Cutoff Values for Screening Post-Intensive Care Syndrome Using the Post-Intensive Care Syndrome Questionnaire
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Setting and Participants
2.3. Measurements
2.3.1. The Post-Intensive Care Syndrome Questionnaire
2.3.2. Criterion Tools for PICS
2.3.3. Tools for Validating Cutoff Values
2.4. Data Collection
2.5. Statistical Analysis
3. Results
3.1. Participant’s Characteristics
3.2. Optimal Cutoff Values for Each Domain of PICSQ
3.3. Comparison of HRQoL and Readmission Rates Between PICS and Non-PICS Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McLarty, J.; Litton, E.; Beane, A.; Aryal, D.; Bailey, M.; Bendel, S.; Burghi, G.; Christensen, S.; Christiansen, C.F.; A Dongelmans, D.; et al. Non-COVID-19 intensive care admissions during the pandemic: A multinational registry-based study. Thorax 2024, 79, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Donovan, J.; Glover, A.; Gregson, J.; Hitchings, A.W.; Wall, E.C.; Heyderman, R.S. A retrospective analysis of 20,178 adult neurological infection admissions to United Kingdom critical care units from 2001 to 2020. BMC Infect. Dis. 2024, 24, 132. [Google Scholar] [CrossRef]
- Kawakami, D.; Fujitani, S.; Morimoto, T.; Dote, H.; Takita, M.; Takaba, A.; Hino, M.; Nakamura, M.; Irie, H.; Adachi, T.; et al. Prevalence of post-intensive care syndrome among Japanese intensive care unit patients: A prospective, multicenter, observational J-PICS study. Crit. Care 2021, 25, 69. [Google Scholar] [CrossRef]
- Kang, J.; Lee, M.H. Incidence rate and risk factors for post-intensive care syndrome subtypes among critical care survivors three months after discharge: A prospective cohort study. Intensive Crit. Care Nurs. 2024, 81, 103605. [Google Scholar] [CrossRef]
- Phillips, E.K.; Monnin, C.; Gregora, A.; Smith, K.; Schultz, A.S.; O’Keefe-McCarthy, S.; Arora, R.C.; Duhamel, T.A.; Chudyk, A.M. A scoping review of incidence and assessment tools for post-intensive care syndrome following cardiac surgery. Intensive Crit. Care Nurs. 2024, 83, 103718. [Google Scholar] [CrossRef]
- Inoue, S.; Nakanishi, N.; Sugiyama, J.; Moriyama, N.; Miyazaki, Y.; Sugimoto, T.; Fujinami, Y.; Ono, Y.; Kotani, J. Prevalence and Long-Term Prognosis of Post-Intensive Care Syndrome after Sepsis: A Single-Center Prospective Observational Study. J. Clin. Med. 2022, 11, 5257. [Google Scholar] [CrossRef]
