Now and the Future: Medications Changing the Landscape of Cardiovascular Disease and Heart Failure Management
Abstract
1. Introduction
2. Current Evidence for GDMT Across the Spectrum of Heart Failure
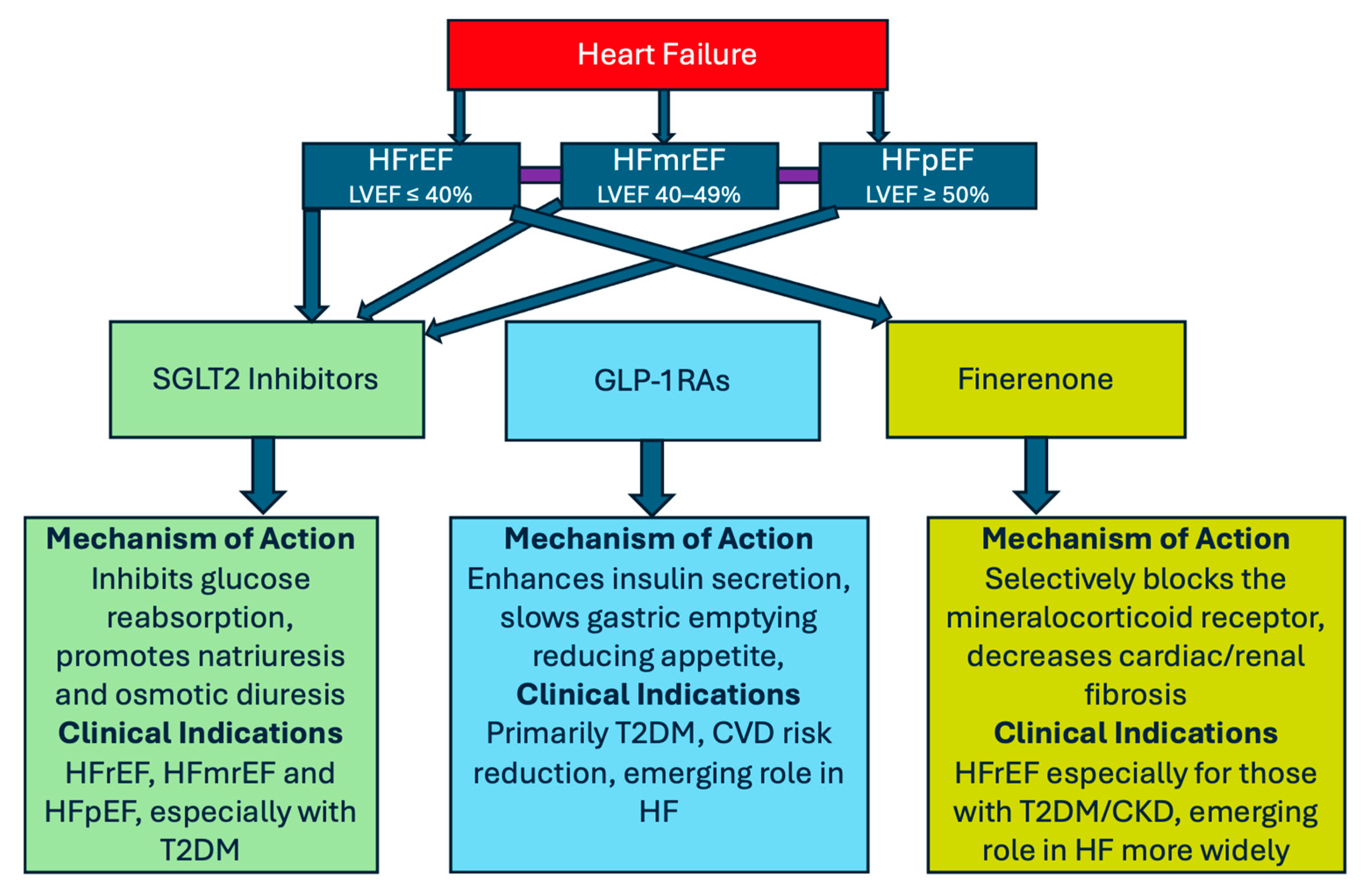
3. Sodium–Glucose Cotransporter-2 (SGLT2) Inhibitors
4. Finerenone—A New Treatment Option for HFpEF and CVD Patients
5. Glucagon-like Peptide One Receptor Agonists (GLP-1RAs)
| Trial | Drug and Class | Primary Condition | Study Population | Key CV Outcomes |
|---|---|---|---|---|
| LEADER (2016) [40] | GLP-1RA—Liraglutide | T2DM patients at high cardiovascular risk with HBA1C ≥ 7.0% | 9340 patients|LVEF: N/A | Liraglutide reduced the risk of CV outcomes. Significant reduction in death from CV and death from all causes compared to placebo. |
| FIGHT (2016) [39] | GLP-1RA—Liraglutide | HFrEF post-hospitalization | 300 patients with LVEF ≤ 40%|LVEF: ≤40% | No significant difference in CV death or HF rehospitalization; trend toward harm in the liraglutide group. |
| LIVE (2017) [38] | GLP-1RA—Liraglutide | Chronic HF (HFrEF and HFpEF) | 241 patients, LVEF < 45%|LVEF: ≤45% | No significant change in LVEF between groups; increased serious adverse cardiac adverse events observed. |
| REWIND (2019) [41] | GLP-1RA—Dulaglutide | T2DM patients at high cardiovascular risk with high HBA1C | 9901 patients|LVEF: N/A | Dulaglutide reduced the risk of CV outcomes compared to placebo, with significant difference in non-fatal stroke outcome. |
| SELECT (2023) [33] | GLP-1RA—Semaglutide | Obesity without diabetes | 17,604 adults with BMI ≥ 27 and CVD|LVEF: N/A | Semaglutide reduced MACEs by 20%, including significant reductions in CV death and non-fatal MI. |
| STEP-HFpEF (2023) [36] | GLP-1RA—Semaglutide | HFpEF with obesity | 529 patients with LVEF ≥ 45%|LVEF: ≥45% | Semaglutide improved KCCQ scores and reduced body weight by 13.3% vs. 2.6% (placebo). |
| OASIS 1 (2023) [46] | GLP-1RA—Semaglutide (Oral) | Obesity | 667 adults with overweight/obesity|LVEF: N/A | Semaglutide 50 mg resulted in 15.1% weight loss vs. 2.4% (placebo). |
