Mitochondrial Bioenergetics and Cardiac Rehabilitation: Bridging Basic Science and Clinical Practice
Abstract
1. Introduction
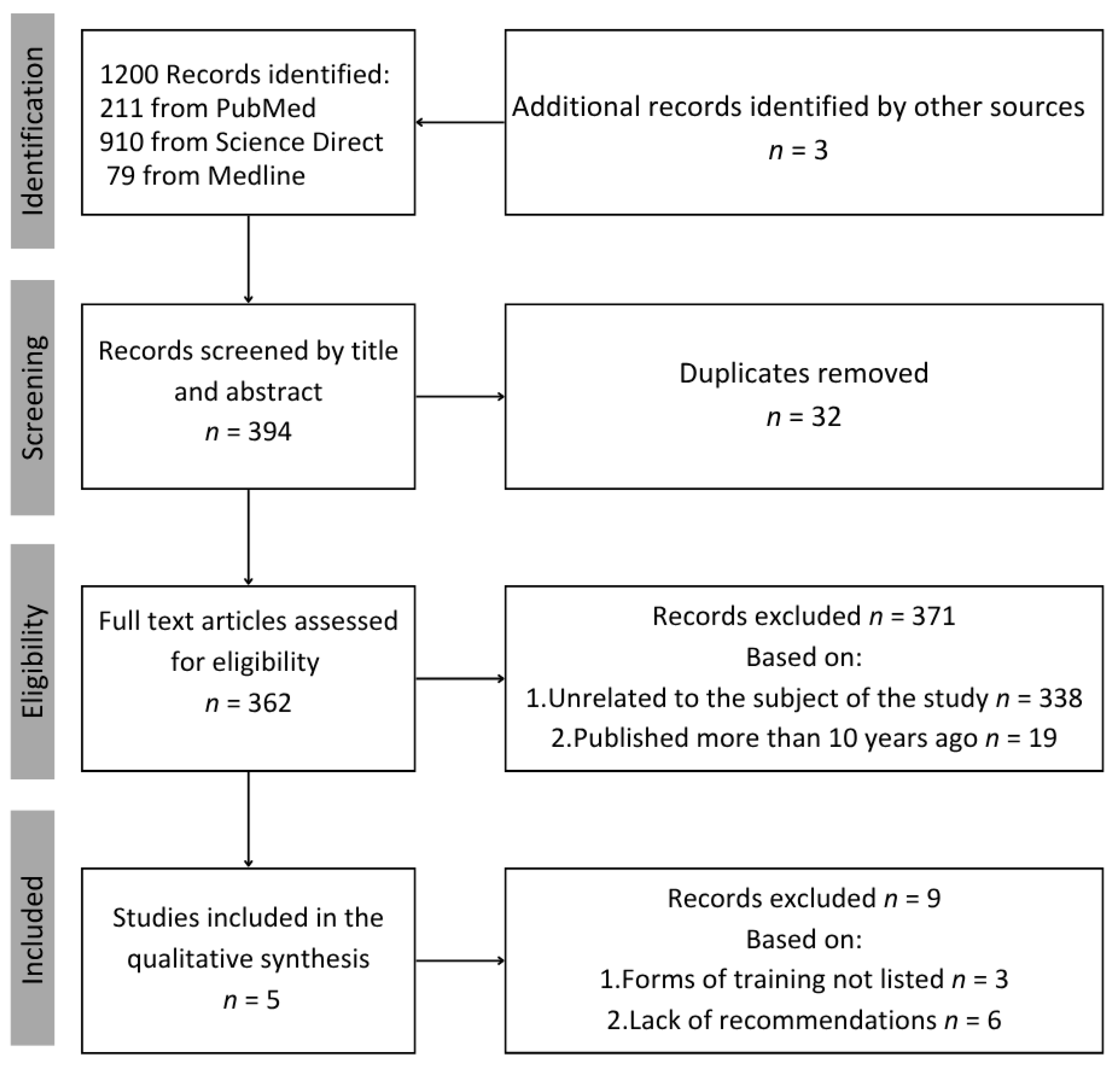
2. Materials and Methods
3. Results
- Breakdown of rehabilitation into stages/phases
- Psychological support
- Training plan
- Multidisciplinary team
- Patient education
- Health monitoring
- Sexual activity
- Pharmacotherapy
- Nutritional counselling
- Daily living and return to work
| Factor/Organization | Cardiac Rehabilitation and Exercise Physiology Section of the Polish Cardiac Society 2021, Poland [2]. | French Society of Cardiology, Groupe Exercise Rehabilitation Sports—Prevention, 2023, France [10]. | American College of Cardiology, American Heart Association, JACC Expert Panel, 2024, US [11]. | Portuguese Society of Cardiology, 2018, Portugal [12]. | European Association for Cardiovascular Prevention and Rehabilitation, 2016, Europe [13]. |
|---|---|---|---|---|---|
| Breakdown of rehabilitation into stages/phases | Stage I—Early (in-hospital) rehabilitation: Begins during hospitalization after a cardiovascular event and continues until the patient is discharged from the hospital. Carried out in the intensive care unit, post-operative care unit, cardiology, internal medicine, or CR. Stage II—Early rehabilitation (outpatient/inpatient): Can be performed entirely in the inpatient setting, in a center/day unit (outpatient), or as a hybrid cardiac telerehabilitation (HCTR). Inpatient form for patients at high cardiovascular risk, with complications after treatment of acute coronary syndromes (ACSs), cardiac surgery, or percutaneous coronary intervention (PCI), with stable, advanced heart failure (HF) in NYHA class III–IV, immediately after heart transplantation (HT), or for those who, for logistical reasons, cannot participate in rehabilitation programs. Stage III—Late (outpatient) rehabilitation: Implemented in a day/outpatient setting Includes health education program for patient and family. Continuous monitoring based on individual needs and cardiac risk profile. Stage III continues for the rest of the patient’s life | No specific breakdown | No specific breakdown | Hospital Phase I: begins 24–48 h after a patient is stabilized following an acute event. Includes early mobilization, low-intensity exercise and education. Early Post-Discharge Phase II: begins within two weeks of hospital discharge or after diagnosis. It can be conducted in the hospital, a specialized CR center, or in the patient’s home. Includes individualized exercise, education, and lifestyle modification. Long-term Phase III: begins after Phase II and continues for the rest of the patient’s life. Aims at long-term maintenance of rehabilitation effects, control of risk factors, and monitoring of health status. | No specific breakdown |
| Psychological suport | Assessment of mental status and development of an individual psychological care plan. Psychological assistance, especially for sleep disorders, anxiety, depression, mental health deterioration, and reduced quality of life. | Psychosocial assessment at the beginning of the rehabilitation process. Access to psychologist during rehabilitation. Mental status monitoring. | Detailed psychological assessment, including depression, perceived stress, anxiety, sexual dysfunction, anger, loneliness, social isolation, and problematic substance abuse. Psychological interventions and patient education. | Assessment and psychological interventions to reduce stress, anxiety, and depression. | Assessment of psychosocial factors. Specialized psychological interventions. |