- Gravante, F.; Trotta, F.; Latina, S.; Simeone, S.; Alvaro, R.; Vellone, E.; Pucciarelli, G. Quality of life in ICU survivors and their relatives with post-intensive care syndrome: A systematic review. Nurs. Crit. Care 2024, 29, 807–823. [Google Scholar] [CrossRef]
- Hiser, S.L.; Fatima, A.; Ali, M.; Needham, D.M. Post-intensive care syndrome (PICS): Recent updates. J. Intensive Care 2023, 11, 23. [Google Scholar] [CrossRef]
- Needham, D.M.; Sepulveda, K.A.; Dinglas, V.D.; Chessare, C.M.; Friedman, L.A.; Bingham, C.O., III; Turnbull, A.E. Core outcome measures for clinical research in acute respiratory failure survivors. An international modified Delphi consensus study. Am. J. Respir. Crit. Care Med. 2017, 196, 1122–1130. [Google Scholar] [CrossRef]
- Spies, C.D.; Krampe, H.; Paul, N.; Denke, C.; Kiselev, J.; Piper, S.K.; Kruppa, J.; Grunow, J.J.; Steinecke, K.; Gülmez, T.; et al. Instruments to measure outcomes of post-intensive care syndrome in outpatient care settings—Results of an expert consensus and feasibility field test. J. Intensive Care Soc. 2021, 22, 159–174. [Google Scholar] [CrossRef]
- Jeong, Y.J.; Kang, J. Development and validation of a questionnaire to measure post-intensive care syndrome. Intensive Crit. Care Nurs. 2019, 55, 102756. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Jeong, Y.J.; Hong, J. Cut-Off Values of the Post-Intensive Care Syndrome Questionnaire for the Screening of Unplanned Hospital Readmission within One Year. J. Korean Acad. Nurs. 2020, 50, 787–798. [Google Scholar] [CrossRef]
- Renner, C.; Jeitziner, M.M.; Albert, M.; Brinkmann, S.; Diserens, K.; Dzialowski, I.; Heidler, M.D.; Lück, M.; Nusser-Müller-Busch, R.; Sandor, P.S.; et al. Guideline on multimodal rehabilitation for patients with post-intensive care syndrome. Crit. Care 2023, 27, 301. [Google Scholar] [CrossRef]
- Clinical Resources—Guidelines. Society of Critical Care Medicine. Available online: https://www.sccm.org/clinical-resources/guidelines (accessed on 2 May 2025).
- Iwashyna, T.J. Trajectories of recovery and dysfunction after acute illness, with implications for clinical trial design. Am. J. Respir. Crit. Care Med. 2012, 186, 302–304. [Google Scholar] [CrossRef]
- Haines, K.J.; Berney, S.; Warrillow, S.; Denehy, L. Long-term recovery following critical illness in an Australian cohort. J. Intensive Care 2018, 6, 8. [Google Scholar] [CrossRef]
- Rai, R.; Singh, R.; Azim, A.; Agarwal, A.; Mishra, P.; Singh, P.K. Impact of Critical Illness on Quality of Life after Intensive Care Unit Discharge. Indian J. Crit. Care Med. 2020, 24, 299–306. [Google Scholar] [CrossRef]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef]
