, 54, 706–709. [Google Scholar] [CrossRef]
- Campbell, M.; Noor, A.A.; Castaneda, M. A Case of Tubo-Ovarian Abscess in a 15-Year-Old Female After Appendectomy Complicated by Peritonitis. Cureus 2021, 13, e20052. [Google Scholar] [CrossRef]
- Goodwin, K.; Fleming, N.; Dumont, T. Tubo-ovarian abscess in virginal adolescent females: A case report and review of the literature. J. Pediatr. Adolesc. Gynecol. 2013, 26, e99–e102. [Google Scholar] [CrossRef]
- Hartmann, K.A.; Lerand, S.J.; Jay, M.S. Tubo-ovarian abscess in virginal adolescents: Exposure of the underlying etiology. J. Pediatr. Adolesc. Gynecol. 2009, 22, e13–e16. [Google Scholar] [CrossRef]
- Rubino, C.; Barbati, F.; Regoli, M.; Bencini, E.; Mattei, A.; Fierro, F.; Brizzi, I.; Indolfi, G. Recurrent Bilateral Salpingitis in a Sexually Inactive Adolescent: Don’t Forget about the Appendix. J. Pediatr. Adolesc. Gynecol. 2021, 34, 217–219. [Google Scholar] [CrossRef] [PubMed]
- Stortini, B.; Dural, O.; Kielly, M.; Fleming, N. Tubo-Ovarian Abscess in a Virginal Adolescent with Labial Agglutination Due to Lichen Sclerosus. J. Pediatr. Adolesc. Gynecol. 2017, 30, 646–648. [Google Scholar] [CrossRef]
- Nishida, N.; Shono, T.; Shono, K.; Hashimoto, Y.; Kawakami, K. Late Occurrence of the Tubo-Ovarian Abscess after Appendectomy for Perforated Appendicitis in a Virginal Adolescent Girl. J. Pediatr. Adolesc. Gynecol. 2022, 35, 509–511. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.M.; Cardosi, R.J.; Barrionuevo, M.J. Tubo-ovarian abscess in an adolescent virgin female. Arch. Pediatr. Adolesc. Med. 1999, 153, 91–92. [Google Scholar] [CrossRef]
- Pomeranz, A.; Korzets, Z.; Eliakim, A.; Pomeranz, M.; Uziel, Y.; Wolach, B. Relapsing Henoch-Schönlein purpura associated with a tubo-ovarian abscess due to Morganella morganii. Am. J. Nephrol. 1997, 17, 471–473. [Google Scholar] [CrossRef] [PubMed]
- Mills, D.; Sharon, B.; Schneider, K. Streptococcus constellatus Tubo-ovarian Abscess in a Non-Sexually Active Adolescent Female. Pediatr. Emerg. Care 2018, 34, e100–e101. [Google Scholar] [CrossRef]
- Cheong, L.H.A.; Emil, S. Non-sexually transmitted tubo-ovarian abscess in an adolescent. J. Pediatr. Surg. Case Rep. 2013, 1, 378–380. [Google Scholar] [CrossRef]
- Boleken, M.E.; Günendi, T.; Yol, C.; Kaya, V.; Kocaman, O.H.; Dörterler, M.E. Xanthogranulomatous Salpingitis Presenting as Pyosalpinx in a Non-Sexually Active Adolescent Girl. J. Pediatr. Adolesc. Gynecol. 2023, 36, 324–327. [Google Scholar] [CrossRef]
- Algren, S.D.; Strickland, J.L. Beta hemolytic streptococcus group f causing pelvic inflammatory disease in a 14-year-old girl. J. Pediatr. Adolesc. Gynecol. 2005, 18, 117–119. [Google Scholar] [CrossRef]
- Kielly, M.; Jamieson, M.A. Pelvic inflammatory disease in virginal adolescent females without tubo-ovarian abscess. J. Pediatr. Adolesc. Gynecol. 2014, 27, e5–e7. [Google Scholar] [CrossRef]
- Murata, T.; Endo, Y.; Furukawa, S.; Ono, A.; Kiko, Y.; Soeda, S.; Watanabe, T.; Takahashi, T.; Fujimori, K. Successful laparoscopic resection of ovarian abscess caused by Staphylococcus aureus in a 13-year-old girl: A case report and review of literature. BMC Womens Health 2021, 21, 198. [Google Scholar] [CrossRef] [PubMed]
