Sonographic Evaluations of the Pubic Symphysis at Different Stages of Pregnancy
Abstract
1. Introduction
2. Materials and Methods
- Ethical issues
- Sonographic examinations, patients
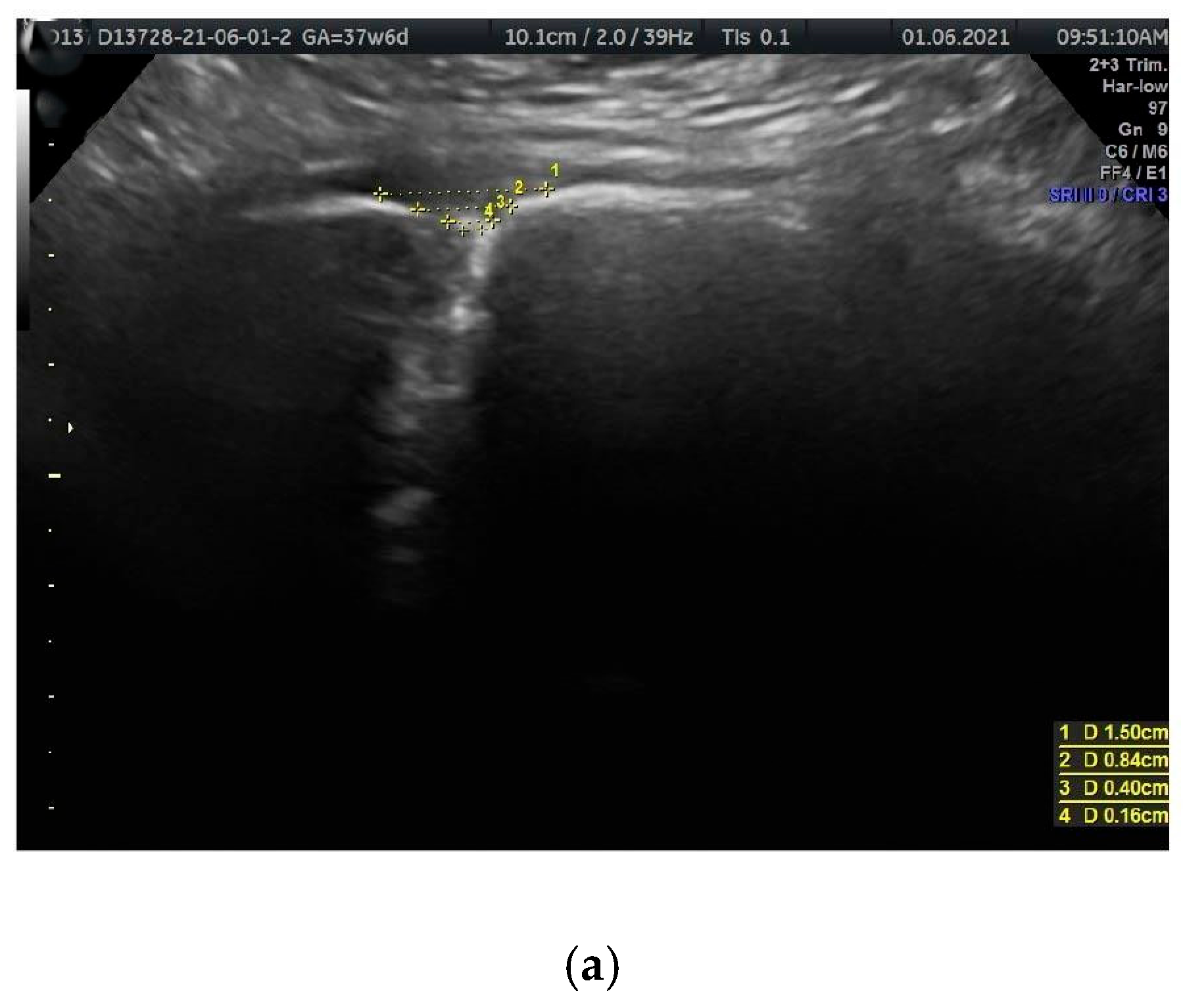
- Ultrasound Visualization
