Tailoring Targeted Temperature Management in Comatose Out-of-Hospital Cardiac Arrest Survivors: A Retrospective Analysis Based on the rCAST Score Classification
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Data Collection
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ichim, C.; Pavel, V.; Mester, P.; Schmid, S.; Todor, S.B.; Stoia, O.; Anderco, P.; Kandulski, A.; Müller, M.; Heumann, P. Assessing Key Factors Influencing Successful Resuscitation Outcomes in Out-of-Hospital Cardiac Arrest (OHCA). J. Clin. Med. 2024, 13, 7399. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-M.; Kim, Y.-J.; Kim, Y.-J.; Kim, W.-Y. Long-term disability level of 1-month survivors after out-of-hospital cardiac arrest: A nationwide cohort study in Korea using data from 2009 to 2018. Signa Vitae 2023, 19, 43. [Google Scholar] [CrossRef]
- Bernard, S.A.; Gray, T.W.; Buist, M.D.; Jones, B.M.; Silvester, W.; Gutteridge, G.; Smith, K. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N. Engl. J. Med. 2002, 346, 557–563. [Google Scholar] [CrossRef]
- Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N. Engl. J. Med. 2002, 346, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Callaway, C.W. Targeted temperature management with hypothermia for comatose patients after cardiac arrest. Clin. Exp. Emerg. Med. 2023, 10, 5. [Google Scholar] [CrossRef]
- Kim, Y.-M.; Jeung, K.W.; Kim, W.Y.; Park, Y.S.; Oh, J.S.; You, Y.H.; Lee, D.H.; Chae, M.K.; Jeong, Y.J.; Kim, M.C. 2020 Korean guidelines for cardiopulmonary resuscitation. Part 5. Post-cardiac arrest care. Clin. Exp. Emerg. Med. 2021, 8, S41. [Google Scholar] [CrossRef]
- Sanfilippo, F.; La Via, L.; Lanzafame, B.; Dezio, V.; Busalacchi, D.; Messina, A.; Ristagno, G.; Pelosi, P.; Astuto, M. Targeted temperature management after cardiac arrest: A systematic review and meta-analysis with trial sequential analysis. J. Clin. Med. 2021, 10, 3943. [Google Scholar] [CrossRef] [PubMed]