- Sakar, M.N.; Gul, T.; Atay, A.E. Tubo-ovarian abscess presenting as an ovarian tumor in a virginal adolescent: A case report. Clin. Exp. Obstet. Gynecol. 2012, 39, 388–389. [Google Scholar] [PubMed]
- Levin, G.; Herzberg, S.; Dior, U.P.; Shushan, A.; Gilad, R.; Benshushan, A.; Rottenstreich, A. The predictive role of CA-125 in the management of tubo-ovarian abscess. A retrospective study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 238, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Workowski, K.A.; Bachmann, L.H.; Chan, P.A.; Johnston, C.M.; Muzny, C.A.; Park, I.; Reno, H.; Zenilman, J.M.; Bolan, G.A. Sexually Transmitted Infections Treatment Guidelines, 2021. MMWR Recomm. Rep. 2021, 70, 1–187. [Google Scholar] [CrossRef]
- Ness, R.B.; Soper, D.E.; Holley, R.L.; Peipert, J.; Randall, H.; Sweet, R.L.; Sondheimer, S.J.; Hendrix, S.L.; Amortegui, A.; Trucco, G.; et al. Effectiveness of inpatient and outpatient treatment strategies for women with pelvic inflammatory disease: Results from the Pelvic Inflammatory Disease Evaluation and Clinical Health (PEACH) Randomized Trial. Am. J. Obstet. Gynecol. 2002, 186, 929–937. [Google Scholar] [CrossRef]
- Risser, W.L.; Risser, J.M.; Risser, A.L. Current perspectives in the USA on the diagnosis and treatment of pelvic inflammatory disease in adolescents. Adolesc. Health Med. Ther. 2017, 8, 87–94. [Google Scholar] [CrossRef]
- Pfeifer, C.M.; Williams, L.E.; Veltkamp, J.G.; Lagomarsino, E.M. Pediatric pyosalpinx without sexually transmitted infection: A report of 3 cases. Radiol. Case Rep. 2019, 14, 501–504. [Google Scholar] [CrossRef]
- Wolff, M.; Balamuth, F.; Sampayo, E.; Mollen, C. Improving Adolescent Pelvic Inflammatory Disease Follow-up From the Emergency Department: Randomized Controlled Trial with Text Messages. Ann. Emerg. Med. 2016, 67, 602–609.e3. [Google Scholar] [CrossRef]
- Solomon, M.; Tuchman, L.; Hayes, K.; Badolato, G.; Goyal, M.K. Pelvic Inflammatory Disease in a Pediatric Emergency Department: Epidemiology and Treatment. Pediatr. Emerg. Care 2019, 35, 389–390. [Google Scholar] [CrossRef]
| Reference | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | Total | Quality |
|---|---|---|---|---|---|---|---|---|---|---|
| Moralioğlu et al. [7] | Y | Y | Y | N | N | N | Y | Y | 5/8 | Moderate |
| Simpson-Camp et al. [17] | Y | Y | N | Y | Y | Y | Y | Y | 7/8 | High |
| McKinnon et al. [6] | Y | N | Y | Y | Y | Y | Y | Y | 7/8 | High |
| Campbell et al. [18] | Y | Y | Y | Y | Y | Y | U | Y | 7/8 | High |
| Goodwin et al. [19] | Y | Y | Y | Y | Y | Y | Y | Y | 8/8 | High |
| Arda et al. [8] | Y | Y | Y | Y | Y | Y | U | Y | 7/8 | High |
| Hartmann et al. [20] | Y | Y | Y | Y | Y | Y | U | Y | 8/8 | High |
| Rubino et al. [21] | Y | Y | Y | N | Y | Y | Y | Y | 7/8 | High |
| Stortini et al. [22] | Y | Y | Y | Y | Y | Y | U | Y | 7/8 | High |
| Nishida et al. [23] | Y | Y | Y | Y | Y | Y | Y | Y | 8/8 | High |
| Moore et al. [24] | Y | Y | Y | Y | Y | Y | Y | Y | 8/8 | High |
| Pomeranz et al. [25] | Y | Y | Y | Y | Y | Y | U | Y | 7/8 | High |
| Mills et al. [26] | Y | Y | Y | Y | Y | Y | U | Y | 7/8 | High |