| Training plan | Individualized exercise program for each patient. The patient must be in a stable clinical condition to begin training. Aerobic endurance training involving large muscle groups—3 days per week/day. Resistance training—2 times a week on non-consecutive days of the week Duration of exercise: 20–30 min minimum (45–60 min preferred) per session. Training intensity should be based on individual exercise tolerance and cardiovascular risk. Train according to the FITT rule (frequency, intensity, time-duration, type of exercise). Energy expenditure during exercise of 1000–2000 kcal/week. | Individual exercise program for each patient Frequency: 3–6 times a week Duration: Warm-up: 5–10 min Exercise proper: 20–45 min —Cooling down: at least 5 min. Type: Aerobic Continuous at moderate intensity Interval Resistance. Respiratory: The program should last at least 12 weeks. | Individualized plan, updated every 30 days with health assessment Aerobic training—3–5 days a week, Moderate intensity (40–59%) and high intensity (60–89%) with effort assessment, 20–60 min. Resistance training—2–3 days a week, on non-consecutive days, 10–15 repetitions with 40–60% 1-RM load (maximum load that can be lifted once) with effort assessment. | Early Phase I—Early mobilization and low-intensity exercises. Intensity: Determined by the subjective feeling of exertion as assessed by the Borg scale, without exceeding the resting heart rate by 20–30 beats per minute, depending on the patient’s clinical condition. Phase II early after hospital discharge. Individualized training program including aerobic and resistance exercises Duration: 8–12 weeks. | Regular physical activity is recommended as a lifelong lifestyle for all men and women, including ≥150 min/week of moderate-intensity activity or ≥75 min/week of vigorous-intensity activity. Aerobic exercise. Resistance training—2–3 sets of 8–12 repetitions at an intensity of 60–80% of 1 repetition performed at a person’s 1-ROM, with a frequency of ≥2 days per week. Neuromotor training. |
| Multidisciplinary team | Physician, physiotherapist, nurse, radiology technician, psychologist, nutritionist, rehabilitation management specialist. | Physician, nurse, physiotherapist, social worker, nutritionist, psychologist, physical activity trainer. | Physician, nurse, physiotherapist, nutritionist, respiratory therapist, behavioral health expert. | Cardiologist, physiotherapist, rehabilitation nurse, nutritionist, psychologist, psychiatrist, and other specialists as needed by the patient. | Physician, physiotherapist, nurse, nutritionist, pharmacist, sports medicine expert. |
| Patient education | Risk factor modification: dyslipidemia, hypertension, diabetes, obesity, smoking, and physical inactivity. Healthy lifestyle and physical activity, pharmacotherapy, symptom self-management, and stress management. | Modification of cardiovascular risk factors. Nutrition education. Physical activity education. | Modification of risk factors: physical activity, diet, mental health, sleep, avoidance of unhealthy behaviors (smoking, alcohol, drug abuse). | Counseling on healthy lifestyle, diet, exercise, returning to work, and managing stress and anxiety. Maintaining long-term control of cardiovascular risk factors and adherence to medications and healthy lifestyles. | Disease knowledge. Modification of risk factors—smoking, unhealthy diet, physical inactivity, hypertension, hyperlipidemia, and diabetes. Stress management education. |
| Health monitoring | Observation of the patient. Assessment of clinical condition before each training—measurement of blood pressure and heart rate. Performing tests: ECG, exercise test, Cardiopulmonary Exercise Test (CPET), 6-min walk test (6-MWT), laboratory tests, transthoracic echocardiography (TTE). | Regular review of risk factors. Monitoring: heart rate and oxygen saturation during exercise. Physical fitness assessment: 6-min walk test, Cardiopulmonary Exercise Test (CPET), ankle–arm index measurement, arterial echo-doppler, Holter-EKG, ambulatory blood pressure monitoring, overnight polygraphy, pulmonary function testing. | Monitor blood pressure and ECG during exercise, especially in high-risk patients. Evaluate progress and adjust exercise plan based on patient response and results achieved. | Risk factor control: Interventions to control hypertension, diabetes, hyperlipidemia, obesity, and smoking. Medical evaluation: Assessment of medical history, risk factors, functional status, and test results (ECG, blood tests, echocardiography). | Monitoring of risk factors: BMI, cholesterol, blood pressure, smoking. Monitoring treatment. |