- Kikutani, K.; Nishikimi, M.; Shimatani, T.; Kyo, M.; Ohshimo, S.; Shime, N. Differential effectiveness of hypothermic targeted temperature management according to the severity of post-cardiac arrest syndrome. J. Clin. Med. 2021, 10, 5643. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Kim, W.Y. Cautious application of targeted temperature management in a real-world OHCA population after “TTM2 trial”. Signa Vitae 2021, 17, 143–144. [Google Scholar] [CrossRef]
- Nishikimi, M.; Ogura, T.; Nishida, K.; Takahashi, K.; Nakamura, M.; Matsui, S.; Matsuda, N.; Iwami, T. External validation of a risk classification at the emergency department of post-cardiac arrest syndrome patients undergoing targeted temperature management. Resuscitation 2019, 140, 135–141. [Google Scholar] [CrossRef]
- Kim, B.; Kwon, H.; Kim, S.-M.; Kim, J.-S.; Ryoo, S.M.; Kim, Y.-J.; Kim, W.Y. Ion Shift Index at the Immediate Post-Cardiac Arrest Period as an Early Prognostic Marker in Out-of-Hospital Cardiac Arrest Survivors. J. Clin. Med. 2022, 11, 6187. [Google Scholar] [CrossRef] [PubMed]
- Nolan, J.P.; Sandroni, C.; Böttiger, B.W.; Cariou, A.; Cronberg, T.; Friberg, H.; Genbrugge, C.; Haywood, K.; Lilja, G.; Moulaert, V.R. European resuscitation council and European society of intensive care medicine guidelines 2021: Post-resuscitation care. Resuscitation 2021, 161, 220–269. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Wu, C.; Gu, S.; Lu, Y.; Wu, H.; Li, C. WHO declares the end of the COVID-19 global health emergency: Lessons and recommendations from the perspective of ChatGPT/GPT-4. Int. J. Surg. 2023, 109, 2859–2862. [Google Scholar] [CrossRef] [PubMed]
- Legriel, S.; Lemiale, V.; Schenck, M.; Chelly, J.; Laurent, V.; Daviaud, F.; Srairi, M.; Hamdi, A.; Geri, G.; Rossignol, T. Hypothermia for neuroprotection in convulsive status epilepticus. N. Engl. J. Med. 2016, 375, 2457–2467. [Google Scholar] [CrossRef]
- Bennet, L.; Dean, J.M.; Wassink, G.; Gunn, A.J. Differential effects of hypothermia on early and late epileptiform events after severe hypoxia in preterm fetal sheep. J. Neurophysiol. 2007, 97, 572–578. [Google Scholar] [CrossRef]