| STEP-HFpEF DM (2024) [37] | GLP-1RA—Semaglutide | HFpEF + diabetes | 616 patients with LVEF ≥ 45%|LVEF: ≥45% | Semaglutide improved KCCQ and reduced body weight by 9.8% vs. 3.4% (placebo). |
| SOUL (2025) [45] | GLP-1RA—Semaglutide (Oral) | T2DM with atherosclerotic (AS) CVD, CKD or both | 9650 patients with T2DM|LVEF: N/A | Semaglutide reduced MACEs by 14% in T2DM with ASCVD/CKD vs. placebo. |
| EMPEROR-Preserved (2021) [15] | SGLT2 inhibitor—Empagliflozin | HFpEF | 5988 patients with LVEF > 40%|LVEF: >40% | Empagliflozin reduced risk of the composite of CV death or hospitalization by 21%; no significant reduction in CV death or death from other causes. |
| PRESERVED-HF (2021) [16] | SGLT2 inhibitor—Dapagliflozin | HFpEF | 324 patients with LVEF ≥4 5%|LVEF: ≥55% | Dapagliflozin improved KCCQ scores (symptoms, physical limitations) vs. placebo at 12 weeks. |
| FINE-HEART (2024) [24] | Non-steroidal MRA—Finerenone | HF + CKD + T2DM | 18,991 patients pooled from FIDELIO-DKD + FIGARO-DKD + FINEARTS HF|LVEF: Mixed (mostly preserved) | Finerenone reduced all-cause death, HF hospitalization, MACEs and renal decline in T2DM + CKD. |
6. Implications for General Medicine When Tackling HF Patients
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CVD | cardiovascular disease |
| CKD | chronic kidney disease |
| T2DM | Type 2 Diabetes Mellitus |
| SGLT2i | sodium–glucose cotransporter receptor 2 inhibitor |
| GLP-1RA | glucagon-like peptide 1 receptor agonist |
| MRA | mineralocorticoid receptor antagonist |
| HF | heart failure |
| MACE | major adverse cardiovascular event |
| CVOT | cardiovascular outcome trial |
| NICE | National Institute for Health and Care Excellence |
| eGFR | estimated glomerular filtration rate |
References
- Cardiovascular Diseases Kill 10000 People in the WHO European Region Every Day, with Men Dying More Frequently than Women. 2024. Available online: https://www.who.int/azerbaijan/news/item/15-05-2024-cardiovascular-diseases-kill-10-000-people-in-the-who-european-region-every-day--with-men-dying-more-frequently-than-women (accessed on 28 April 2025).