- Measurements (in mm)
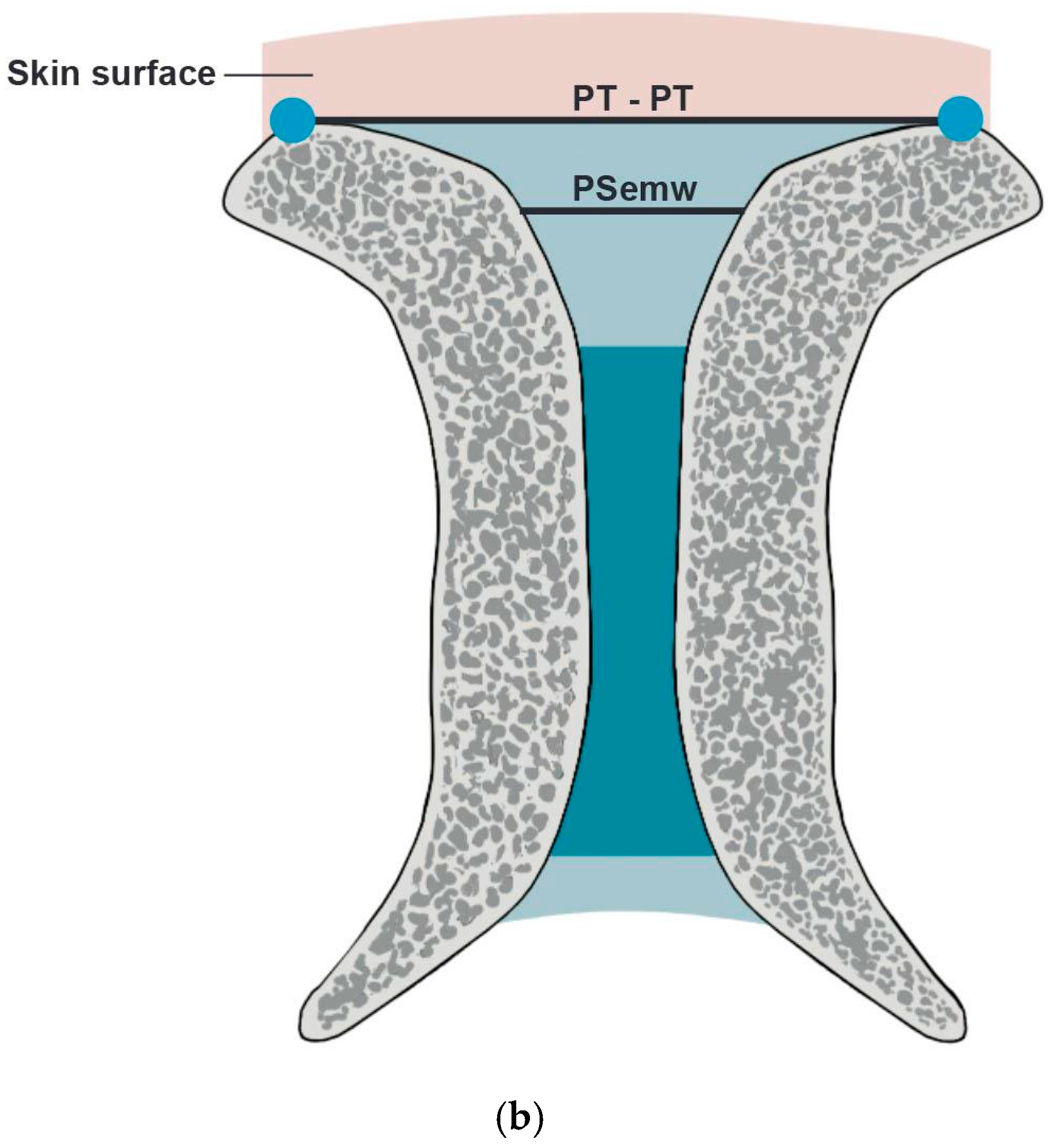
- PS entry middle width (PSemw): The distance measured at the midpoint of the PS entrance.
- Intertubercular distance (PT-PT): The span between the right and left PTs.