| Sexual activity | Information on how to implement physical activity and return to sexual activity is included in patient education. | Therapy related to sexual activity must be offered to both men and women, taking into account the psychological dimension and individual wishes of the patients. Determine the cause of the difficulty: may be due to the disease itself, comorbidities, and medications used (beta-blockers, diuretics, antihypertensives). Possible use of phosphodiesterase inhibitors. Patients with unstable cardiovascular disease or symptoms should not engage in sexual activity until their condition has stabilized. | Psychosocial assessment, including sexual dysfunction. Psychosocial interventions—sex and intimacy education and counseling. | Patient education includes counseling on returning to sexual activity | Possible occurrence of erectile dysfunction due to a cardiovascular event in the future. Modification of risk factors: hypercholesterolemia, hypertension, insulin resistance and diabetes, smoking, obesity, metabolic syndrome, sedentary lifestyle, and depression. Recommend pharmacotherapy. |
| Pharmacotherapy | Analysis of existing pharmacotherapy with the possibility of its modification depending on clinical condition and in accordance with general recommendations. Pharmacotherapy education. | Monitor drug therapy: Optimize drug therapy, including anticoagulant therapy, and monitor potassium levels and kidney function after each change in therapy. | Evaluate current treatment and modify if necessary. Patient education—importance of adherence to medication recommendations. | Optimization of pharmacotherapy (phase II). Pharmacologic compliance education. | Optimization of pharmacotherapy. Adherence to recommendations. A multicomponent pill (polypill) may be considered to improve adherence to prescribed pharmacotherapy. |
| Nutritional counselling | Recognize and counteract the effects of malnutrition. Education about healthy nutrition by a nutritionist. | Mediterranean diet. Eating at least 5 servings of fruits and vegetables per day. Limiting the intake of salt and products containing simple sugars. Avoiding processed foods. Nutrition education. | Diet evaluation. Discussion of eating habits. Nutrition education. Individualized nutrition plan. | Dietary counseling, including assessment and advice from dietitians. | Individual dietary recommendations. Avoiding overeating. When following a healthy diet, supplements are not recommended. Limit salt intake. Mediterranean diet. Nutrition education. |
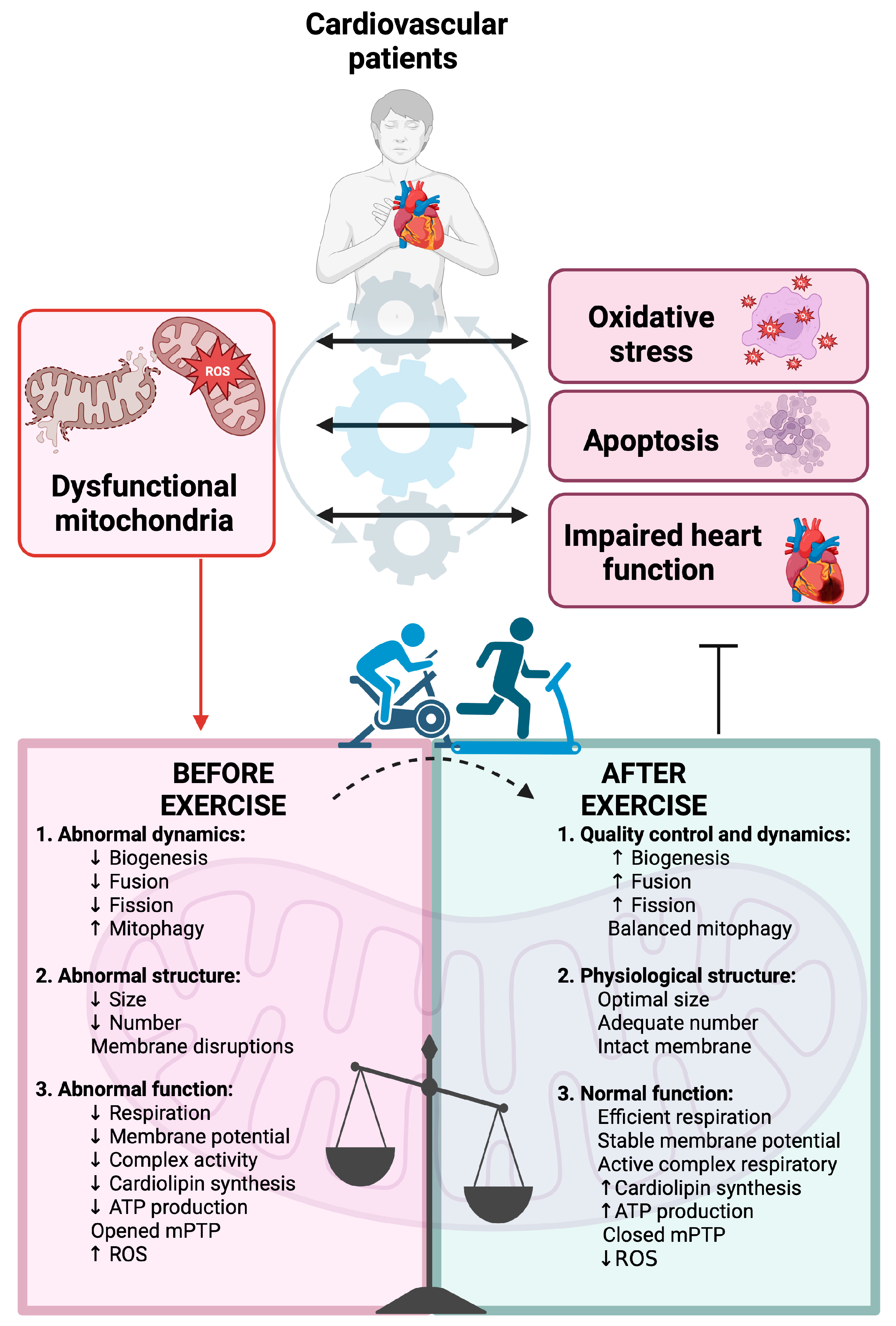
4. Mitochondria Overview in Cardiology and Cardiac Rehabilitation (CR)
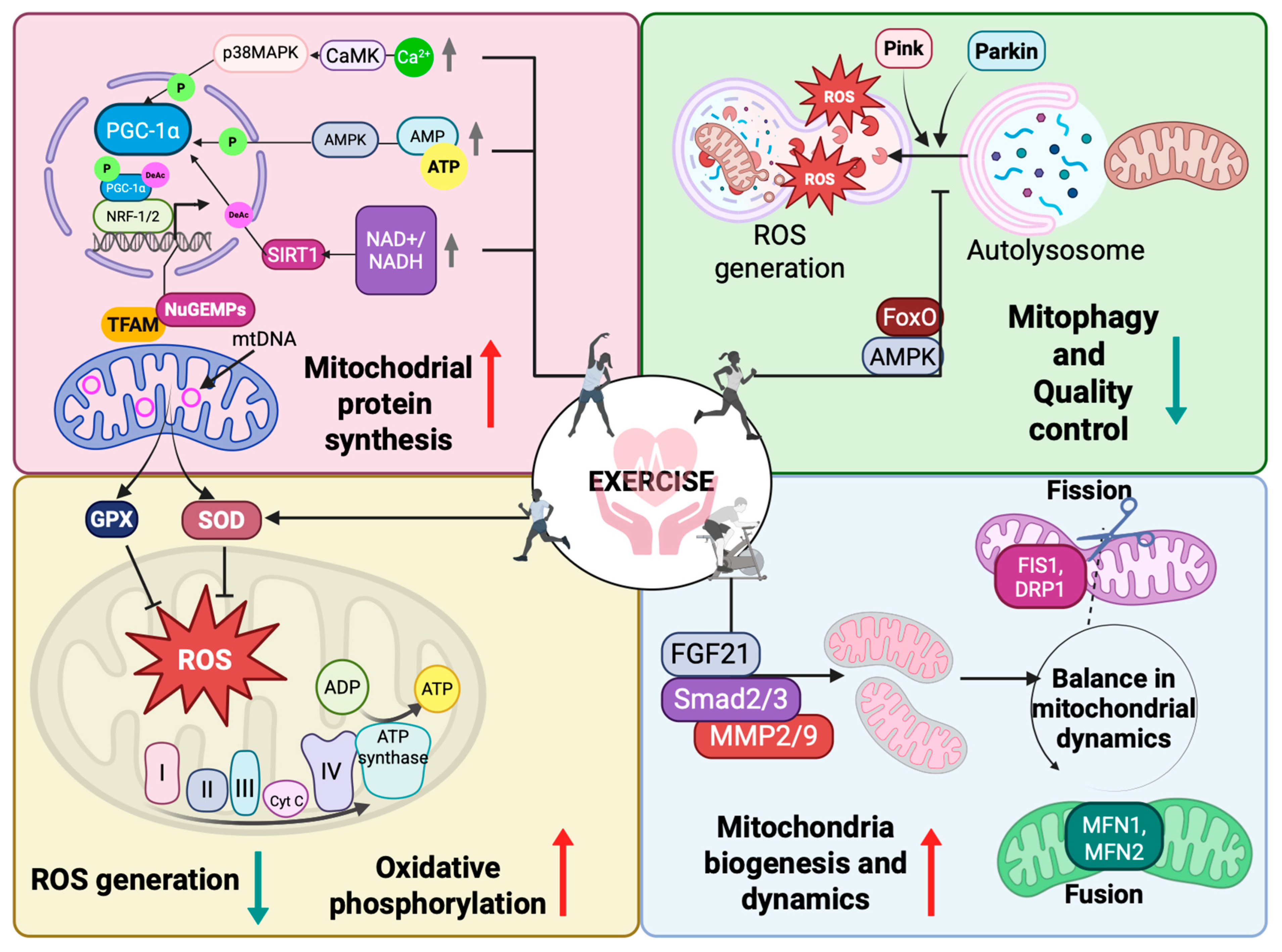
4.1. Mitochondria Biogenesis
4.2. Mitochondria Fusion and Fission
4.3. Mitophagy
4.4. Effects of Physical Exercise on Mitochondria Biogenesis and Functionality in Cardiological Patients
4.5. Non-Invasive Potential Methods for Assessing Mitochondrial Adaptation
5. Limitations
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ambrosetti, M.; Abreu, A.; Corrà, U.; Davos, C.H.; Hansen, D.; Frederix, I.; Iliou, M.C.; Pedretti, R.F.; Schmid, J.-P.; Vigorito, C. Secondary prevention through comprehensive cardiovascular rehabilitation: From knowledge to implementation. 2020 update. A position paper from the Secondary Prevention and Rehabilitation Section of the European Association of Preventive Cardiology. Eur. J. Prev. Cardiol. 2021, 28, 460–495. [Google Scholar] [CrossRef] [PubMed]
- Jegier, A.; Szalewska, D.; Mawlichanów, A.; Bednarczyk, T.; Eysymontt, Z.; Gałaszek, M.; Mamcarz, A.; Mierzyńska, A.; Piotrowicz, E.; Piotrowicz, R. Comprehensive cardiac rehabilitation as the keystone in the secondary prevention of cardiovascular disease. Pol. Heart J. 2021, 79, 901–916. [Google Scholar] [CrossRef] [PubMed]