- GBD 2021 Diabetes Collaborators. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2023, 402, 203–234. [Google Scholar] [CrossRef] [PubMed]
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.M.; Divers, J.; Isom, S.; Saydah, S.; Imperatore, G.; Pihoker, C.; Marcovina, S.M.; Mayer-Davis, E.J.; Hamman, R.F.; Dolan, L.; et al. Trends in Prevalence of Type 1 and Type 2 Diabetes in Children and Adolescents in the US, 2001-2017. JAMA 2021, 326, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.N.; Zhao, D.; Allison, M.A.; Guallar, E.; Sharma, K.; Criqui, M.H.; Cushman, M.; Blumenthal, R.S.; Michos, E.D. Adiposity and Incident Heart Failure and its Subtypes: MESA (Multi-Ethnic Study of Atherosclerosis). JACC Heart Fail. 2018, 6, 999–1007. [Google Scholar] [CrossRef]
- Mazin, I.; Chernomordik, F.; Fefer, P.; Matetzky, S.; Beigel, R. The Impact of Novel Anti-Diabetic Medications on CV Outcomes: A New Therapeutic Horizon for Diabetic and Non-Diabetic Cardiac Patients. J. Clin. Med. 2022, 11, 1904. [Google Scholar] [CrossRef]
- Chertow, G.M.; Correa-Rotter, R.; Vart, P.; Jongs, N.; McMurray, J.J.V.; Rossing, P.; Langkilde, A.M.; Sjöström, C.D.; Toto, R.D.; Wheeler, D.C.; et al. Effects of Dapagliflozin in Chronic Kidney Disease, with and without Other Cardiovascular Medications: DAPA-CKD Trial. J. Am. Heart Assoc. 2023, 12, e028739. [Google Scholar] [CrossRef]
- Solomon, S.D.; McMurray, J.J.V.; Vaduganathan, M.; Claggett, B.; Jhund, P.S.; Desai, A.S.; Henderson, A.D.; Lam, C.S.P.; Pitt, B.; Senni, M.; et al. Finerenone in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N. Engl. J. Med. 2024, 391, 1475–1485. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2023, 44, 3627–3639. [Google Scholar] [CrossRef]
- Docherty, K.F.; Bayes-Genis, A.; Butler, J.; Coats, A.J.S.; Drazner, M.H.; Joyce, E.; Lam, C.S.P. The four pillars of HFrEF therapy: Is it time to treat heart failure regardless of ejection fraction? Eur. Heart J. Suppl. J. Eur. 2022, 24 (Suppl. L), L10–L19. [Google Scholar] [CrossRef]
- Solomon, S.D.; Vaduganathan, M.L.; Claggett, B.; Packer, M.; Zile, M.; Swedberg, K.; Rouleau, J.A.; Pfeffer, M.; Desai, A.; Lund, L.H.; et al. Sacubitril/Valsartan Across the Spectrum of Ejection Fraction in Heart Failure. Circulation 2020, 141, 352–361. [Google Scholar] [CrossRef]