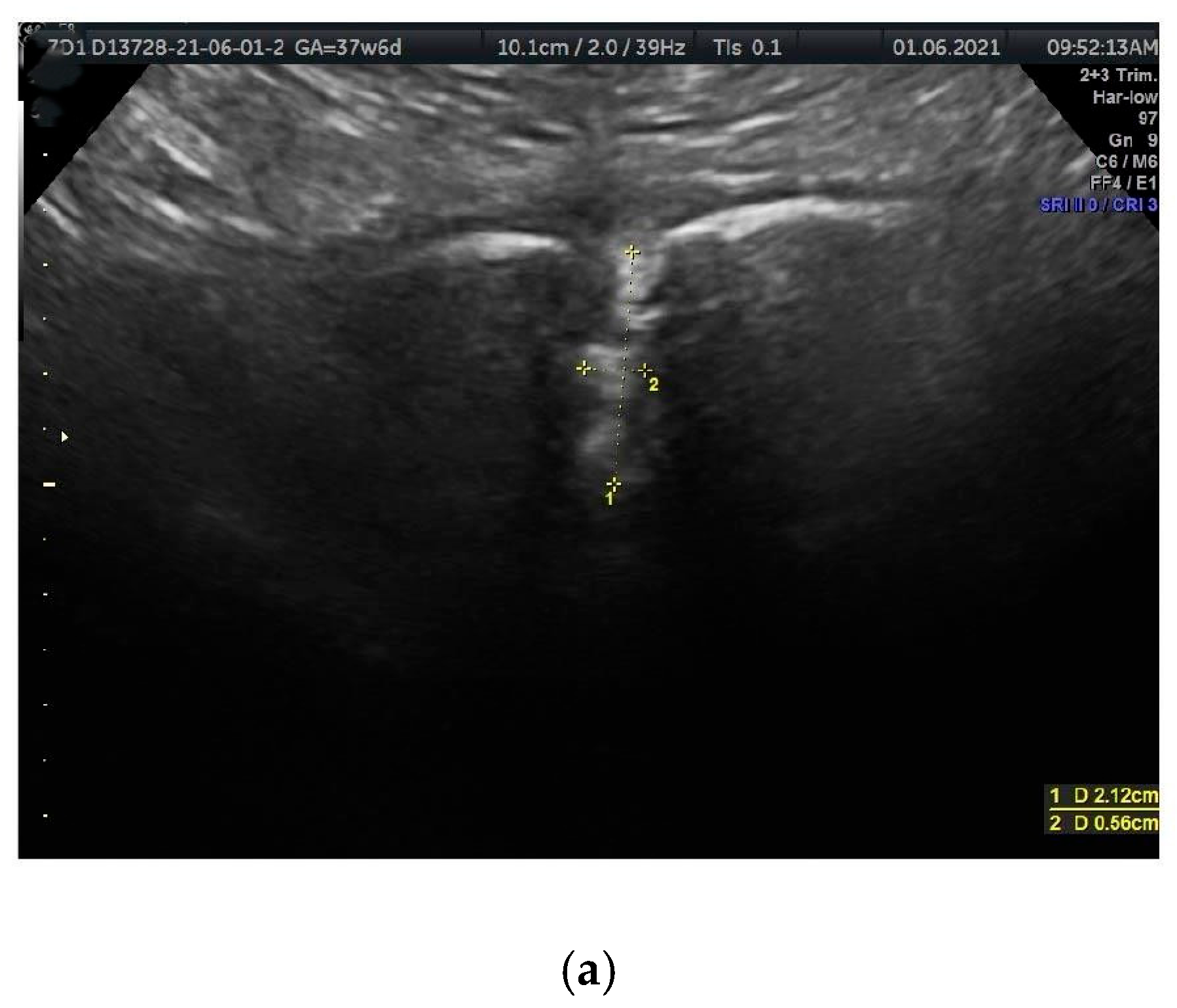
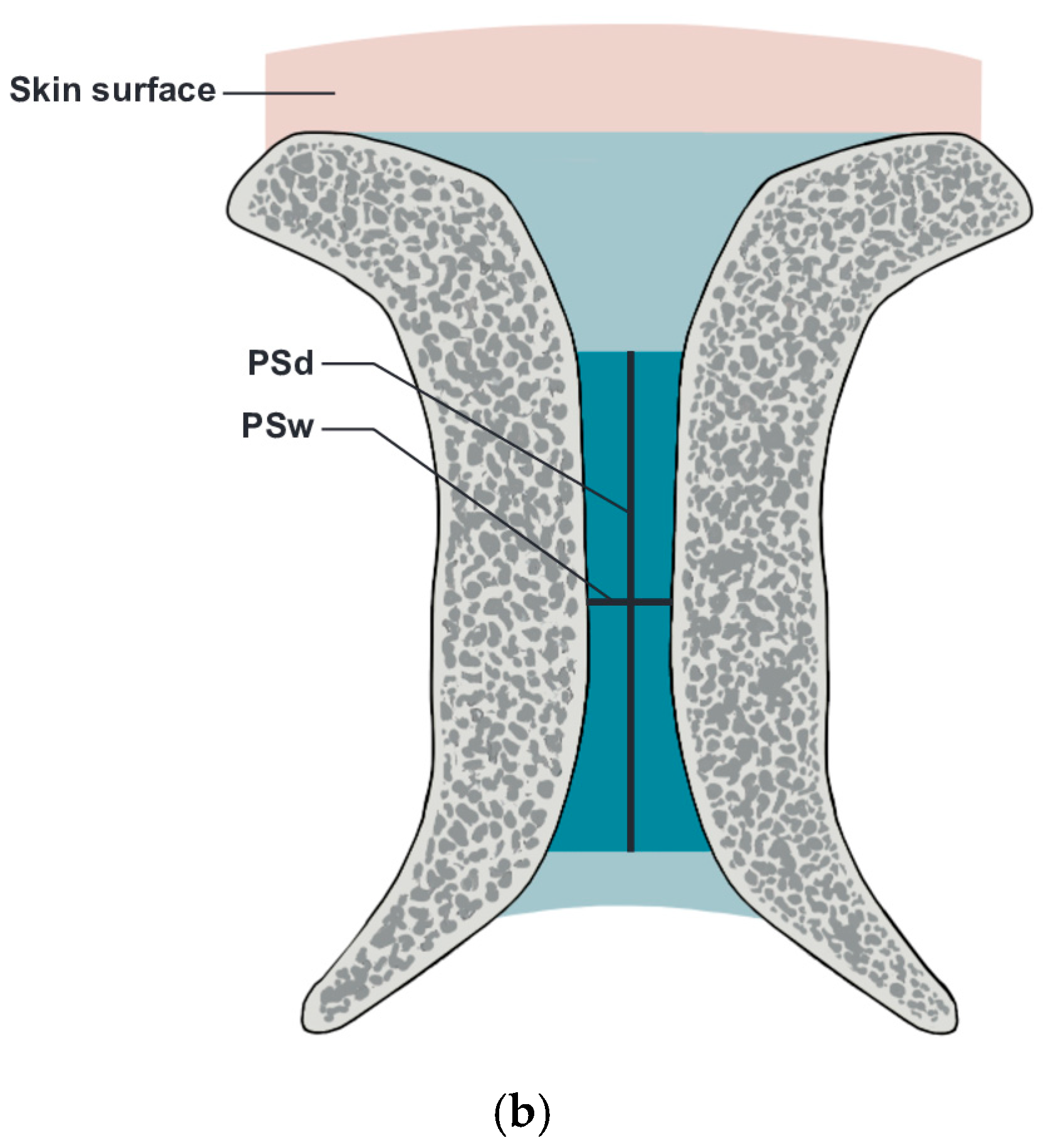
- PS width (PSw): The span between the right and left symphysial surfaces, measured at the midpoint of the PS. Another term for this is the PS width across the PS.
- PS depth (PSd): The distance between the proximal and distal borders of the PS disc.
3. Results
- Interpretation of results
- Generally, heavier fetuses are associated with increased PS entry and PS parameters, except for PT span.
- Older females tend to have longer PT-PT distances. No statistically significant correlation was found with other PS parameters.
- Heavier women showed wider PS entry and PS discs, along with PT-PT distance. Similar trends were observed with weight gained during pregnancy.
- Height: Patients’ mean height (166.5 ± 5.7 cm) versus PS parameters (PSemw: 6.6 ± 3.4 mm, PSw: 6.5 ± 2.8 mm, PSd: 14.6 ± 4.8 mm, PT-PT: 15.3 ± 5.4 mm).