- Cowie, A.; Buckley, J.; Doherty, P.; Furze, G.; Hayward, J.; Hinton, S.; Jones, J.; Speck, L.; Dalal, H.; Mills, J. Standards and core components for cardiovascular disease prevention and rehabilitation. Heart 2019, 105, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Lim, A.Y.; Chen, Y.-C.; Hsu, C.-C.; Fu, T.-C.; Wang, J.-S. The effects of exercise training on mitochondrial function in cardiovascular diseases: A systematic review and meta-analysis. Int. J. Mol. Sci. 2022, 23, 12559. [Google Scholar] [CrossRef]
- Zeng, Z.; Yuan, Q.; Zu, X.; Liu, J. Insights into the role of mitochondria in vascular calcification. Front. Cardiovasc. Med. 2022, 9, 879752. [Google Scholar] [CrossRef]
- Dalal, J.; Low, L.-P.; Van Phuoc, D.; Abdul Rahman, A.R.; Reyes, E.; Ann Soenarta, A.; Tomlinson, B. The use of medications in the secondary prevention of coronary artery disease in the Asian region. Curr. Med. Res. Opin. 2015, 31, 423–433. [Google Scholar] [CrossRef]
- Pavy, B.; Iliou, M.-C.; Vergès-Patois, B.; Brion, R.; Monpère, C.; Carré, F.; Aeberhard, P.; Argouach, C.; Borgne, A.; Consoli, S. French Society of Cardiology guidelines for cardiac rehabilitation in adults. Arch. Cardiovasc. Dis. 2012, 105, 309–328. [Google Scholar] [CrossRef]
- Mamataz, T.; Uddin, J.; Alam, S.I.; Taylor, R.S.; Pakosh, M.; Grace, S.L. Effects of cardiac rehabilitation in low- and middle-income countries: A systematic review and meta-analysis of randomised controlled trials. Prog. Cardiovasc. Dis. 2022, 70, 119–174. [Google Scholar] [CrossRef]
- Dibben, G.O.; Faulkner, J.; Oldridge, N.; Rees, K.; Thompson, D.R.; Zwisler, A.-D.; Taylor, R.S. Exercise-based cardiac rehabilitation for coronary heart disease: A meta-analysis. Eur. Heart J. 2023, 44, 452–469. [Google Scholar] [CrossRef]
- Bigot, M.; Guy, J.M.; Monpere, C.; Cohen-Solal, A.; Pavy, B.; Iliou, M.C.; Bosser, G.; Corone, S.; Douard, H.; Farrokhi, T. Cardiac rehabilitation recommendations of the Group Exercise Rehabilitation Sports–Prevention (GERS-P) of the French Society of Cardiology: 2023 update. Arch. Cardiovasc. Dis. 2024, 117, 521–541. [Google Scholar] [CrossRef]
- Brown, T.M.; Pack, Q.R.; Aberegg, E.; Brewer, L.C.; Ford, Y.R.; Forman, D.E.; Gathright, E.C.; Khadanga, S.; Ozemek, C.; Thomas, R.J. Core components of cardiac rehabilitation programs: 2024 update: A scientific statement from the American Heart Association and the American Association of Cardiovascular and Pulmonary Rehabilitation. Circulation 2024, 150, e328–e347. [Google Scholar] [CrossRef] [PubMed]
- Abreu, A.; Mendes, M.; Dores, H.; Silveira, C.; Fontes, P.; Teixeira, M.; Santa Clara, H.; Morais, J. Mandatory criteria for cardiac rehabilitation programs: 2018 guidelines from the Portuguese Society of Cardiology. Rev. Port. Cardiol. 2018, 37, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.-T.; Corrà, U.; Cosyns, B.; Deaton, C. 2016 European Guidelines on cardiovascular disease prevention in clinical practice. Pol. Heart J. 2016, 74, 821–936. [Google Scholar] [CrossRef] [PubMed]
- Zielińska-Nowak, E.; Cichon, N.; Saluk-Bijak, J.; Bijak, M.; Miller, E. Nutritional supplements and neuroprotective diets and their potential clinical significance in post-stroke rehabilitation. Nutrients 2021, 13, 2704. [Google Scholar] [CrossRef] [PubMed]
- Lavie, C.J.; Milani, R.V. Cardiac rehabilitation and exercise training in secondary coronary heart disease prevention. Prog. Cardiovasc. Dis. 2011, 53, 397–403. [Google Scholar] [CrossRef]
- Kirkman, D.L.; Lee, D.-c.; Carbone, S. Resistance exercise for cardiac rehabilitation. Prog. Cardiovasc. Dis. 2022, 70, 66–72. [Google Scholar] [CrossRef]
- Xanthos, P.D.; Gordon, B.A.; Kingsley, M.I. Implementing resistance training in the rehabilitation of coronary heart disease: A systematic review and meta-analysis. Int. J. Cardiol. 2017, 230, 493–508. [Google Scholar] [CrossRef]
- Jelleyman, C.; Yates, T.; O’Donovan, G.; Gray, L.J.; King, J.A.; Khunti, K.; Davies, M.J. The effects of high-intensity interval training on glucose regulation and insulin resistance: A meta-analysis. Obes. Rev. 2015, 16, 942–961. [Google Scholar] [CrossRef]
- Ruivo, J.; Moholdt, T.; Abreu, A. Overview of Cardiac Rehabilitation following post-acute myocardial infarction in European Society of Cardiology member countries. Eur. J. Prev. Cardiol. 2023, 30, 758–768. [Google Scholar] [CrossRef]