- Pitt, B.; Pfeffer, M.A.; Assmann, S.F.; Boineau, R.; Anand, I.S.; Claggett, B.; Clausell, N.; Desai, A.S.; Diaz, R.; Fleg, J.L.; et al. Spironolactone for heart failure with preserved ejection fraction. N. Engl. J. Med. 2014, 370, 1383–1392. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, M.A.; Claggett, B.; Assmann, S.F.; Boineau, R.; Anand, I.S.; Clausell, N.; Desai, A.S.; Diaz, R.; Fleg, J.L.; Gordeev, I.; et al. Regional variation in patients and outcomes in the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) trial. Circulation 2015, 131, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Cleland, J.G.F.; Bunting, K.V.; Flather, M.D.; Altman, D.G.; Holmes, J.; Coats, A.J.S.; Manzano, L.; McMurray, J.J.V.; Ruschitzka, F.; van Veldhuisen, D.J.; et al. Beta-blockers for heart failure with reduced, mid-range, and preserved ejection fraction: An individual patient-level analysis of double-blind randomized trials. Eur. Heart J. 2018, 39, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner-La Rocca, H.P.; Choi, D.J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef]
- Nassif, M.E.; Windsor, S.L.; Borlaug, B.A.; Kitzman, D.W.; Shah, S.J.; Tang, F.; Khariton, Y.; Malik, A.O.; Khumri, T.; Umpierrez, G.; et al. The SGLT2 inhibitor dapagliflozin in heart failure with preserved ejection fraction: A multicenter randomized trial. Nat. Med. 2021, 27, 1954–1960. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Stefánsson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.F.; Mann, J.F.E.; McMurray, J.J.V.; Lindberg, M.; Rossing, P.; et al. Dapagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2020, 383, 1436–1446. [Google Scholar] [CrossRef]
- Alfano, G.; Perrone, R.; Fontana, F.; Ligabue, G.; Giovanella, S.; Ferrari, A.; Gregorini, M.; Cappelli, G.; Magistroni, R.; Donati, G. Rethinking Chronic Kidney Disease in the Aging Population. Life 2022, 12, 1724. [Google Scholar] [CrossRef]
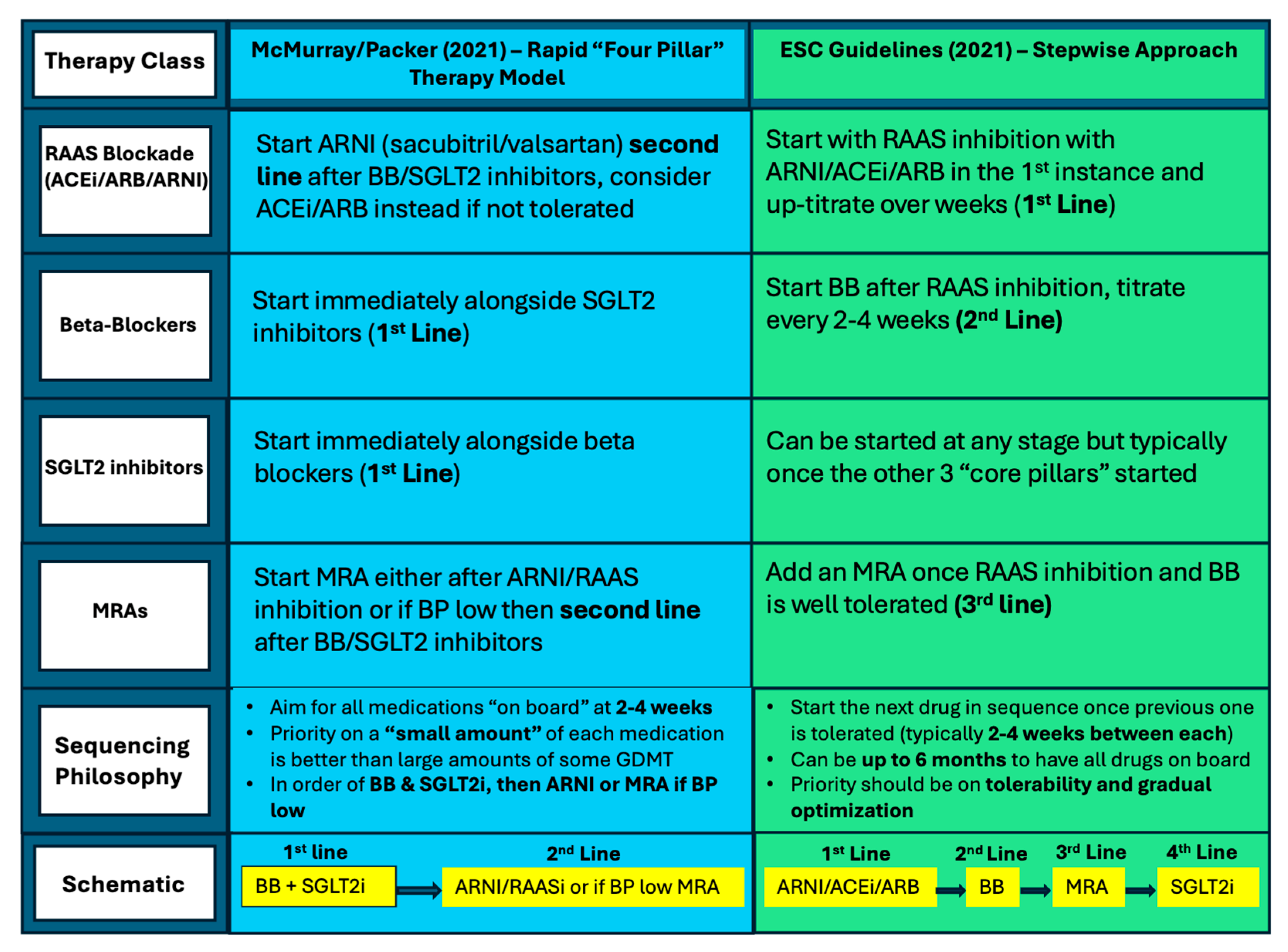
- McMurray, J.J.V.; Packer, M. How Should We Sequence the Treatments for Heart Failure and a Reduced Ejection Fraction?: A Redefinition of Evidence-Based Medicine. Circulation 2021, 143, 875–877. [Google Scholar] [CrossRef]