- Guluma, K.Z.; Oh, H.; Yu, S.-W.; Meyer, B.C.; Rapp, K.; Lyden, P.D. Effect of endovascular hypothermia on acute ischemic edema: Morphometric analysis of the ICTuS trial. Neurocrit. Care 2008, 8, 42–47. [Google Scholar] [CrossRef]
- Andrews, P.J.; Sinclair, H.L.; Rodriguez, A.; Harris, B.A.; Battison, C.G.; Rhodes, J.K.; Murray, G.D. Hypothermia for intracranial hypertension after traumatic brain injury. N. Engl. J. Med. 2015, 373, 2403–2412. [Google Scholar] [CrossRef]
- Bacher, A.; Illievich, U.M.; Fitzgerald, R.; Ihra, G.; Spiss, C.K. Changes in oxygenation variables during progressive hypothermia in anesthetized patients. J. Neurosurg. Anesthesiol. 1997, 9, 205–210. [Google Scholar] [CrossRef]
- Callaway, C.W.; Coppler, P.J.; Faro, J.; Puyana, J.S.; Solanki, P.; Dezfulian, C.; Doshi, A.A.; Elmer, J.; Frisch, A.; Guyette, F.X. Association of initial illness severity and outcomes after cardiac arrest with targeted temperature management at 36 °C or 33 °C. JAMA Netw. Open 2020, 3, e208215. [Google Scholar] [CrossRef]
- Nishikimi, M.; Ogura, T.; Nishida, K.; Takahashi, K.; Fukaya, K.; Liu, K.; Nakamura, M.; Matsui, S.; Matsuda, N. Differential effect of mild therapeutic hypothermia depending on the findings of hypoxic encephalopathy on early CT images in patients with post-cardiac arrest syndrome. Resuscitation 2018, 128, 11–15. [Google Scholar] [CrossRef]
- Nishikimi, M.; Matsuda, N.; Matsui, K.; Takahashi, K.; Ejima, T.; Liu, K.; Ogura, T.; Higashi, M.; Umino, H.; Makishi, G. CAST: A new score for early prediction of neurological outcomes after cardiac arrest before therapeutic hypothermia with high accuracy. Intensive Care Med. 2016, 42, 2106–2107. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nishikimi, M.; Matsuda, N.; Matsui, K.; Takahashi, K.; Ejima, T.; Liu, K.; Ogura, T.; Higashi, M.; Umino, H.; Makishi, G. A novel scoring system for predicting the neurologic prognosis prior to the initiation of induced hypothermia in cases of post-cardiac arrest syndrome: The CAST score. Scand. J. Trauma. Resusc. Emerg. Med. 2017, 25, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, Y.; Nishikimi, M.; Matsui, K.; Numaguchi, A.; Nishida, K.; Emoto, R.; Matsui, S.; Matsuda, N. The rCAST score is useful for estimating the neurological prognosis in pediatric patients with post-cardiac arrest syndrome before ICU admission: External validation study using a nationwide prospective registry. Resuscitation 2021, 168, 103–109. [Google Scholar] [CrossRef]
- Chen, C.-H.; Wang, C.-J.; Wang, I.-T.; Yang, S.-H.; Wang, Y.-H.; Lin, C.-Y. Does One Size Fit All? External Validation of the rCAST Score to Predict the Hospital Outcomes of Post-Cardiac Arrest Patients Receiving Targeted Temperature Management. J. Clin. Med. 2022, 12, 242. [Google Scholar] [CrossRef]
- Misumi, K.; Hagiwara, Y.; Kimura, T.; Hifumi, T.; Inoue, A.; Sakamoto, T.; Kuroda, Y.; Ogura, T. External validation of the CAST and rCAST score in patients with out-of-hospital cardiac arrest who underwent ECPR: A secondary analysis of the SAVE-J II study. J. Am. Heart Assoc. 2023, 13, e031035. [Google Scholar] [CrossRef]
- Kim, N.; Kitlen, E.; Garcia, G.; Khosla, A.; Miller, P.E.; Johnson, J.; Wira, C.; Greer, D.M.; Gilmore, E.J.; Beekman, R. Validation of the rCAST score and comparison to the PCAC and FOUR scores for prognostication after out-of-hospital cardiac arrest. Resuscitation 2023, 188, 109832. [Google Scholar] [CrossRef]
- Nishikimi, M.; Ogura, T.; Nishida, K.; Hayashida, K.; Emoto, R.; Matsui, S.; Matsuda, N.; Iwami, T. Outcome related to level of targeted temperature management in postcardiac arrest syndrome of low, moderate, and high severities: A nationwide multicenter prospective registry. Crit. Care Med. 2021, 49, e741–e750. [Google Scholar] [CrossRef]
- Delhaye, C.; Mahmoudi, M.; Waksman, R. Hypothermia therapy: Neurological and cardiac benefits. J. Am. Coll. Cardiol. 2012, 59, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, J.H.; Kjaergaard, J.; Graff, C.; Pehrson, S.; Erlinge, D.; Wanscher, M.; Køber, L.; Bro-Jeppesen, J.; Søholm, H.; Winther-Jensen, M. Ventricular ectopic burden in comatose survivors of out-of-hospital cardiac arrest treated with targeted temperature management at 33 °C and 36 °C. Resuscitation 2016, 102, 98–104. [Google Scholar] [CrossRef]