- Brown, D.A.; Perry, J.B.; Allen, M.E.; Sabbah, H.N.; Stauffer, B.L.; Shaikh, S.R.; Cleland, J.G.; Colucci, W.S.; Butler, J.; Voors, A.A. Mitochondrial function as a therapeutic target in heart failure. Nat. Rev. Cardiol. 2017, 14, 238–250. [Google Scholar] [CrossRef]
- Qu, K.; Yan, F.; Qin, X.; Zhang, K.; He, W.; Dong, M.; Wu, G. Mitochondrial dysfunction in vascular endothelial cells and its role in atherosclerosis. Front. Physiol. 2022, 13, 1084604. [Google Scholar] [CrossRef] [PubMed]
- Ham, P.B., III; Raju, R. Mitochondrial function in hypoxic ischemic injury and influence of aging. Prog. Neurobiol. 2017, 157, 92–116. [Google Scholar] [CrossRef] [PubMed]
- Porter, C.; Reidy, P.T.; Bhattarai, N.; Sidossis, L.S.; Rasmussen, B.B. Resistance exercise training alters mitochondrial function in human skeletal muscle. Med. Sci. Sports Exerc. 2015, 47, 1922–1931. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Radak, Z.; Ji, L.L.; Jackson, M. Reactive oxygen species promote endurance exercise-induced adaptations in skeletal muscles. J. Sport Health Sci. 2024, 13, 780–792. [Google Scholar] [CrossRef]
- Huang, Y.; Zhou, B. Mitochondrial dysfunction in cardiac diseases and therapeutic strategies. Biomedicines 2023, 11, 1500. [Google Scholar] [CrossRef]
- Yan, W.; Diao, S.; Fan, Z. The role and mechanism of mitochondrial functions and energy metabolism in the function regulation of the mesenchymal stem cells. Stem Cell Res. Ther. 2021, 12, 140. [Google Scholar] [CrossRef]
- Lax, N.Z.; Turnbull, D.M.; Reeve, A.K. Mitochondrial mutations: Newly discovered players in neuronal degeneration. Neurosci. 2011, 17, 645–658. [Google Scholar] [CrossRef]
- Abu Shelbayeh, O.; Arroum, T.; Morris, S.; Busch, K.B. PGC-1α is a master regulator of mitochondrial lifecycle and ROS stress response. Antioxidants 2023, 12, 1075. [Google Scholar] [CrossRef]
- Scarpulla, R.C.; Vega, R.B.; Kelly, D.P. Transcriptional integration of mitochondrial biogenesis. Trends Endocrinol. Metab. 2012, 23, 459–466. [Google Scholar] [CrossRef]
- Willows, R.; Sanders, M.J.; Xiao, B.; Patel, B.R.; Martin, S.R.; Read, J.; Wilson, J.R.; Hubbard, J.; Gamblin, S.J.; Carling, D. Phosphorylation of AMPK by upstream kinases is required for activity in mammalian cells. Biochem. J. 2017, 474, 3059–3073. [Google Scholar] [CrossRef]
- Irrcher, I.; Ljubicic, V.; Kirwan, A.F.; Hood, D.A. AMP-activated protein kinase-regulated activation of the PGC-1α promoter in skeletal muscle cells. PLoS ONE 2008, 3, e3614. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Goldberg, A.L. SIRT1 protein, by blocking the activities of transcription factors FoxO1 and FoxO3, inhibits muscle atrophy and promotes muscle growth. J. Biol. Chem. 2013, 288, 30515–30526. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Marcos, P.J.; Auwerx, J. Regulation of PGC-1α, a nodal regulator of mitochondrial biogenesis. Am. J. Clin. Nutr. 2011, 93, 884S–890S. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.-F.; Drumea, K.; Mott, S.; Wang, J.; Rosmarin, A.G. GABP transcription factor (nuclear respiratory factor 2) is required for mitochondrial biogenesis. Mol. Cell. Biol. 2014, 34, 3194–3201. [Google Scholar] [CrossRef]
- Satoh, J.-i.; Kawana, N.; Yamamoto, Y. Pathway analysis of ChIP-Seq-based NRF1 target genes suggests a logical hypothesis of their involvement in the pathogenesis of neurodegenerative diseases. Gene Regul. Syst. Biol. 2013, 7, GRSB.S13204. [Google Scholar] [CrossRef]
- Biswas, M.; Chan, J.Y. Role of Nrf1 in antioxidant response element-mediated gene expression and beyond. Toxicol. Appl. Pharmacol. 2010, 244, 16–20. [Google Scholar] [CrossRef]
- Piantadosi, C.A.; Carraway, M.S.; Babiker, A.; Suliman, H.B. Heme oxygenase-1 regulates cardiac mitochondrial biogenesis via Nrf2-mediated transcriptional control of nuclear respiratory factor-1. Circ. Res. 2008, 103, 1232–1240. [Google Scholar] [CrossRef]
- Malhotra, D.; Portales-Casamar, E.; Singh, A.; Srivastava, S.; Arenillas, D.; Happel, C.; Shyr, C.; Wakabayashi, N.; Kensler, T.W.; Wasserman, W.W. Global mapping of binding sites for Nrf2 identifies novel targets in cell survival response through ChIP-Seq profiling and network analysis. Nucleic Acids Res. 2010, 38, 5718–5734. [Google Scholar] [CrossRef]