- D’Amario, D.; Rodolico, D.; Delvinioti, A.; Laborante, R.; Iacomini, C.; Masciocchi, C.; Restivo, A.; Ciliberti, G.; Galli, M.; Paglianiti, A.D.; et al. Eligibility for the 4 Pharmacological Pillars in Heart Failure with Reduced Ejection Fraction at Discharge. J. Am. Heart Assoc. 2023, 12, e029071. [Google Scholar] [CrossRef]
- Armillotta, M.; Angeli, F.; Paolisso, P.; Belmonte, M.; Raschi, E.; Di Dalmazi, G.; Amicone, S.; Canton, L.; Fedele, D.; Suma, N.; et al. Cardiovascular therapeutic targets of sodium-glucose co-transporter 2 (SGLT2) inhibitors beyond heart failure. Pharmacol. Ther. 2025, 270, 108861. [Google Scholar] [CrossRef]
- Mariani, M.V.; Lavalle, C.; Palombi, M.; Pierucci, N.; Trivigno, S.; D’Amato, A.; Filomena, D.; Cipollone, P.; Laviola, D.; Piro, A.; et al. SGLT2i reduce arrhythmic events in heart failure patients with cardiac implantable electronic devices. ESC Heart Fail. 2025, 12, 2125–2133. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.S.; Jhund, P.S.; Claggett, B.L.; Vaduganathan, M.; Miao, Z.M.; Kondo, T.; Barkoudah, E.; Brahimi, A.; Connolly, E.; Finn, P.; et al. Effect of Dapagliflozin on Cause-Specific Mortality in Patients with Heart Failure Across the Spectrum of Ejection Fraction: A Participant-Level Pooled Analysis of DAPA-HF and DELIVER. JAMA Cardiol. 2022, 7, 1227–1234. [Google Scholar] [CrossRef] [PubMed]
- Vaduganathan, M.; Filippatos, G.; Claggett, B.L.; Desai, A.S.; Jhund, P.S.; Henderson, A.; Brinker, M.; Kolkhof, P.; Schloemer, P.; Lay-Flurrie, J.; et al. Finerenone in heart failure and chronic kidney disease with type 2 diabetes: FINE-HEART pooled analysis of cardiovascular, kidney and mortality outcomes. Nat. Med. 2024, 30, 3758–3764. [Google Scholar] [CrossRef] [PubMed]
- Di Lullo, L.; Lavalle, C.; Scatena, A.; Mariani, M.V.; Ronco, C.; Bellasi, A. Finerenone: Questions and Answers-The Four Fundamental Arguments on the New-Born Promising Non-Steroidal Mineralocorticoid Receptor Antagonist. J. Clin. Med. 2023, 12, 3992. [Google Scholar] [CrossRef]
- National Institute for Health, (NICE) CE. Finerenone for Treating Heart Failure with Preserved or Mildly Reduced Ejection Fraction [ID6514] (November 2024). Available online: https://www.nice.org.uk/guidance/awaiting-development/gid-ta11651 (accessed on 28 April 2025).