- Kim, M.-J.; Kim, Y.-J.; Yum, M.-S.; Kim, W.Y. Alpha-power in electroencephalography as good outcome predictor for out-of-hospital cardiac arrest survivors. Sci. Rep. 2022, 12, 10907. [Google Scholar] [CrossRef]
- Metter, R.B.; Rittenberger, J.C.; Guyette, F.X.; Callaway, C.W. Association between a quantitative CT scan measure of brain edema and outcome after cardiac arrest. Resuscitation 2011, 82, 1180–1185. [Google Scholar] [CrossRef]
- Kim, S.H.; Choi, S.P.; Park, K.N.; Youn, C.S.; Oh, S.H.; Choi, S.M. Early brain computed tomography findings are associated with outcome in patients treated with therapeutic hypothermia after out-of-hospital cardiac arrest. Scand. J. Trauma. Resusc. Emerg. Med. 2013, 21, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.-W.; Yi, H.; Bae, H.-J.; Kim, Y.-J.; Sohn, C.-H.; Ahn, S.; Lim, K.-S.; Kim, N.; Kim, W.-Y. Prediction of Neurologically Intact Survival in Cardiac Arrest Patients without Pre-Hospital Return of Spontaneous Circulation: Machine Learning Approach. J. Clin. Med. 2021, 10, 1089. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Kim, M.-J.; Kim, Y.H.; Youn, C.S.; Cho, I.S.; Kim, S.J.; Wee, J.H.; Park, Y.S.; Oh, J.S.; Lee, D.H. Background frequency can enhance the prognostication power of EEG patterns categories in comatose cardiac arrest survivors: A prospective, multicenter, observational cohort study. Crit. Care 2021, 25, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Deye, N.; Cariou, A.; Girardie, P.; Pichon, N.; Megarbane, B.; Midez, P.; Tonnelier, J.-M.; Boulain, T.; Outin, H.; Delahaye, A. Endovascular versus external targeted temperature management for patients with out-of-hospital cardiac arrest: A randomized, controlled study. Circulation 2015, 132, 182–193. [Google Scholar] [CrossRef]