| Cheong et al. [27] | Y | Y | Y | Y | Y | Y | Y | Y | 8/8 | High |
| Fink et al. [5] | Y | Y | Y | Y | Y | Y | Y | Y | 8/8 | High |
| Boleken et al. [28] | Y | Y | Y | Y | Y | Y | Y | Y | 8/8 | High |
| Algren et al. [29] | Y | Y | Y | Y | Y | Y | Y | Y | 8/8 | High |
| Kielly et al. [30] | Y | Y | Y | Y | Y | Y | Y | Y | 8/8 | High |
| Murata et al. [31] | Y | Y | Y | Y | Y | Y | Y | Y | 8/8 | High |
| Sakar et al. [32] | Y | Y | N | Y | Y | Y | Y | Y | 6/8 | Moderate |
| Reference | Age (Years) | Diagnosis | Clinical Presentation | Coexisting Conditions | Imaging |
|---|---|---|---|---|---|
| Moralioğlu et al. [7] | 13 | Right-sided hydrosalpinx | Abdominal pain | Hirschsprung disease (HD) | CT scan: septated cyst measuring 10 × 6 × 7 cm3 in size |
| Moralioğlu et al. [7] | 14 | Right-sided pyosalpinx | Abdominal pain, vomiting, fever | Rectovestibular fistula, anal atresia, sigmoid resection, uterus bicornis unicollis, septate vagina | Ultrasound scan: cystic lesion (10.5 cm × 7.5 cm) with internal septations in the right adnexal region |
| Simpson-Camp et al. [17] | 14 | Left TOA | Fatigue, fever, pelvic pain, abdominal fullness, dysuria | Not mentioned | Ultrasound scan: complex right adnexal mass measuring 12.5 cm/9.6 cm/11 cm with multicystic areas and septations |
| McKinnon et al. [6] | 13 | Bilateral pyosalpinx | Nausea, vomiting, fever, diffuse abdominal and pelvic pain | Obesity, asthma, type 1 diabetes mellitus | Ultrasound scan: left ovarian cyst (4.38 × 3.42 cm2) with smooth contours, reduced venous Doppler flow, moderate amount of free fluid |
| Campbell et al. [18] | 15 | Right pyosalpinx | Abdominal pain | Recent appendectomy postoperative peritonitis | Ultrasound scan: right annexation lesion adjacent to the right ovary measuring 7.1 cm × 4.3 cm × 4.3 cm |
| Goodwin et al. [19] | 13 | Bilateral TOA | Abdominal pain, vomiting | Constipation | Abdominal radiography: air fluid levels US scan: intestinal occlusion and potential perforation |
| Arda et al. [8] | 15 | Right TOA | Right lower abdominal pain, dysuria, fever | Urinary tract infections | US and CT scan: 6 × 2.5 × 3 cm3 abscess originating in the right tubo-ovarian structures |
| Hartmann et al. [20] | 16 | Right TOA | Abdominal pain in the right lower quadrant, fever | Inflammatory bowel disease, Candida vaginitis | US scan: small right ovarian cyst CT scan: small irregular fluid collections extending into the pelvis, anterior and superior to the uterus with inflammation of the right ovary |
| Hartmann et al. [20] | 12 | Bilateral TOA | Diffuse lower abdomen pain, nausea, vomiting, fever | Obesity, type 2 diabetes mellitus, constipation, recurrent UTI | CT scan: echogenic debris at the center of the lower pelvis, suggestive of large dominant cyst and inflammation of the left ovary |
| Rubino et al. [21] | 16 | Bilateral salpingitis | Lower quadrant abdominal pain, fever, leukorrhea | Chronic appendicitis | MRI scan: bilateral salpingitis |
| Stortini et al. [22] | 14 | Bilateral TOA | Acute urinary retention | Lichen sclerosus Recurrent UTI | MRI scan: severe inflammatory changes of the pelvis, TOAs mainly in the right ovary and both fallopian tubes |