- Sabina, M.; Trube, J.; Shah, S.; Lurie, A.; Grimm, M.; Bizanti, A. Finerenone: A Third-Generation MRA and Its Impact on Cardiovascular Health-Insights from Randomized Controlled Trials. J. Clin. Med. 2024, 13, 6398. [Google Scholar] [CrossRef]
- Randomized Trial to Determine the Efficacy and Safety of Finerenone on Morbidity and Mortality Among Heart Failure Patients with Left Ventricular Ejection Fraction Greater than or Equal to 40% Hospitalized Due to an Episode of Acute Decompensated Heart Failure (REDEFINE-HF); ClinicalTrials.gov Identifier: NCT06008197. EudraCT:2023-508581-15-00. Available online: https://clinicaltrials.gov/ct2/show/NCT06008197 (accessed on 4 May 2025).
- A Study to Evaluate Finerenone on Clinical Efficacy and Safety in Patients with Heart Failure Who Are Intolerant or Not Eligible for Treatment with Steroidal Mineralocorticoid Receptor Antagonists (FINALITY-HF); ClinicalTri-als.gov Identifier: NCT06033950. Available online: https://clinicaltrials.gov/ct2/show/NCT06033950 (accessed on 4 May 2025).
- A Study to Determine the Efficacy and Safety of Finerenone and SGLT2i in Combination in Hospitalized Patients with Heart Failure (CONFIRMATION-HF) (CONFIRMATION); ClinicalTrials.gov Identifier: NCT06024746. Available online: https://clinicaltrials.gov/ct2/show/NCT06024746 (accessed on 4 May 2025).
- National Institute for Health, (NICE) CE. Semaglutide for Managing Overweight and Obesity (TA875). 2023. Available online: https://www.nice.org.uk/guidance/TA875/chapter/1-Recommendations (accessed on 28 April 2025).
- Ferhatbegović, L.; Mršić, D.; Macić-Džanković, A. The benefits of GLP1 receptors in cardiovascular diseases. Front. Clin. Diabetes Healthc. 2023, 4, 1293926. [Google Scholar] [CrossRef]
- Lincoff, A.M.; Brown-Frandsen, K.; Colhoun, H.M.; Deanfield, J.; Emerson, S.S.; Esbjerg, S.; Hardt-Lindberg, S.; Hovingh, G.K.; Kahn, S.E.; Kushner, R.F.; et al. Semaglutide and Cardiovascular Outcomes in Obesity without Diabetes. N. Engl. J. Med. 2023, 389, 2221–2232. [Google Scholar] [CrossRef]
- Ryan, D.H.; Lingvay, I.; Deanfield, J.; Kahn, S.E.; Barros, E.; Burguera, B.; Colhoun, H.M.; Cercato, C.; Dicker, D.; Horn, D.B.; et al. Long-term weight loss effects of semaglutide in obesity without diabetes in the SELECT trial. Nat. Med. 2024, 30, 2049–2057. [Google Scholar] [CrossRef]
- U.S Food and Drug Administration. FDA Approves First Treatment to Reduce Risk of Serious Heart Problems Specifically in Adults with Obesity or Overweight. 2024. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-reduce-risk-serious-heart-problems-specifically-adults-obesity-or (accessed on 28 April 2025).