- Look, X.; Li, H.; Ng, M.; Lim, E.T.S.; Pothiawala, S.; Tan, K.B.K.; Sewa, D.W.; Shahidah, N.; Pek, P.P.; Ong, M.E.H. Randomized controlled trial of internal and external targeted temperature management methods in post-cardiac arrest patients. Am. J. Emerg. Med. 2018, 36, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Kirkegaard, H.; Søreide, E.; De Haas, I.; Pettilä, V.; Taccone, F.S.; Arus, U.; Storm, C.; Hassager, C.; Nielsen, J.F.; Sørensen, C.A. Targeted temperature management for 48 vs 24 hours and neurologic outcome after out-of-hospital cardiac arrest: A randomized clinical trial. JAMA 2017, 318, 341–350. [Google Scholar] [CrossRef]
- Callaway, C.W.; Donnino, M.W.; Fink, E.L.; Geocadin, R.G.; Golan, E.; Kern, K.B.; Leary, M.; Meurer, W.J.; Peberdy, M.A.; Thompson, T.M. Part 8: Post–cardiac arrest care: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2015, 132, S465–S482. [Google Scholar] [CrossRef]
- Nolan, J.P.; Soar, J.; Cariou, A.; Cronberg, T.; Moulaert, V.R.; Deakin, C.D.; Bottiger, B.W.; Friberg, H.; Sunde, K.; Sandroni, C. European resuscitation council and European society of intensive care medicine 2015 guidelines for post-resuscitation care. Intensive Care Med. 2015, 41, 2039–2056. [Google Scholar] [CrossRef]
- Kim, Y.-M.; Park, K.N.; Choi, S.P.; Lee, B.K.; Park, K.; Kim, J.; Kim, J.H.; Chung, S.P.; Hwang, S.O. Part 4. Post-cardiac arrest care: 2015 Korean guidelines for cardiopulmonary resuscitation. Clin. Exp. Emerg. Med. 2016, 3, S27. [Google Scholar] [CrossRef]
- Merchant, R.M.; Topjian, A.A.; Panchal, A.R.; Cheng, A.; Aziz, K.; Berg, K.M.; Lavonas, E.J.; Magid, D.J. on behalf of Adult Basic and Advanced Life Support, Pediatric Basic and Advanced Life Support, Neonatal Life Support, Resuscitation Education Science, and Systems of Care Writing. Part 1: Executive summary: 2020 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2020, 142, S337–S357. [Google Scholar] [CrossRef] [PubMed]
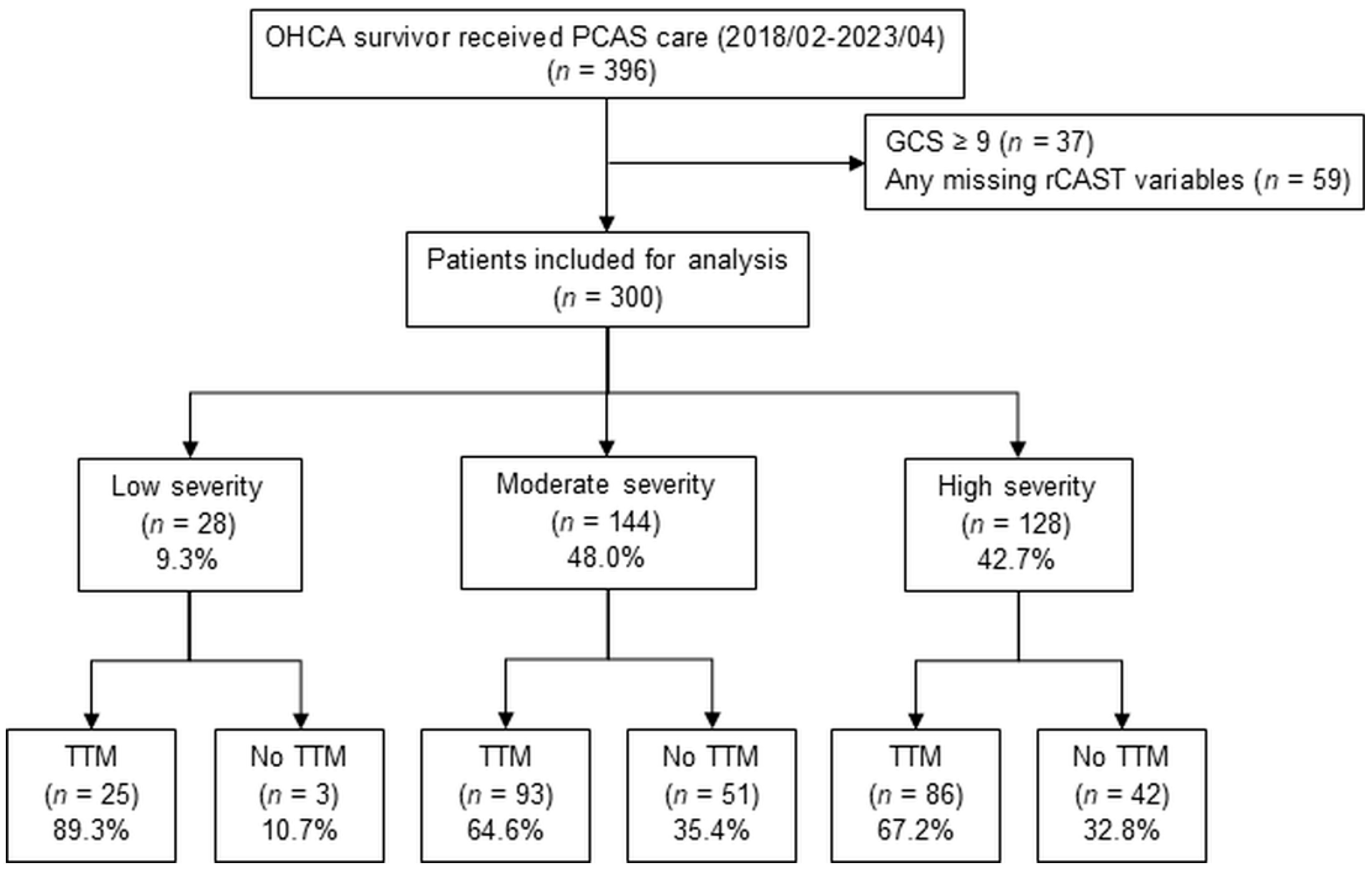
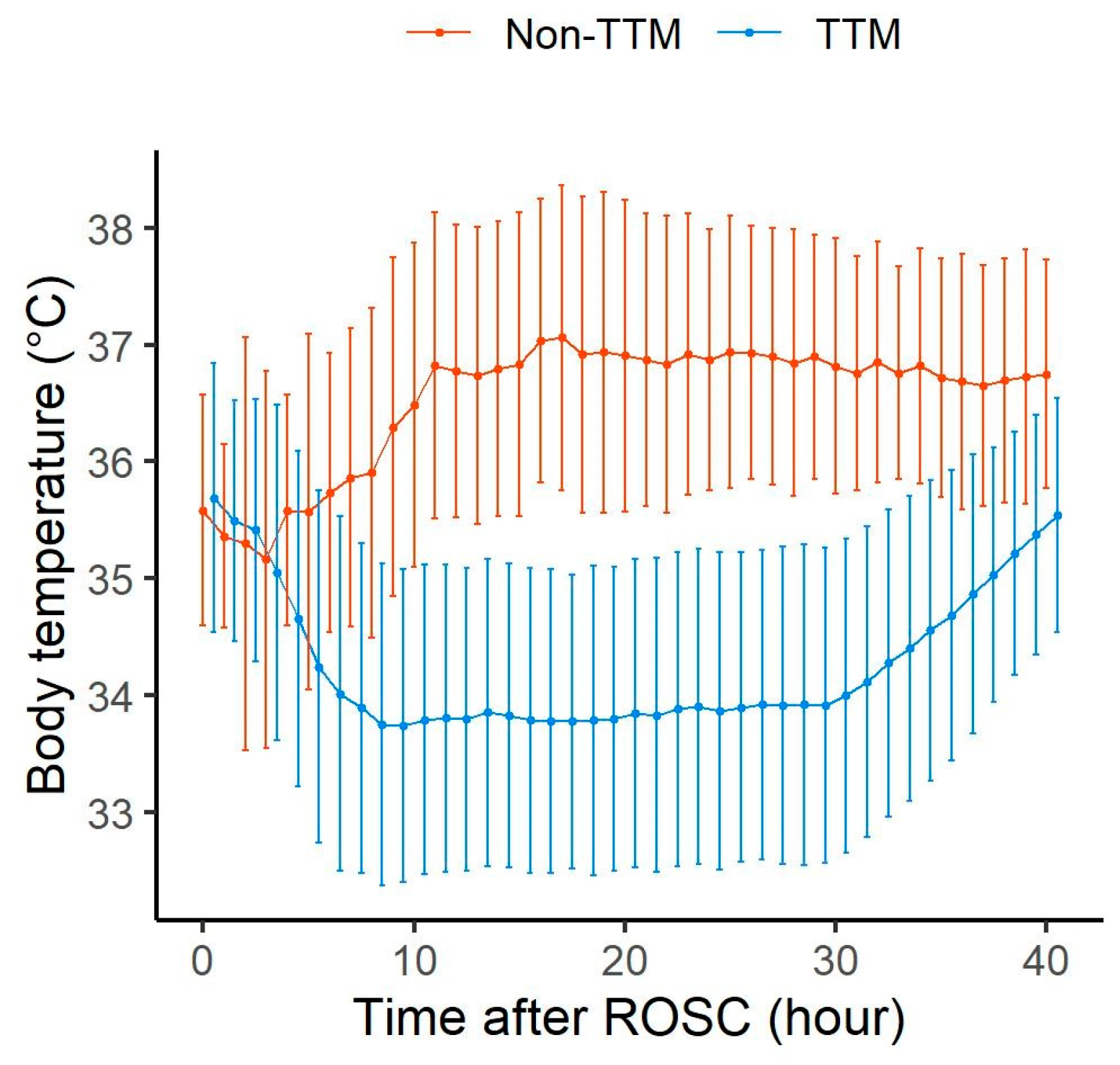
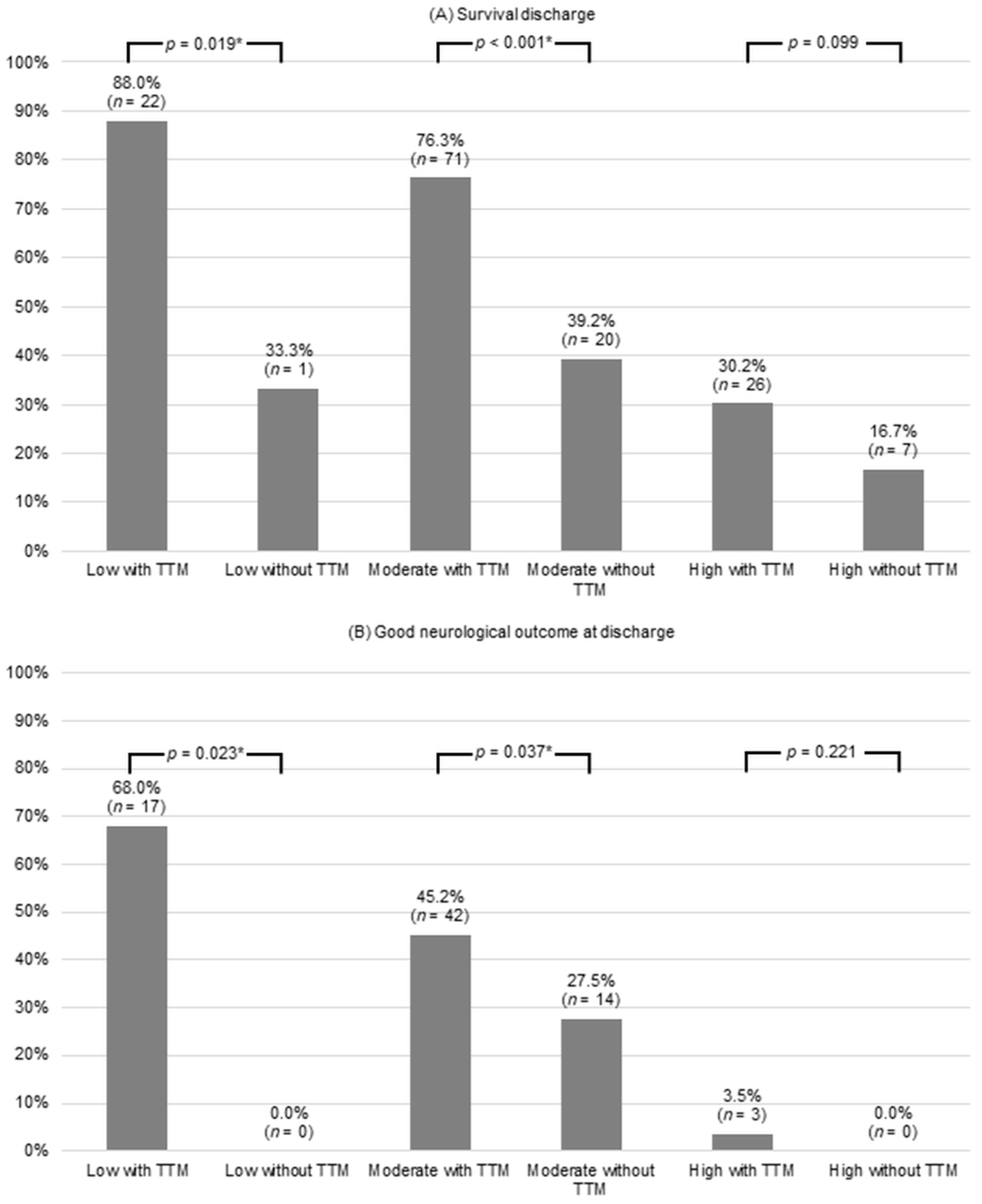
| Characteristics | Total (n = 300) | Low (n = 28) | Moderate (n = 144) | High (n = 128) | p-Value |
|---|---|---|---|---|---|
| Age, years | 65.5 (51.5–74.00) | 64.5 (56.0–72.5) | 66.0 (52.0–74.0) | 65.5 (50.0–75.0) | 0.829 |