| Nishida et al. [23] | 15 | Right TOA | Recurrent fever, right lower quadrant pain | Appendectomy | CT scan: cystic structures with thickened walls in the right pelvis |
| Moore et al. [24] | 15 | Left TOA | Abdominal pain, nausea, vomiting, dysuria and fever | Obesity, cystitis | US scan: enlarged heterogenous uterus, small fluid collection in the fundus MRI scan: abovementioned findings, poorly defined soft tissue changes |
| Pomeranz et al. [25] | 15 | Left TOA | Abdominal pain, vomiting, purpuric rash, hematuria | Recurrent episodes of Henoch–Schönlein purpura | US scan; multiloculated mass localized to the left ovary CT scan: left semisolid ovarian mass |
| Mills et al. [26] | 13 | Bilateral tubo-ovarian abscess | Intermittent abdominal pain for several months | Appendicitis | CT scan: 5.2 × 5.8 × 5.3-cm3 multiloculated cystic mass with surrounding inflammation and adjacent peripherally enhancing fluid |
| Cheong et al. [27] | 13 | Left-sided pyosalpinx | Fever, anorexia, vomiting, abdominal pain, vaginal discharge | Peritonitis | US scan: retrouterine heterogenous collection measuring 10.8 × 11.4 cm2 that was compatible with an abscess secondary to perforated appendicitis |
| Fink et al. [5] | 11 | Left tubo-ovarian abscess | Left lower abdominal pain, blood-streaked emesis, anorexia | Enuresis, obesity | CT scan: Lower abdominal mesentery heterogenous complex lesion MRI scan: hydrosalpinx with thick peripheral enhancement |
| Boleken et al. [28] | 15 | Left-sided pyosalpinx | Left lower quadrant abdominal pain | Chronic constipation | Ultrasound scan: thick-walled, dense cystic mass of 10 × 10 cm2 CT scan: 9.3 × 10 × 11 cm3 cystic lesion, suggesting a pyosalpinx |
| Algren et al. [29] | 14 | Bilateral hydrosalpinges; right TOA | Abdominal pain, dysuria, nausea, vomiting, diarrhea, weight loss, fevers, night sweats, fatigue | Gastroenteritis | US scan: large complex (mostly solid) pelvic mass of 10.8 × 7.9 × 9.9 cm3. CT scan: multiloculated fluid collection |
| Kielly et al. [30] | 15 | Pelvic inflammatory disease | General malaise, diarrhea, right lower quadrant pain. | Fitz–Hugh–Curtis syndrome | Ultrasound and CT scan: moderate amount of free fluid in the pelvis and right lower quadrant |
| Murata et al. [31] | 13 | Right TOA | Fever | None | Ultrasound scan: pelvic mass measuring 6 cm CT scan: unilateral and unilocular ovarian mass |
| Sakar et al. [32] | 13 | Subacute salpingitis, Left TOA | Abdominal pain, menstrual disorder | None | US scan: semisolid, hyperechogenic mass of 57 × 73 mm2 in the left adnexal area CT scan: dense cystic semisolid mass (7 × 6.4 cm2) with thickened walls and peripheral contrast |
| Reference | Surgical Management | Microorganism Cultured | Antibiotic Regimens | Follow-Up/ Recurrence |
|---|---|---|---|---|
| Moralioğlu et al. [7] | Exploratory laparotomy: right salpingectomy | Not mentioned | Not mentioned | No further complications |
| Moralioğlu et al. [7] | Exploratory laparotomy: right salpingectomy | Escherichia coli | Inpatient: IV ceftriaxone/metronidazole | No further complications |