- Hughes, D.C.; Ellefsen, S.; Baar, K. Adaptations to endurance and strength training. Cold Spring Harb. Perspect. Med. 2018, 8, a029769. [Google Scholar] [CrossRef]
- Adebayo, M.; Singh, S.; Singh, A.P.; Dasgupta, S. Mitochondrial fusion and fission: The fine-tune balance for cellular homeostasis. FASEB J. 2021, 35, e21620. [Google Scholar] [CrossRef]
- Thomas, K.J.; Cookson, M.R. The role of PTEN-induced kinase 1 in mitochondrial dysfunction and dynamics. Int. J. Biochem. Cell Biol. 2009, 41, 2025–2035. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.; Burke, N.; Dongworth, R.; Hausenloy, D. Mitochondrial fusion and fission proteins: Novel therapeutic targets for combating cardiovascular disease. Br. J. Pharmacol. 2014, 171, 1890–1906. [Google Scholar] [CrossRef] [PubMed]
- Geisler, S.; Holmström, K.M.; Skujat, D.; Fiesel, F.C.; Rothfuss, O.C.; Kahle, P.J.; Springer, W. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat. Cell Biol. 2010, 12, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Di Cesare, M.; Perel, P.; Taylor, S.; Kabudula, C.; Bixby, H.; Gaziano, T.A.; McGhie, D.V.; Mwangi, J.; Pervan, B.; Narula, J. The heart of the world. Glob. Heart 2024, 19, 11. [Google Scholar] [CrossRef]
- Zhang, C.-Y.; Li, K.-L.; Zhao, X.-X.; Zhang, Z.-Y.; Yin, A.-W.; Wang, R.-X. The Role and Underlying Mechanisms of Exercise in Heart Failure. Rev. Cardiovasc. Med. 2024, 25, 285. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Y.; Zhang, J.; Jia, D. Exercise Alleviates Cardiovascular Diseases by Improving Mitochondrial Homeostasis. J. Am. Heart Assoc. 2024, 13, e036555. [Google Scholar] [CrossRef]
- Lozano, O.; Marcos, P.; Salazar-Ramirez, F.d.J.; Lázaro-Alfaro, A.F.; Sobrevia, L.; García-Rivas, G. Targeting the mitochondrial Ca2+ uniporter complex in cardiovascular disease. Acta Physiol. 2023, 237, e13946. [Google Scholar] [CrossRef]
- Ikeda, Y.; Sciarretta, S.; Nagarajan, N.; Rubattu, S.; Volpe, M.; Frati, G.; Sadoshima, J. New insights into the role of mitochondrial dynamics and autophagy during oxidative stress and aging in the heart. Oxidative Med. Cell. Longev. 2014, 2014, 210934. [Google Scholar] [CrossRef]
- Mendoza, A.; Patel, P.; Robichaux, D.; Ramirez, D.; Karch, J. Inhibition of the mPTP and lipid peroxidation is additively protective against I/R injury. Circ. Res. 2024, 134, 1292–1305. [Google Scholar] [CrossRef]
- Yang, J.; Guo, Q.; Feng, X.; Liu, Y.; Zhou, Y. Mitochondrial dysfunction in cardiovascular diseases: Potential targets for treatment. Front. Cell Dev. Biol. 2022, 10, 841523. [Google Scholar] [CrossRef]
- Rocca, C.; Soda, T.; De Francesco, E.M.; Fiorillo, M.; Moccia, F.; Viglietto, G.; Angelone, T.; Amodio, N. Mitochondrial dysfunction at the crossroad of cardiovascular diseases and cancer. J. Transl. Med. 2023, 21, 635. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.; Li, H.; Liao, P.; Chen, L.; Pan, Y.; Zheng, Y.; Zhang, C.; Liu, D.; Zheng, M.; Gao, J. Mitochondrial dysfunction: Mechanisms and advances in therapy. Signal Transduct. Target. Ther. 2024, 9, 124. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Diaz, J.; Izquierdo, D.; Torres-Martos, Á.; Baig, A.T.; Aguilera, C.M.; Ruiz-Ojeda, F.J. Impact of physical activity and exercise on the epigenome in skeletal muscle and effects on systemic metabolism. Biomedicines 2022, 10, 126. [Google Scholar] [CrossRef] [PubMed]
- MacInnis, M.J.; Zacharewicz, E.; Martin, B.J.; Haikalis, M.E.; Skelly, L.E.; Tarnopolsky, M.A.; Murphy, R.M.; Gibala, M.J. Superior mitochondrial adaptations in human skeletal muscle after interval compared to continuous single-leg cycling matched for total work. J. Physiol. 2017, 595, 2955–2968. [Google Scholar] [CrossRef]
- Meinild Lundby, A.K.; Jacobs, R.; Gehrig, S.; De Leur, J.; Hauser, M.; Bonne, T.; Flück, D.; Dandanell, S.; Kirk, N.; Kaech, A. Exercise training increases skeletal muscle mitochondrial volume density by enlargement of existing mitochondria and not de novo biogenesis. Acta Physiol. 2018, 222, e12905. [Google Scholar] [CrossRef]