- BMI during pregnancy: Patients’ mean BMI (24.2 ± 4.2) versus PS parameters (PSemw: 5.6 ± 2.4 mm, PSw: 6.0 ± 2.4 mm, PSd: 14.3 ± 4.1 mm, PT-PT 15.3 ± 5.4 mm).
- Interpretation of results
- Singular Pregnancies: Fetal Weight:PSemw: Statistically significant differences were observed for fetuses weighing 1001–2000 g.PSw: statistically significant differences were revealed between
- Fetuses weighing under 1000 g versus those weighing more than 3000 g
- Fetuses weighing 1001–2000 g versus those weighing more than 3000 g
No statistically significant differences were found for PSd or PT-PT.
- Interpretation of results
4. Discussion
5. Conclusions
6. Strengths
7. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PS | Pubic symphysis |
| US | Ultrasonography |
| PSemw | Pubic symphysis entry middle width |
| PT-PT | Intertubercular distance |
| PSw | Pubic symphysis width |
| PSd | Pubic symphysis depth |
References
- Becker, I.; Stringer, M.D.; Jeffery, R.; Woodley, S.J. Sonographic anatomy of the pubic symphysis in healthy nulliparous women. Clin. Anat. 2014, 27, 1058–1067. [Google Scholar] [CrossRef] [PubMed]
- Becker, I.; Woodley, S.J.; Stringer, M.D. The adult human pubic symphysis: A systematic review. J. Anat. 2010, 217, 475–487. [Google Scholar] [CrossRef]
- Mathieu, T.; Van Glabbeek, F.; Van Nassauw, L.; Van Den Plas, K.; Denteneer, L.; Stassijns, G. New insights into the musculotendinous and ligamentous attachments at the pubic symphysis: A systematic review. Ann. Anat. 2022, 244, 151959. [Google Scholar] [CrossRef]
- Mazura, M.; Hromadka, R.; Kopriva, T.; Benes, M.; Kachlik, D. The interpubic cavity: A scoping review. Clin. Anat. 2023, 36, 1104–1108. [Google Scholar] [CrossRef]
- So, C.C.; Niakan, L.S.; Garza-Gongora, R.D. Imaging of the Pubic Symphysis: Anatomy and Pathologic Conditions. Radiographics 2023, 43, e220058. [Google Scholar] [CrossRef] [PubMed]
- Hwang, K.; Wu, X.; Park, C.Y. Width of pubic symphysis relating to age and sex in Koreans. J. Orthop. Surg. Res. 2021, 16, 430. [Google Scholar] [CrossRef] [PubMed]
- Pieroh, P.; Li, Z.L.; Kawata, S.; Ogawa, Y.; Josten, C.; Steinke, H.; Dehghani, F.; Itoh, M. The topography and morphometrics of the pubic ligaments. Ann. Anat. 2021, 236, 151698. [Google Scholar] [CrossRef]
- Parker, J.M.; Bhattacharjee, M. Peripartum diastasis of the symphysis pubis. N. Engl. J. Med. 2009, 361, 1886. [Google Scholar] [CrossRef]
- Yoo, J.J.; Ha, Y.C.; Lee, Y.K.; Hong, J.S.; Kang, B.J.; Koo, K.H. Incidence and risk factors of symptomatic peripartum diastasis of pubic symphysis. J. Korean Med. Sci. 2014, 29, 281–286. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.Q.; Tian, M.R.; Wang, N.; Zheng, Z.C. Role of relaxin in diastasis of the pubic symphysis peripartum. World J. Clin. Cases 2021, 9, 91–101. [Google Scholar] [CrossRef]
- Albert, H.; Godskesen, M.; Westergaard, J. Prognosis in four syndromes of pregnancy-related pelvic pain. Acta Obstet. Gynecol. Scand. 2001, 80, 505–510. [Google Scholar]
- Bastiaanssen, J.M.; de Bie, R.A.; Bastiaenen, C.H.; Essed, G.G.; van den Brandt, P.A. A historical perspective on pregnancy-related low back and/or pelvic girdle pain. Eur. J. Obstet. Gynecol. Reprod. Biol. 2005, 120, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Kristiansson, P.; Zöller, B.; Dahl, N.; Kalliokoski, P.; Hallqvist, J.; Li, X. Heredity of pregnancy-related pelvic girdle pain in Sweden. Acta Obstet. Gynecol. Scand. 2013, 102, 1250–1258. [Google Scholar] [CrossRef]