- Foa, E.B.; Cashman, L.; Jaycox, L.; Perry, K. The validation of a self-report measure of posttraumatic stress disorder: The Posttraumatic Diagnostic Scale. Psychol. Assess. 1997, 9, 445. [Google Scholar] [CrossRef]
- Ahn, H.N. An Exploratory Study on The Effects of Psychological Trauma on Posttraumatic Stress Symptoms and Personality Characteristics in Adolescents. Korean J. Counsel. Psychother. 2005, 17, 217–231. [Google Scholar]
- McCarthy, S. Post-traumatic Stress Diagnostic Scale (PDS). Occup. Med. 2008, 58, 379. [Google Scholar] [CrossRef]
- Won, C.W.; Rho, Y.G.; Kim, S.Y.; Cho, B.R.; Lee, Y.S. The validity and reliability of Korean Activities of Daily Living (K-ADL) scale. Hanguk Nonyonhak 2002, 6, 98–106. [Google Scholar]
- Jung, Y.S. Oral health factors affecting the nutritional status of the elderly. J. Korean Soc. Dent. Hyg. 2018, 18, 903–910. [Google Scholar] [CrossRef]
- Zietemann, V.; Kopczak, A.; Muller, C.; Wollenweber, F.A.; Dichgans, M. Validation of the Telephone Interview of Cognitive Status and Telephone Montreal Cognitive Assessment Against Detailed Cognitive Testing and Clinical Diagnosis of Mild Cognitive Impairment After Stroke. Stroke 2017, 48, 2952–2957. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bedirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Ware, J.E.; Sherbourne, C.D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care 1992, 30, 473–483. [Google Scholar] [CrossRef]
- Fischer, J.E.; Bachmann, L.M.; Jaeschke, R. A readers’ guide to the interpretation of diagnostic test properties: Clinical example of sepsis. Intensive Care Med. 2003, 29, 1043–1051. [Google Scholar] [CrossRef]
- Akobeng, A.K. Understanding diagnostic tests 3: Receiver operating characteristic curves. Acta Paediatr. 2007, 96, 644–647. [Google Scholar] [CrossRef]
- Elkins, M. Research Note: Interpreting confidence intervals. J. Physiother. 2024, 70, 319–323. [Google Scholar] [CrossRef]
- Milton, A.; Brück, E.; Schandl, A.; Bottai, M.; Sackey, P. Early psychological screening of intensive care unit survivors: A prospective cohort study. Crit. Care 2017, 21, 273. [Google Scholar] [CrossRef]
- Sheeran, T.; Zimmerman, M. Screening for posttraumatic stress disorder in a general psychiatric outpatient setting. J. Consult. Clin. Psychol. 2002, 70, 961–966. [Google Scholar] [CrossRef]