- Serpiello, F.R.; Mckenna, M.J.; Bishop, D.J.; Aughey, R.J.; Caldow, M.K.; Cameron-Smith, D.; Stepto, N.K. Repeated sprints alter signaling related to mitochondrial biogenesis in humans. Med. Sci. Sports Exerc. 2012, 44, 827–834. [Google Scholar] [CrossRef]
- Campos, J.C.; Marchesi Bozi, L.H.; Krum, B.; Grassmann Bechara, L.R.; Ferreira, N.D.; Arini, G.S.; Albuquerque, R.P.; Traa, A.; Ogawa, T.; van der Bliek, A.M. Exercise preserves physical fitness during aging through AMPK and mitochondrial dynamics. Proc. Natl. Acad. Sci. USA 2023, 120, e2204750120. [Google Scholar] [CrossRef]
- O’Neill, H.M.; Maarbjerg, S.J.; Crane, J.D.; Jeppesen, J.; Jørgensen, S.B.; Schertzer, J.D.; Shyroka, O.; Kiens, B.; Van Denderen, B.J.; Tarnopolsky, M.A. AMP-activated protein kinase (AMPK) β1β2 muscle null mice reveal an essential role for AMPK in maintaining mitochondrial content and glucose uptake during exercise. Proc. Natl. Acad. Sci. USA 2011, 108, 16092–16097. [Google Scholar] [CrossRef]
- Lantier, L.; Fentz, J.; Mounier, R.; Leclerc, J.; Treebak, J.T.; Pehmøller, C.; Sanz, N.; Sakakibara, I.; Saint-Amand, E.; Rimbaud, S. AMPK controls exercise endurance, mitochondrial oxidative capacity, and skeletal muscle integrity. FASEB J. 2014, 28, 3211–3224. [Google Scholar] [CrossRef]
- Ju, J.-s.; Jeon, S.-i.; Park, J.-y.; Lee, J.-y.; Lee, S.-c.; Cho, K.-j.; Jeong, J.-m. Autophagy plays a role in skeletal muscle mitochondrial biogenesis in an endurance exercise-trained condition. J. Physiol. Sci. 2016, 66, 417–430. [Google Scholar] [CrossRef]
- Lin, J.; Zhang, X.; Sun, Y.; Xu, H.; Li, N.; Wang, Y.; Tian, X.; Zhao, C.; Wang, B.; Zhu, B. Exercise ameliorates muscular excessive mitochondrial fission, insulin resistance and inflammation in diabetic rats via irisin/AMPK activation. Sci. Rep. 2024, 14, 10658. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Diaz-Canestro, C.; Wang, Y.; Tse, M.A.; Xu, A. Exerkines and cardiometabolic benefits of exercise: From bench to clinic. EMBO Mol. Med. 2024, 16, 432–444. [Google Scholar] [CrossRef] [PubMed]
- Jia, D.; Hou, L.; Lv, Y.; Xi, L.; Tian, Z. Postinfarction exercise training alleviates cardiac dysfunction and adverse remodeling via mitochondrial biogenesis and SIRT1/PGC-1α/PI3K/Akt signaling. J. Cell. Physiol. 2019, 234, 23705–23718. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Sun, Y.; Tan, Y.; Zhang, Z.; Hou, Z.; Gao, C.; Feng, P.; Zhang, X.; Yi, W.; Gao, F. Short-duration swimming exercise after myocardial infarction attenuates cardiac dysfunction and regulates mitochondrial quality control in aged mice. Oxidative Med. Cell. Longev. 2018, 2018, 4079041. [Google Scholar] [CrossRef]
- Mendham, A.E.; Goedecke, J.H.; Zeng, Y.; Larsen, S.; George, C.; Hauksson, J.; Fortuin-de Smidt, M.C.; Chibalin, A.V.; Olsson, T.; Chorell, E. Exercise training improves mitochondrial respiration and is associated with an altered intramuscular phospholipid signature in women with obesity. Diabetologia 2021, 64, 1642–1659. [Google Scholar] [CrossRef]
- Burtscher, J.; Soltany, A.; Visavadiya, N.P.; Burtscher, M.; Millet, G.P.; Khoramipour, K.; Khamoui, A.V. Mitochondrial stress and mitokines in aging. Aging Cell 2023, 22, e13770. [Google Scholar] [CrossRef]
- Li, H.; Liu, G.; Wang, B.; Momeni, M.R. Exosomes and microRNAs as mediators of the exercise. Eur. J. Med. Res. 2025, 30, 38. [Google Scholar] [CrossRef]
- Gaál, Z.; Fodor, J.; Oláh, T.; Szabó, I.G.; Balatoni, I.; Csernoch, L. Implication of microRNAs as messengers of exercise adaptation in junior female triathlonists. Sci. Rep. 2024, 14, 22858. [Google Scholar] [CrossRef]
- Song, R.; Hu, X.-Q.; Zhang, L. Mitochondrial MiRNA in cardiovascular function and disease. Cells 2019, 8, 1475. [Google Scholar] [CrossRef]
- Carrer, M.; Liu, N.; Grueter, C.E.; Williams, A.H.; Frisard, M.I.; Hulver, M.W.; Bassel-Duby, R.; Olson, E.N. Control of mitochondrial metabolism and systemic energy homeostasis by microRNAs 378 and 378. Proc. Natl. Acad. Sci. USA 2012, 109, 15330–15335. [Google Scholar] [CrossRef]