- Kosiborod, M.N.; Abildstrøm, S.Z.; Borlaug, B.A.; Butler, J.; Rasmussen, S.; Davies, M.; Hovingh, G.K.; Kitzman, D.W.; Lindegaard, M.L.; Møller, D.V.; et al. Semaglutide in Patients with Heart Failure with Preserved Ejection Fraction and Obesity. N. Engl. J. Med. 2023, 389, 1069–1084. [Google Scholar] [CrossRef]
- Kosiborod, M.N.; Petrie, M.C.; Borlaug, B.A.; Butler, J.; Davies, M.J.; Hovingh, G.K.; Kitzman, D.W.; Møller, D.V.; Treppendahl, M.B.; Verma, S.; et al. Semaglutide in Patients with Obesity-Related Heart Failure and Type 2 Diabetes. N. Engl. J. Med. 2024, 390, 1394–1407. [Google Scholar] [CrossRef]
- Jorsal, A.; Kistorp, C.; Holmager, P.; Tougaard, R.S.; Nielsen, R.; Hänselmann, A.; Nilsson, B.; Møller, J.E.; Hjort, J.; Rasmussen, J.; et al. Effect of liraglutide, a glucagon-like peptide-1 analogue, on left ventricular function in stable chronic heart failure patients with and without diabetes (LIVE)-a multicentre, double-blind, randomised, placebo-controlled trial. Eur. J. Heart Fail. 2017, 19, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Neves, J.S.; Vasques-Nóvoa, F.; Borges-Canha, M.; Leite, A.R.; Sharma, A.; Carvalho, D.; Packer, M.; Zannad, F.; Leite-Moreira, A.; Ferreira, J.P. Risk of adverse events with liraglutide in heart failure with reduced ejection fraction: A post hoc analysis of the FIGHT trial. Diabetes Obes. Metab. 2023, 25, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Marso, S.P.; Daniels, G.H.; Brown-Frandsen, K.; Kristensen, P.; Mann, J.F.; Nauck, M.A.; Nissen, S.E.; Pocock, S.; Poulter, N.R.; Ravn, L.S.; et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Gerstein, H.C.; Colhoun, H.M.; Dagenais, G.R.; Diaz, R.; Lakshmanan, M.; Pais, P.; Probstfield, J.; Riesmeyer, J.S.; Riddle, M.C.; Rydén, L.; et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): A double-blind, randomised placebo-controlled trial. Lancet 2019, 394, 121–130. [Google Scholar] [CrossRef]
- Wilding, J.P.H.; Batterham, R.L.; Calanna, S.; Davies, M.; Van Gaal, L.F.; Lingvay, I.; McGowan, B.M.; Rosenstock, J.; Tran, M.T.D.; Wadden, T.A.; et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N. Engl. J. Med. 2021, 384, 989–1002. [Google Scholar] [CrossRef]
- Wilding, J.P.H.; Batterham, R.L.; Davies, M.; Van Gaal, L.F.; Kandler, K.; Konakli, K.; Lingvay, I.; McGowan, B.M.; Oral, T.K.; Rosenstock, J.; et al. Weight regain and cardiometabolic effects after withdrawal of semaglutide: The STEP 1 trial extension. Diabetes Obes. Metab. 2022, 24, 1553–1564. [Google Scholar] [CrossRef]
- McGuire, D.K.; Busui, R.P.; Deanfield, J.; Inzucchi, S.E.; Mann, J.F.E.; Marx, N.; Mulvagh, S.L.; Poulter, N.; Engelmann, M.D.M.; Hovingh, G.K.; et al. Effects of oral semaglutide on cardiovascular outcomes in individuals with type 2 diabetes and established atherosclerotic cardiovascular disease and/or chronic kidney disease: Design and baseline characteristics of SOUL, a randomized trial. Diabetes Obes. Metab. 2023, 25, 1932–1941. [Google Scholar] [CrossRef]
- McGuire, D.K.; Marx, N.; Mulvagh, S.L.; Deanfield, J.E.; Inzucchi, S.E.; Pop-Busui, R.; Mann, J.F.E.; Emerson, S.S.; Poulter, N.R.; Engelmann, M.D.M.; et al. Oral Semaglutide and Cardiovascular Outcomes in High-Risk Type 2 Diabetes. N. Engl. J. Med. 2025, 392, 2001–2012. [Google Scholar] [CrossRef]
- Knop, F.K.; Aroda, V.R.; do Vale, R.D.; Holst-Hansen, T.; Laursen, P.N.; Rosenstock, J.; Rubino, D.M.; Garvey, W.T.; OASIS 1 Investigators. Oral semaglutide 50 mg taken once per day in adults with overweight or obesity (OASIS 1): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2023, 402, 705–719. [Google Scholar] [CrossRef]
- Rahman, I.; Barwell, J. Genomic medicine for the 21st century. Ann. R. Coll. Surg. Engl. 2024, 106, 295–299. [Google Scholar] [CrossRef]