| Male | 209 (69.7) | 21 (75.0) | 96 (66.7) | 92 (71.9) | 0.526 |
| Arrest etiology, trauma | 26 (8.7) | 0 (0.0) | 9 (6.3) | 17 (13.3) | 0.028 * |
| Witnessed | 225 (75.0) | 24 (85.7) | 117 (81.3) | 84 (65.6) | 0.005 * |
| Bystander CPR | 209 (69.7) | 20 (71.4) | 101 (70.1) | 88 (68.8) | 0.948 |
| Shockable rhythm | 100 (33.3) | 20 (71.4) | 57 (39.6) | 23 (18.0) | <0.001 * |
| Duration of resuscitation, min | 26.5 (13.0–39.0) | 12.0 (6.5–16.5) | 17.5 (10.0–33.0) | 35.5 (27.5–45.5) | <0.001 * |
| Past medical history | |||||
| Hypertension | 101 (33.7) | 8 (28.6) | 54 (37.5) | 39 (31.0) | 0.432 |
| Diabetes mellitus | 86 (28.7) | 7 (25.0) | 41 (28.5) | 38 (30.2) | 0.853 |
| Heart disease | 82 (27.3) | 13 (46.4) | 43 (29.9) | 26 (20.6) | 0.015 * |
| Stroke | 17 (5.7) | 0 (0.0) | 12 (8.3) | 5 (4.0) | 0.119 |
| Serum pH | 7.014 (6.847–7.162) | 7.291 (7.211–7.371) | 7.109 (7.022–7.216) | 6.847 (6.732–6.938) | <0.001 * |
| Serum lactate | 10.2 (7.4–14.1) | 6.8 (3.5–9.5) | 8.6 (6.6–12.0) | 13.3 (10.1–15.0) | <0.001 * |
| PLR | 133 (44.3) | 23 (82.1) | 85 (59.0) | 25 (19.5) | <0.001 * |
| Motor score of GCS | 1 (1–1) | 4 (3–4) | 1 (1–1) | 1 | <0.001 * |
| TTM | 204 (68.0) | 25 (89.3) | 93 (64.6) | 86 (67.2) | 0.036 * |
| Outcome | |||||
| Survival to discharge | 147 (49.0) | 23 (82.1) | 91 (63.2) | 33 (25.8) | <0.001 * |
| CPC 1, 2 at discharge | 76 (25.3) | 17 (60.7) | 56 (38.9) | 3 (2.3) | <0.001 * |
| Characteristics | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | aOR | 95% CI | p-Value | |
| Age, years | 0.98 | 0.97–1.00 | 0.021 * | 0.97 | 0.95–0.99 | 0.010 * |
| Male | 1.19 | 0.67–2.12 | 0.553 | |||
| Arrest etiology, trauma | 0.51 | 0.17–1.53 | 0.222 | |||
| Witnessed | 1.86 | 0.95–3.61 | 0.066 | |||
| Bystander CPR | 1.09 | 0.62–1.93 | 0.761 | |||
| Duration of resuscitation, min | 0.92 | 0.90–0.95 | <0.001 * | 0.95 | 0.92–0.98 | <0.001 * |
| Past medical history | ||||||
| Hypertension | 1.02 | 0.59–1.77 | 0.946 | |||
| Diabetes mellitus | 0.64 | 0.35–1.18 | 0.148 | |||