| Simpson-Camp et al. [17] | Exploratory laparotomy: abscess drainage | Streptococcus viridans | Preoperatively: IV cefazolin (single doses) Postoperatively: IV doxycycline/cefoxitin changed to cefotaxime × 14 days | Superficial fluid collection at the inferior portion of her wound developed on 30th post-operative day |
| McKinnon et al. [6] | Diagnostic laparoscopy: bilateral salpingostomies, drainage | Fusobacterium nucleatum | Inpatient: IV cefoxitin/doxycycline × 10 days Outpatient: PO metronidazole × 1 month | Resolution of symptoms on day 1 postoperatively |
| Campbell et al. [18] | Diagnostic laparoscopy: abscess drainage | Negative | Inpatient: IV doxycycline/metronidazole/cefoxitin | No further complications |
| Goodwin et al. [19]. | Exploratory laparotomy: abscess drainage | Ampicillin-sensitive Escherichia coli | Preoperatively: IV gentamycin (5 mg/kg)/metronidazole (500 mg). Postoperatively: IV clindamycin (40 mg/kg/d)/gentamycin (5 mg/kg/d) | Complete resolution of bilateral TOA on ultrasound scan at 3 months |
| Arda et al. [8] | Diagnostic laparoscopy: Abscess drainage | Escherichia coli | Inpatient: IV ceftriaxone (100 mg/kg, 24 h)/amikacin (15 mg/kg, 12 h) | No further complications |
| Hartmann et al. [20] | Diagnostic laparoscopy | Bacteroides uniformis, Coagulase negative Staphylococcus, Streptococcus milleri | Inpatient: IV doxycycline/gentamycin/cefotaxime/metronidazole doxycycline Outpatient: PO Doxycycline/Metronidazole × 14 days | No further complications |
| Hartmann et al. [20] | Diagnostic laparoscopy | Escherichia coli | Inpatient: IV doxycycline/gentamycin/cefotaxime/metronidazole Outpatient: PO doxycycline/metronidazole × 14 days | Persistence of hydrosalpinx |
| Rubino et al. [21] | Diagnostic laparoscopy: adhesiolysis; appendectomy | Not mentioned | Inpatient: IV ceftriaxone/metronidazole Outpatient: PO azithromycin × 14 days | Persistence of hydrosalpinx |
| Stortini et al. [22] | Abscess drainage by interventional radiology | Streptococcus anginosus Peptostreptococcus anaerobius | Inpatient: IV tobramycin (7.5 mg/kg/d)/metronidazole (30 mg/kg/d) × 14 days Outpatient: PO amoxicillin/clavulanic acid (1500 mg/d) × 10 days | No further complications |
| Nishida et al. [23] | Diagnostic laparoscopy: abscess drainage | Negative | Inpatient: IV Cefmetazole | Recurrence of TOA after 2 months |
| Moore et al. [24] | Diagnostic laparoscopy, exploratory laparotomy: left salpingo-oophorectomy | Escherichia coli | Preoperatively: IV ceftazidime for pyelonephritis | Sepsis, wound infection Recurrent intra-abdominal abscess |
| Pomeranz et al. [25] | Exploratory laparotomy: left salpingo-oophorectomy | Morganella morganii | Inpatient: IV ampicillin/gentamicin/metronidazole | No further complications |
| Mills et al. [26] | Exploratory laparotomy: abscess drainage | Streptococcus constellatus | Inpatient: IV piperacillin/tazobactam × 12 days | No further complications |
| Cheong et al. [27] | Diagnostic laparoscopy: left salpingectomy | Streptococcus viridans Peptostreptococcus | Inpatient: IV piperacillin/tazobactam × 5 days Outpatient: PO amoxicillin/clavulanic acid × 14 days | Resolution of bilateral TOA on ultrasound scan at 2 months |