- Yamamoto, H.; Morino, K.; Nishio, Y.; Ugi, S.; Yoshizaki, T.; Kashiwagi, A.; Maegawa, H. MicroRNA-494 regulates mitochondrial biogenesis in skeletal muscle through mitochondrial transcription factor A and Forkhead box j3. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E1419–E1427. [Google Scholar] [CrossRef] [PubMed]
- Nahálková, J. Focus on molecular functions of anti-aging deacetylase SIRT3. Biochemistry 2022, 87, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Geng, L.; Zhang, T.; Liu, W.; Chen, Y. miR-494-3p modulates the progression of in vitro and in vivo Parkinson’s disease models by targeting SIRT3. Neurosci. Lett. 2018, 675, 23–30. [Google Scholar] [PubMed]
- He, T.; Sha, J.; Hu, Y.; Shao, C.; Zhou, Y.; Chen, L.; Yao, J.; Gao, J. Single-Cell Sequencing Identifies the Crucial Role of Mitochondrial Fission-Fusion Balance in Cardiac Hypertrophy Progression. bioRxiv 2025. [Google Scholar] [CrossRef]
- Pribil Pardun, S.; Bhat, A.; Anderson, C.P.; Allen, M.F.; Bruening, W.; Jacob, J.; Pendyala, V.V.; Yu, L.; Bruett, T.; Zimmerman, M.C. Electrical Pulse Stimulation Protects C2C12 Myotubes against Hydrogen Peroxide-Induced Cytotoxicity via Nrf2/Antioxidant Pathway. Antioxidants 2024, 13, 716. [Google Scholar]
- Hernandez, G.; Thornton, C.; Stotland, A.; Lui, D.; Sin, J.; Ramil, J.; Magee, N.; Andres, A.; Quarato, G.; Carreira, R.S. MitoTimer: A novel tool for monitoring mitochondrial turnover. Autophagy 2013, 9, 1852–1861. [Google Scholar]
- Laker, R.C.; Xu, P.; Ryall, K.A.; Sujkowski, A.; Kenwood, B.M.; Chain, K.H.; Zhang, M.; Royal, M.A.; Hoehn, K.L.; Driscoll, M. A novel MitoTimer reporter gene for mitochondrial content, structure, stress, and damage in vivo. J. Biol. Chem. 2014, 289, 12005–12015. [Google Scholar]
- Supervia, M.; Turk-Adawi, K.; Lopez-Jimenez, F.; Pesah, E.; Ding, R.; Britto, R.R.; Bjarnason-Wehrens, B.; Derman, W.; Abreu, A.; Babu, A.S. Nature of cardiac rehabilitation around the globe. eClinicalMedicine 2019, 13, 46–56. [Google Scholar] [CrossRef]
- Maruf, F.A.; Mohammed, J. Unmet Needs for Cardiac Rehabilitation in Africa: A Perennial Gap in the Management of Individuals with Cardiac Diseases. High Blood Press. Cardiovasc. Prev. 2023, 30, 199–206. [Google Scholar] [CrossRef]
- Dalal, J.J.; Almahmeed, W.; Krittayaphong, R.; Nicholls, S.J.; Soomro, K.; Yeo, K. Consensus recommendations of the Asia Pacific Cardiometabolic Consortium on Secondary Prevention Strategies in Myocardial Infarction: Recommendations on pharmacotherapy, lifestyle modification and cardiac rehabilitation. J. Asian Pac. Soc. Cardiol. 2023, 2, e01. [Google Scholar] [CrossRef]
| Author, Year, Country | Resistance Training | Aerobic Training | High-Intensity Interval Training |
|---|---|---|---|
| Jegier, 2021, Poland [2]. |
|
|
|
| Bigot, 2024, France [10]. |
|
Session structure: Each session should include:
|
|
| Brown, US, 2024 [11]. |
|
Intensity:
|
|
| Abreu, 2018, Portugal [12]. |
|
|
|
| Piepoli, 2016, Europe [13]. |
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dziedzic, A.; Marek, K.; Niebrzydowski, P.; Szalewska, D.; Nowak, P.; Miller, E. Mitochondrial Bioenergetics and Cardiac Rehabilitation: Bridging Basic Science and Clinical Practice. J. Clin. Med. 2025, 14, 3949. https://doi.org/10.3390/jcm14113949
Dziedzic A, Marek K, Niebrzydowski P, Szalewska D, Nowak P, Miller E. Mitochondrial Bioenergetics and Cardiac Rehabilitation: Bridging Basic Science and Clinical Practice. Journal of Clinical Medicine. 2025; 14(11):3949. https://doi.org/10.3390/jcm14113949
Chicago/Turabian StyleDziedzic, Angela, Klaudia Marek, Piotr Niebrzydowski, Dominika Szalewska, Patrycja Nowak, and Elżbieta Miller. 2025. "Mitochondrial Bioenergetics and Cardiac Rehabilitation: Bridging Basic Science and Clinical Practice" Journal of Clinical Medicine 14, no. 11: 3949. https://doi.org/10.3390/jcm14113949
APA StyleDziedzic, A., Marek, K., Niebrzydowski, P., Szalewska, D., Nowak, P., & Miller, E. (2025). Mitochondrial Bioenergetics and Cardiac Rehabilitation: Bridging Basic Science and Clinical Practice. Journal of Clinical Medicine, 14(11), 3949. https://doi.org/10.3390/jcm14113949