- Sward, L.; Manning, N.; Murchison, A.B.; Ghahremani, T.; McCaulley, J.A.; Magann, E.F. Pelvic Girdle Pain in Pregnancy: A Review. Obstet. Gynecol. Surv. 2023, 78, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Agten, C.A.; Metzler, C.; Rosskopf, A.B.; Zanetti, M.; Binkert, C.A.; Prentl, E.; Pfirrmann, C.W.A. MR imaging of pubic symphysis after uncomplicated vaginal delivery and planned caesarean delivery in the first postpartum week. Clin. Imaging 2019, 56, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Alicioglu, B.; Kartal, O.; Gurbuz, H.; Sut, N. Symphysis pubis distance in adults: A retrospective computed tomography study. Surg. Radiol. Anat. 2008, 30, 153–157, Erratum in Surg. Radiol. Anat. 2008, 30, 281. [Google Scholar] [CrossRef]
- Aydın, S.; Bakar, R.Z.; Aydın, Ç.A.; Özcan, P. Assessment of postpartum symphysis pubis distention with 3D ultrasonography: A novel method. Clin. Imaging 2016, 40, 185–190. [Google Scholar] [CrossRef]
- Bauman, M.; Marinaro, J.; Tawil, I.; Crandall, C.; Rosenbaum, L.; Paul, I. Ultrasonographic determination of pubic symphyseal widening in trauma: The FAST-PS study. J. Emerg. Med. 2011, 40, 528–533. [Google Scholar] [CrossRef]
- Culligan, P.; Hill, S.; Heit, M. Rupture of the symphysis pubis during vaginal delivery followed by two subsequent uneventful pregnancies. Obstet. Gynecol. 2002, 100 Pt 2, 1114–1117. [Google Scholar]
- Herren, C.; Sobottke, R.; Dadgar, A.; Ringe, M.J.; Graf, M.; Keller, K.; Eysel, P.; Mallmann, P.; Siewe, J. Peripartum pubic symphysis separation—Current strategies in diagnosis and therapy and presentation of two cases. Injury 2015, 46, 1074–1080. [Google Scholar] [CrossRef]
- Shnaekel, K.L.; Magann, E.F.; Ahmadi, S. Pubic Symphysis Rupture and Separation During Pregnancy. Obstet. Gynecol. Surv. 2015, 70, 713–718. [Google Scholar] [CrossRef]
- Hermann, K.G.; Halle, H.; Reisshauer, A.; Schink, T.; Vsianska, L.; Mühler, M.R.; Lembcke, A.; Hamm, B.; Bollow, M. Peripartum changes of the pelvic ring: Usefulness of magnetic resonance imaging. Rofo 2007, 179, 1243–1250. (In German) [Google Scholar] [CrossRef] [PubMed]
- Mazura, M.; Kachlik, D.; Blankova, A.; Malikova, H.; Whitley, A.; Landor, I.; Dzupa, V. A Morphologic Analysis of the Pubic Symphysis Using CT and MRI. J. Am. Acad. Orthop. Surg. 2022, 30, e939–e948. [Google Scholar] [CrossRef]
- Wurdinger, S.; Humbsch, K.; Reichenbach, J.R.; Peiker, G.; Seewald, H.J.; Kaiser, W.A. MRI of the pelvic ring joints postpartum: Normal and pathological findings. J. Magn. Reson. Imaging 2002, 15, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Kalenderer, Ö.; Turgut, A.; Bacaksız, T.; Bilgin, E.; Kumbaracı, M.; Akkan, H.A. Evaluation of symphysis pubis and sacroiliac joint distances in skeletally immature patients: A computerized tomography study of 1020 individuals. Acta Orthop. Traumatol. Turc. 2017, 51, 150–154. [Google Scholar] [CrossRef]
- Wozniak, S.; Piatek, A.; Kurc-Darak, B.; Lazarchuk, I.; Zazga, M.; Domagala, Z.; Florjanski, J. Abstract 75. Pubic symphysis during singular and twin pregnancies. A pilot study. DAG. Available online: https://anatomische-gesellschaft.de/events/tagungen/116-jahrestagung-der-anatomischen-gesellschaft-berlin-20-23-09-2022/ (accessed on 1 April 2025).