- Ibrahim, A.R.N.; Orayj, K.M. Impact of ADA Guidelines and Medication Shortage on GLP-1 Receptor Agonists Prescribing Trends in the UK: A Time-Series Analysis with Country-Specific Insights. J. Clin. Med. 2024, 13, 6256. [Google Scholar] [CrossRef] [PubMed]
- Iacobucci, G. UK clinics told to stop prescribing antidiabetes drugs for weight loss, after shortages. BMJ 2023, 382, 1693. [Google Scholar] [CrossRef] [PubMed]
- Botana López, M.; Camafort Babkowski, M.; Campuzano Ruiz, R.; Cebrián Cuenca, A.; Gargallo Fernández, M.; David de Paz, H.; Redondo-Antón, J.; Artime, E.; Díaz-Cerezo, S.; Rubio de Santos, M. Barriers and Strategies to Optimize the Use of Glucagon-Like Peptide 1 Receptor Agonists in People with Type 2 Diabetes and High Cardiovascular Risk or Established Cardiovascular Disease: A Delphi Consensus in Spain. Adv. Ther. 2024, 41, 3569–3584. [Google Scholar] [CrossRef] [PubMed]
- Arillotta, D.; Floresta, G.; Guirguis, A.; Corkery, J.M.; Catalani, V.; Martinotti, G.; Sensi, S.L.; Schifano, F. GLP-1 Receptor Agonists and Related Mental Health Issues; Insights from a Range of Social Media Platforms Using a Mixed-Methods Approach. Brain Sci. 2023, 13, 1503. [Google Scholar] [CrossRef]
- Unlu, O.; Levitan, E.B.; Reshetnyak, E.; Kneifati-Hayek, J.; Diaz, I.; Archambault, A.; Chen, L.; Hanlon, J.T.; Maurer, M.S.; Safford, M.M.; et al. Polypharmacy in Older Adults Hospitalized for Heart Failure. Circulation Heart Fail. 2020, 13, e006977. [Google Scholar] [CrossRef]
- Onyebeke, C.; Zhang, D.; Musse, M.; Unlu, O.; Nahid, M.; Ambrosy, A.P.; Levitan, E.B.; Safford, M.M.; Goyal, P. Polypharmacy and Guideline-Directed Medical Therapy Initiation Among Adults Hospitalized with Heart Failure. JACC Adv. 2024, 3, 101126. [Google Scholar] [CrossRef]
- Stolfo, D.; Iacoviello, M.; Chioncel, O.; Anker, M.S.; Bayes-Genis, A.; Braunschweig, F.; Cannata, A.; El Hadidi, S.; Filippatos, G.; Jhund, P.; et al. How to handle polypharmacy in heart failure. A clinical consensus statement of the Heart Failure Association of the ESC. Eur. J. Heart Fail. 2025, 27, 747–759. [Google Scholar] [CrossRef]
- Banerjee, D.; Ali, M.A.; Wang, A.Y.; Jha, V. Acute kidney injury in acute heart failure-when to worry and when not to worry? Nephrol. Dial. Transplant. 2024, 40, 10–18. [Google Scholar] [CrossRef]
- National Institute for Health, (NICE) CE. Heart Failure—Chronic: SGLT2 Inhibitors. NICE Guidelines. 2025. Available online: https://cks.nice.org.uk/topics/heart-failure-chronic/prescribing-information/sglt-2-inhibitors/ (accessed on 28 April 2025).
- Murphy, D.; Banerjee, D. Hyperkalaemia in Heart Failure: Consequences for Outcome and Sequencing of Therapy. Curr. Heart Fail. Rep. 2022, 19, 191–199. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oswald, T.; Coombs, S.; Ellery, S.; Liu, A. Now and the Future: Medications Changing the Landscape of Cardiovascular Disease and Heart Failure Management. J. Clin. Med. 2025, 14, 3948. https://doi.org/10.3390/jcm14113948
Oswald T, Coombs S, Ellery S, Liu A. Now and the Future: Medications Changing the Landscape of Cardiovascular Disease and Heart Failure Management. Journal of Clinical Medicine. 2025; 14(11):3948. https://doi.org/10.3390/jcm14113948
Chicago/Turabian StyleOswald, Thomas, Steven Coombs, Susan Ellery, and Alexander Liu. 2025. "Now and the Future: Medications Changing the Landscape of Cardiovascular Disease and Heart Failure Management" Journal of Clinical Medicine 14, no. 11: 3948. https://doi.org/10.3390/jcm14113948
APA StyleOswald, T., Coombs, S., Ellery, S., & Liu, A. (2025). Now and the Future: Medications Changing the Landscape of Cardiovascular Disease and Heart Failure Management. Journal of Clinical Medicine, 14(11), 3948. https://doi.org/10.3390/jcm14113948