- O’Caoimh, R.; Timmons, S.; Molloy, D.W. Screening for Mild Cognitive Impairment: Comparison of “MCI Specific” Screening Instruments. J. Alzheimers Dis. 2016, 51, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Swift, A.; Heale, R.; Twycross, A. What are sensitivity and specificity? Evid. Based Nurs. 2020, 23, 2–4. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Jeong, Y.J.; Hong, J. The effect of postintensive care syndrome on the quality of life of intensive care unit survivors: A secondary analysis. Aust. Crit. Care 2021, 34, 246–253. [Google Scholar] [CrossRef]
- Prescott, H.C.; Langa, K.M.; Iwashyna, T.J. Readmission diagnoses after hospitalization for severe sepsis and other acute medical conditions. JAMA 2015, 313, 1055–1057. [Google Scholar] [CrossRef]
- Donaghy, E.; Salisbury, L.; Lone, N.I.; Lee, R.; Ramsey, P.; Rattray, J.E.; Walsh, T.S. Unplanned early hospital readmission among critical care survivors: A mixed methods study of patients and carers. BMJ Qual. Saf. 2018, 27, 915–927. [Google Scholar] [CrossRef]
- Dimopoulos, S.; Leggett, N.E.; Deane, A.M.; Haines, K.J.; Abdelhamid, Y.A. Models of intensive care unit follow-up care and feasibility of intervention delivery: A systematic review. Aust. Crit. Care 2024, 37, 508–516. [Google Scholar] [CrossRef]
- Kang, J.; Lee, K.M. Three-year mortality, readmission, and medical expenses in critical care survivors: A population-based cohort study. Aust. Crit. Care 2024, 37, 251–257. [Google Scholar] [CrossRef]
- Marshall, D.C.; Hatch, R.A.; Gerry, S.; Young, J.D.; Watkinson, P. Conditional survival with increasing duration of ICU admission: An observational study of three intensive care databases. Crit. Care Med. 2020, 48, 91–97. [Google Scholar] [CrossRef]
| Variables | Categories | n (%) | M ± SD |
|---|---|---|---|
| Gender | Male | 282 (59.4) | |
| Female | 193 (40.6) | ||
| Age (years) | <60 | 199 (41.9) | 60.48 ± 13.12 |
| ≥60 | 276 (58.1) | ||
| Awareness of ICU | Yes | 373 (78.5) | |
| admission | No | 102 (21.5) | |
| ICU admission route | Emergency department | 234 (49.3) | |
| Others | 241 (50.7) | ||
| Reasons for ICU | Postoperative monitoring | 146 (30.7) | |
| admission | Neurologic disease | 118 (24.8) | |
| Cardiovascular disease | 99 (20.8) | ||
| Other medical conditions | 77 (16.3) | ||
| Physical injury | 35 (7.4) | ||
| Types of ICU | Surgical | 200 (42.1) | |
| Cardiovascular | 93 (19.6) | ||
| Neurological | 87 (18.3) | ||
| Medical | 46 (9.7) | ||
| Others | 49 (10.3) | ||
| Disease severity | APACHE II | 275 (57.9) | 11.51 ± 6.02 |
| at admission | SAPS III | 151 (31.8) | 33.16 ± 15.36 |
| Not recorded | 49 (10.3) | ||