- Zhang, S.; Dumas, G.; Hemmerich, A. Measurement of pubic symphysis width in different birthing positions using ultrasound. J. Biomech. 2020, 113, 110114. [Google Scholar] [CrossRef] [PubMed]
- De Maeseneer, M.; Forsyth, R.; Provyn, S.; Milants, A.; Lenchik, L.; De Smet, A.; Marcelis, S.; Shahabpour, M. MR imaging-anatomical-histological evaluation of the abdominal muscles, aponeurosis, and adductor tendon insertions on the pubic symphysis: A cadaver study. Eur. J. Radiol. 2019, 118, 107–113. [Google Scholar] [CrossRef]
- Osterhoff, G.; Ossendorf, C.; Ossendorf-Kimmich, N.; Zimmermann, R.; Wanner, G.A.; Simmen, H.P.; Werner, C.M. Surgical stabilization of postpartum symphyseal instability: Two cases and a review of the literature. Gynecol. Obstet. Investig. 2011, 73, 1–7. [Google Scholar] [CrossRef]
- Gupta, J.K.; Sood, A.; Hofmeyr, G.J.; Vogel, J.P. Position in the second stage of labour for women without epidural anaesthesia. Cochrane Database Syst. Rev. 2017, 5, CD002006. [Google Scholar] [CrossRef]
- Mezian, K.; Ricci, V.; Güvener, O.; Jačisko, J.; Novotný, T.; Kara, M.; Chang, K.V.; Naňka, O.; Pirri, C.; Stecco, C.; et al. EURO-MUSCULUS/USPRM Dynamic Ultrasound Protocols for (Adult) Hip. Am. J. Phys. Med. Rehabil. 2022, 101, e162–e168. [Google Scholar] [CrossRef]
- Dudley, N.J. A systematic review of the ultrasound estimation of fetal weight. Ultrasound Obs. Gynecol. 2005, 25, 80–89. [Google Scholar] [CrossRef] [PubMed]
- La Verde, M.; De Franciscis, P.; Torre, C.; Celardo, A.; Grassini, G.; Papa, R.; Cianci, S.; Capristo, C.; Morlando, M.; Riemma, G. Accuracy of Fetal Biacromial Diameter and Derived Ultrasonographic Parameters to Predict Shoulder Dystocia: A Prospective Observational Study. Int. J. Environ. Res. Public Health 2022, 19, 5747. [Google Scholar] [CrossRef] [PubMed]
- Youssef, A.E.A.; Amin, A.F.; Khalaf, M.; Khalaf, M.S.; Ali, M.K.; Abbas, A.M. Fetal biacromial diameter as a new ultrasound measure for prediction of macrosomia in term pregnancy: A prospective observational study. J. Matern. Fetal Neonatal. Med. 2019, 32, 2674–2679. [Google Scholar] [CrossRef] [PubMed]

| Pubic Symphysis Sonographic Parameters | Fetal Weight: 2504.3 ± 1014.4 (Mean ± sd) gram | |
|---|---|---|
| Mean ± sd; mm | P; r | |
| PSemw | 6.5 ± 3.4 | ˂0.001; 0.28 |
| PSw | 6.4 ± 2.9 | ˂0.001; 0,29 |
| PSd | 14.8 ± 4.8 | 0.03; 0.15 |
| PT-PT | 15.8 ± 5.3 | 0.05; 0.05 |
| Pubic Symphysis Sonographic Parameters | Age ** 30.3 ± 5.3 (Mean ± sd) Years | Weight * 65.1 ± 15.3 (Mean ± sd) kgs | Weight Gained ** (Mean ± sd) 12 ± 5.7 kgs |
|---|---|---|---|
| PSemw | 6.5 ± 3.3 | 6.4 ± 3.3 p = 0.02; r = 0.16 | 6.4 ± 3.3 p = ˂ 0.001; r = 0.24 |