| Fink et al. [5] | Diagnostic laparoscopy: abscess drainage; | Streptococcus bovi, Bacteroides thetaiotaomicron | Inpatient: cefoxitin (2000 mg)/doxycycline (100 mg), ampicillin/sulbactam (2000 mg) Outpatient: PO amoxicillin/clavulanic × 14 days | Recurrence of left tubo-ovarian abscess after 1 month |
| Boleken et al. [28] | Exploratory laparotomy: left salpingectomy | Escherichia coli | Inpatient: IV broad-spectrum as preoperative preparation Outpatient: PO meropenem (40 mg/kg/d) | Resolution of symptoms after 2 days postoperatively |
| Algren et al. [29] | Diagnostic laparoscopy, exploratory laparotomy: right salpingo-oophorectomy | Beta Hemolytic Streptococcus Group F | Ampicillin, gentamycin, and Clindamycin (preoperatively); metronidazole and ceftriaxone (postoperatively) later changed to clindamycin and ciprofloxacin | Resolution on CT scan after 1 month |
| Kielly et al. [30] | Diagnostic laparoscopy | Not mentioned | Preoperatively: ceftriaxone 2 g intravenously (IV), metronidazole 500 mg IV, and vancomycin 1 g IV Postoperatively: ceftriaxone 2 g IV × 24 h, metronidazole 500 mg IV × 8 h, and doxycycline 100 mg IV × 12 h | Suspected pulmonary embolism; Severe secondary dysmenorrhea Recurrence 1 year post-operatively |
| Murata et al. [31] | Diagnostic laparoscopy: right salpingo-oophorectomy | Methicillin-susceptible, Staphylococcus aureus | Inpatient: IV cefmetazole for 5 days, 2 g/day Outpatient: PO cefaclor at a dose of 900 mg/day × 14 days | Resolution on MRI scan after 1 month |
| Sakar et al. [32] | Exploratory laparotomy: Abscess drainage | Not mentioned | Inpatient: IV ceftriaxone (2 g/day)/metronidazole (500 mg/day) Outpatient: PO metronidazole/cefuroxime × 14 days | Resolution of symptoms after 7 days postoperatively |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Surd, A.; Mureșan, R.; Oprea, A.; Snakovszki, K.; Sur, L.M.; Usatiuc, L.-O.; Ciongradi, C.-I.; Sârbu, I. Diagnostic Challenges and Management Strategies of Pelvic Inflammatory Disease in Sexually Inactive Pediatric and Adolescent Patients: A Systematic Review of Case Reports. J. Clin. Med. 2025, 14, 3971. https://doi.org/10.3390/jcm14113971
Surd A, Mureșan R, Oprea A, Snakovszki K, Sur LM, Usatiuc L-O, Ciongradi C-I, Sârbu I. Diagnostic Challenges and Management Strategies of Pelvic Inflammatory Disease in Sexually Inactive Pediatric and Adolescent Patients: A Systematic Review of Case Reports. Journal of Clinical Medicine. 2025; 14(11):3971. https://doi.org/10.3390/jcm14113971
Chicago/Turabian StyleSurd, Adrian, Rodica Mureșan, Andreea Oprea, Kriszta Snakovszki, Lucia Maria Sur, Lia-Oxana Usatiuc, Carmen-Iulia Ciongradi, and Ioan Sârbu. 2025. "Diagnostic Challenges and Management Strategies of Pelvic Inflammatory Disease in Sexually Inactive Pediatric and Adolescent Patients: A Systematic Review of Case Reports" Journal of Clinical Medicine 14, no. 11: 3971. https://doi.org/10.3390/jcm14113971
APA StyleSurd, A., Mureșan, R., Oprea, A., Snakovszki, K., Sur, L. M., Usatiuc, L.-O., Ciongradi, C.-I., & Sârbu, I. (2025). Diagnostic Challenges and Management Strategies of Pelvic Inflammatory Disease in Sexually Inactive Pediatric and Adolescent Patients: A Systematic Review of Case Reports. Journal of Clinical Medicine, 14(11), 3971. https://doi.org/10.3390/jcm14113971