| ICU length of stay | <4 | 306 (64.4) | 4.63 ± 7.90 |
| (days) | ≥4 | 169 (35.6) | |
| Discharge place | Home | 407 (85.7) | |
| Others | 68 (14.3) |
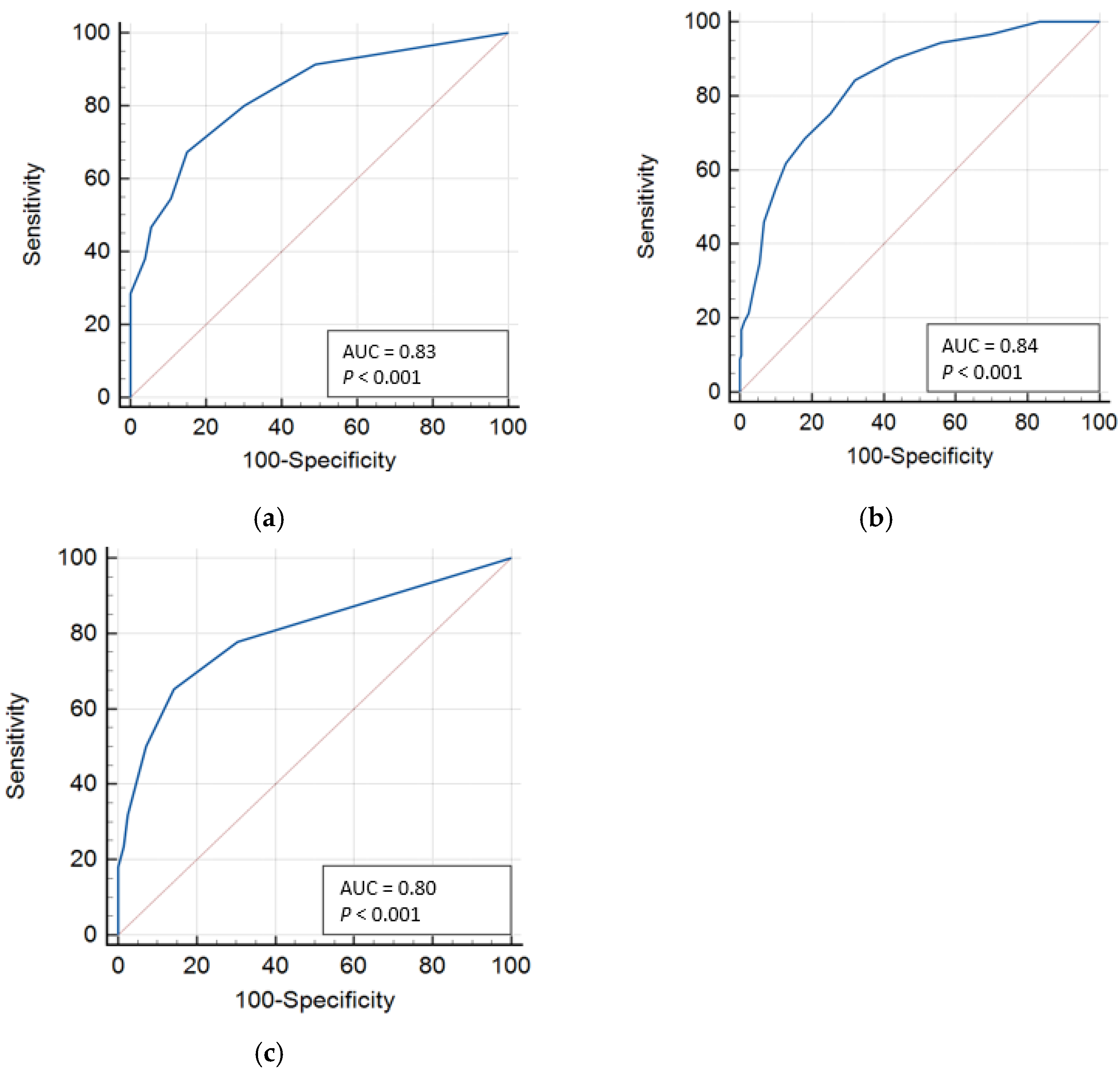
| Variable | AUC | 95% CI | Cutoff Value | Sensitivity (%) | Specificity (%) | Youden Index (J) | LR+ |
|---|---|---|---|---|---|---|---|
| PICS mental | 0.83 | 0.80 to 0.87 | ≥2 | 80.0 | 69.9 | 0.50 | 2.65 |
| ≥3 | 67.3 | 84.9 | 0.52 | 4.47 | |||
| ≥4 | 54.7 | 89.2 | 0.44 | 5.08 | |||
| PICS physical | 0.84 | 0.80 to 0.87 | ≥6 | 75.3 | 74.8 | 0.50 | 2.99 |
| ≥7 | 68.5 | 81.9 | 0.50 | 3.78 | |||
| ≥8 | 61.8 | 87.1 | 0.49 | 4.81 | |||
| PICS cognitive | 0.80 | 0.75 to 0.84 | ≥1 | 77.8 | 69.5 | 0.47 | 2.55 |
| ≥2 | 65.3 | 85.7 | 0.51 | 4.55 | |||
| ≥3 | 50.0 | 92.8 | 0.43 | 6.97 |
| Variables | Categories | PICS Mental | PICS Physical | PICS Cognitive | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| <3 | ≥3 | t or χ2 (p) | <7 | ≥7 | t or χ2 (p) | <2 | ≥2 | t or χ2 (p) | ||
| M ± SD or n (%) | M ± SD or n (%) | M ± SD or n (%) | ||||||||
| Health-related Quality of Life | PCS | 46.24 ± 9.66 | 37.73 ± 9.69 | 8.15 (<0.001) | 47.05 ± 8.90 | 33.64 ± 7.86 | 14.43 (<0.001) | 45.75 ± 9.70 | 36.85 ± 9.83 | 7.78 (<0.001) |
| MCS | 54.71 ± 8.64 | 42.24 ± 11.56 | 10.71 (<0.001) | 53.09 ± 9.56 | 44.67 ± 13.16 | 5.95 (<0.001) | 52.68 ± 9.91 | 45.30 ± 13.16 | 5.03 (<0.001) | |
| Readmission rate | Yes | 52 (18.1) | 37 (30.3) | 7.50 (0.006) | 55 (17.9) | 34 (33.3) | 10.69 (0.001) | 58 (18.4) | 31 (33.0) | 9.02 (0.003) |
| No | 235 (81.9) | 85 (69.7) | 252 (82.1) | 68 (66.7) | 257 (81.6) | 63 (67.0) | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, J.; Kang, J. Cutoff Values for Screening Post-Intensive Care Syndrome Using the Post-Intensive Care Syndrome Questionnaire. J. Clin. Med. 2025, 14, 3897. https://doi.org/10.3390/jcm14113897
Hong J, Kang J. Cutoff Values for Screening Post-Intensive Care Syndrome Using the Post-Intensive Care Syndrome Questionnaire. Journal of Clinical Medicine. 2025; 14(11):3897. https://doi.org/10.3390/jcm14113897
Chicago/Turabian StyleHong, Jiwon, and Jiyeon Kang. 2025. "Cutoff Values for Screening Post-Intensive Care Syndrome Using the Post-Intensive Care Syndrome Questionnaire" Journal of Clinical Medicine 14, no. 11: 3897. https://doi.org/10.3390/jcm14113897
APA StyleHong, J., & Kang, J. (2025). Cutoff Values for Screening Post-Intensive Care Syndrome Using the Post-Intensive Care Syndrome Questionnaire. Journal of Clinical Medicine, 14(11), 3897. https://doi.org/10.3390/jcm14113897