| PSw | 6.4 ± 2.9 | 6.3 ± 2.6 p = 0.01; r = 0.17 | 6.3 ± 2.6 p = ˂ 0.001; r = 0.19 |
| PSd | 14.6 ± 4.9 | 14.8 ± 4.9 | 14.8 ± 4.9 |
| PT-PT | 15.1 ± 5.4 p = 0.03; r = 0.15 | 15.7 ± 5.3 | 15.7 ± 5.3 |
| Measured Parameter | Singular Pregnancy: Fetal Weight. Mean± sd mm (No.: 197) | p * | |||
|---|---|---|---|---|---|
| Under/or 1000 g (19) | 1001–2000 g (28) | 2001–3000 g (70) | More than 3000 g (80) | ||
| PSemw | 4.7 ± 1.7 mm | 5.5 ± 3.0 mm * p = 0.02 | 6.4 ± 3.1 mm | 7.4 ± 3.9 mm * p = 0.02 | H = 11.2 |
| PSw | 4.56 ± 1.5 mm * p = 0.02 | 5.51 ± 2.6 mm * p = 0.02 | 6.4 ± 3.1 mm. | 7.33 ± 3.9 mm * p = 0.02 | H = 13.6 |
| PSd | 13.9 ± 4.7 mm | 13.6 ± 4.7 mm | 14.8 ± 4.9 mm | 15.6 ± 5.2 mm | H = 8.2 p = 0.05 |
| PT-PT | 15.2 ± 5.1 mm | 16.0 ± 5.2 mm | 15.3 ± 5.2 mm | 15.8 ± 5.4 mm | H = 1.12 p = 0.7 |
| Measured Parameter | Singular Pregnancy Mean ± sd mm (No.: 197) | p * | |||||
|---|---|---|---|---|---|---|---|
| Fetal Weight ≤ 2000 g (47) | Fetal weight > 2000 g (150) | ||||||
| Nulliparity (33) | Multiparity (14) | Nulliparity (81) | Multiparity (69) | ||||
| PSemw | 4.5 ± 2.4 mm | 4.3 ± 2.7 mm | H = 0.32 p = 0.57 | 5.0 ± 3.1 mm | 4.7 ± 2.8 mm | H = 0.64 p = 0.42 | p > 0.05 |
| PSw | 4.7 ± 2.6 mm | 6.3 ± 2.9 mm | H = 0.01 p = 0.97 | 5.8 ± 2.9 mm | 6.4 ± 3.6 mm | H = 0.04 p = 0.83 | p > 0.05 |
| PSd | 13.4 ± 4.7 mm | 15.0 ± 4.7 mm | H = 0.57 p = 0.45 | 13.3 ± 4.9 mm | 14.6 ± 4.8 mm | H = 1.56 p = 0.21 | p > 0.05 |
| PT-PT | 15.9 ± 7.2 mm | 13.4 ± 6.2 mm | H = 8.66 p = 0.03 * | 14.3 ± 5.9 mm | 16.6 ± 7.6 mm | H = 0.98 p = 0.32 | p = 0.03 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wozniak, S.; Piatek, A.; Kurc-Darak, B.; Domagala, Z.; Paulsen, F.; Florjanski, J. Sonographic Evaluations of the Pubic Symphysis at Different Stages of Pregnancy. J. Clin. Med. 2025, 14, 3898. https://doi.org/10.3390/jcm14113898
Wozniak S, Piatek A, Kurc-Darak B, Domagala Z, Paulsen F, Florjanski J. Sonographic Evaluations of the Pubic Symphysis at Different Stages of Pregnancy. Journal of Clinical Medicine. 2025; 14(11):3898. https://doi.org/10.3390/jcm14113898
Chicago/Turabian StyleWozniak, Slawomir, Aleksandra Piatek, Bozena Kurc-Darak, Zygmunt Domagala, Friedrich Paulsen, and Jerzy Florjanski. 2025. "Sonographic Evaluations of the Pubic Symphysis at Different Stages of Pregnancy" Journal of Clinical Medicine 14, no. 11: 3898. https://doi.org/10.3390/jcm14113898
APA StyleWozniak, S., Piatek, A., Kurc-Darak, B., Domagala, Z., Paulsen, F., & Florjanski, J. (2025). Sonographic Evaluations of the Pubic Symphysis at Different Stages of Pregnancy. Journal of Clinical Medicine, 14(11), 3898. https://doi.org/10.3390/jcm14113898