| Heart disease | 1.20 | 0.68–2.13 | 0.535 | |||
| Stroke | 0.89 | 0.28–2.83 | 0.848 | |||
| PLR | 15.44 | 7.50–31.90 | <0.001 * | 6.74 | 2.96–15.36 | <0.001 * |
| rCAST | ||||||
| Low | 64.39 | 16.31–254.29 | <0.001 * | 13.86 | 3.82–50.25 | <0.001 * |
| Moderate | 26.52 | 8.04–87.43 | <0.001 * | 13.73 | 2.99–63.08 | 0.001 * |
| High | Reference | <0.001 * | Reference | <0.001 * | ||
| TTM | 2.56 | 1.35–4.85 | 0.003 * | 3.51 | 1.51–8.15 | 0.003 * |
| Dataset | Variable | Good Neurological Outcomes at Discharge | ||
|---|---|---|---|---|
| OR | 95% CI | p-Value | ||
| Crude | Non-TTM | Reference | ||
| TTM | 2.18 | 1.04–4.55 | 0.039 * | |
| IPTW | Non-TTM | Reference | ||
| TTM | 2.31 | 1.09–4.91 | 0.029 * | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, H.; Park, H.; Kim, D.; Kim, S.-M.; Kim, J.-S.; Kim, Y.-J.; Kim, W.Y. Tailoring Targeted Temperature Management in Comatose Out-of-Hospital Cardiac Arrest Survivors: A Retrospective Analysis Based on the rCAST Score Classification. J. Clin. Med. 2025, 14, 3931. https://doi.org/10.3390/jcm14113931
Kwon H, Park H, Kim D, Kim S-M, Kim J-S, Kim Y-J, Kim WY. Tailoring Targeted Temperature Management in Comatose Out-of-Hospital Cardiac Arrest Survivors: A Retrospective Analysis Based on the rCAST Score Classification. Journal of Clinical Medicine. 2025; 14(11):3931. https://doi.org/10.3390/jcm14113931
Chicago/Turabian StyleKwon, Hyojeong, Hanna Park, Dongju Kim, Sang-Min Kim, June-Sung Kim, Youn-Jung Kim, and Won Young Kim. 2025. "Tailoring Targeted Temperature Management in Comatose Out-of-Hospital Cardiac Arrest Survivors: A Retrospective Analysis Based on the rCAST Score Classification" Journal of Clinical Medicine 14, no. 11: 3931. https://doi.org/10.3390/jcm14113931
APA StyleKwon, H., Park, H., Kim, D., Kim, S.-M., Kim, J.-S., Kim, Y.-J., & Kim, W. Y. (2025). Tailoring Targeted Temperature Management in Comatose Out-of-Hospital Cardiac Arrest Survivors: A Retrospective Analysis Based on the rCAST Score Classification. Journal of Clinical Medicine, 14(11), 3931. https://doi.org/10.3390/jcm14113931






