Short-Term Effects of Spinal Manual Therapy on the Nervous System in Managing Musculoskeletal Pain: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Registration
2.2. Search Strategy
2.3. Eligibility Criteria
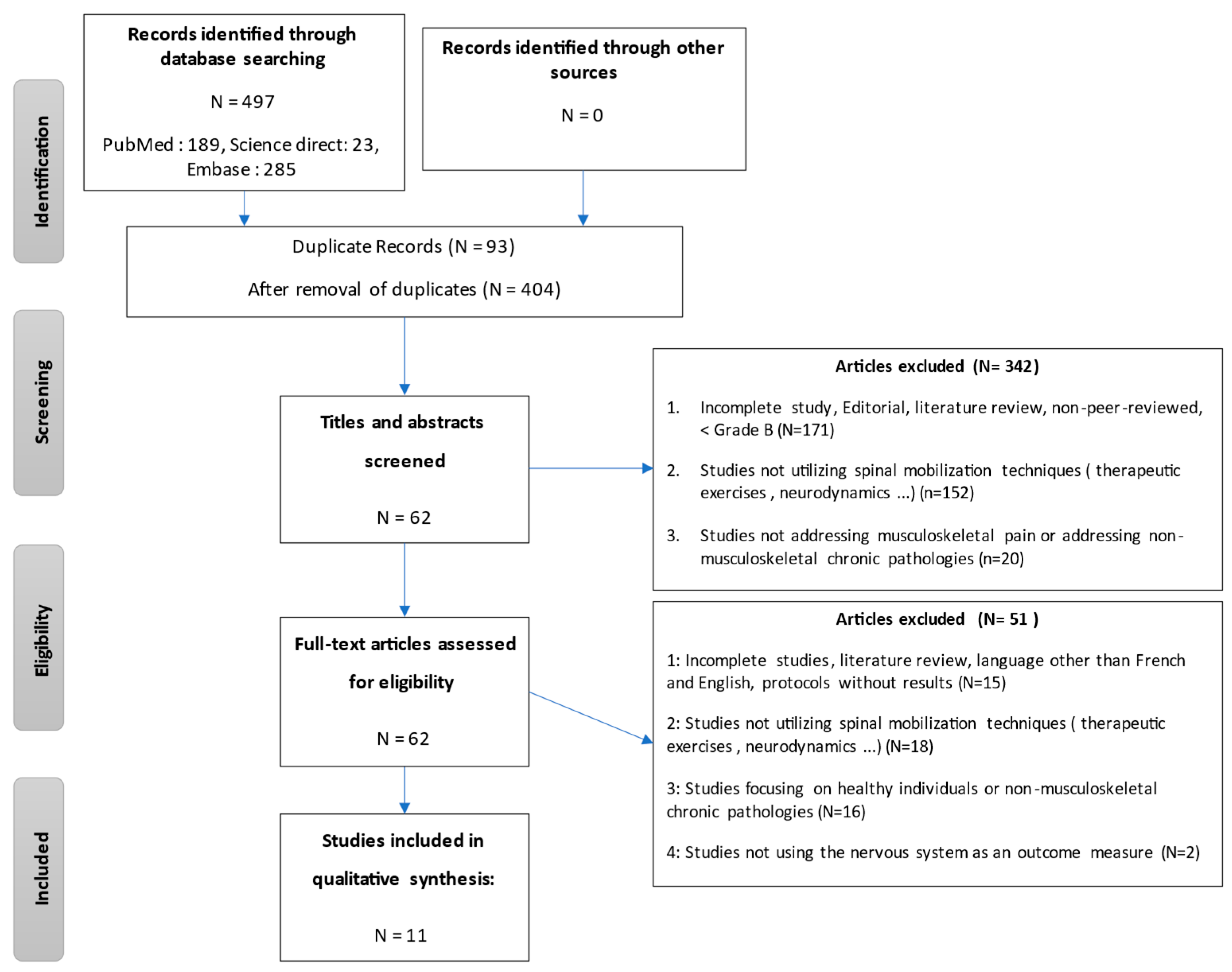
2.4. Selection Process
2.5. Data Extraction
2.6. Methodological Quality Assessment and Risk of Bias
2.7. Data Synthesis
3. Results
3.1. Study Selection
3.2. Study Characteristics
3.3. Main Findings
3.4. Methodological Quality
3.5. Risk of Bias
4. Discussion
4.1. Interpretation of Findings
4.2. Relevance to Clinical Practice
4.3. Recommendations for Future Research
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| PICO | Formulation | Associated Keywords |
|---|---|---|
| Population | Adults experiencing musculoskeletal pain | Musculoskeletal pain |
| Intervention | Spinal manual therapy, including mobilization or manipulation | Manual therapy; spinal manipulation; musculoskeletal manipulation |
| Comparison | Comparison across included studies | – |
| Outcomes | Effects of spinal manual therapy on the nervous system and pain perception | Nervous system; autonomic nervous system; central nervous system; spinal cord; peripheral nervous system; neurophysiology; pain |
| ((manual therapy) OR (musculoskeletal manipulation)) AND (musculoskeletal pain) AND (pain) AND ((nervous system) OR (autonomic nervous system) OR (central nervous system) OR (spinal cord) OR (peripheral nervous system) OR (neurophysiology)) | ||
| Studies (Years) | 1. | 2. | 3. | 4 | 5. | 6. | 7. | 8. | 9. | 10. | 11. | Score Total (/10) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bakken et al. (2021) [6] | * | * | * | * | - | - | * | * | * | * | * | 8 |
| Barassi et al. (2018) [7] | - | * | * | * | - | - | - | * | * | * | - | 6 |
| Bialosky et al. (2014) [12] | * | * | * | * | * | - | - | * | * | * | * | 8 |
| Fisher et al. (2016) [9] | - | * | - | * | - | - | * | * | * | * | * | 7 |
| Gay et al. (2014) [10] | * | * | * | * | - | * | * | * | * | * | - | 8 |
| Haider et al. (2018) [13] | - | * | - | * | - | - | - | * | - | * | * | 5 |
| La Touche et al. (2013) [14] | * | * | * | * | * | - | * | * | - | * | * | 8 |
| Peña-Salinas et al. (2017) [15] | * | * | * | * | - | - | * | * | * | * | * | 8 |
| Rodrigues et al. (2021) [16] | * | * | * | * | - | - | * | * | - | * | - | 7 |
| Sillevis et al. (2011) [17] | * | * | * | * | - | - | - | * | - | * | - | 5 |
| Weber et al. (2019) [18] | * | * | * | * | * | * | - | - | - | * | * | 8 |
| Studies | Pedro Scale | Duration | Intervention | Frequency (Intervention Duration) | Intensity | Groups Types | Volume | Others |
|---|---|---|---|---|---|---|---|---|
| Bakken et al. [6] | 8 | Exercises: 5 min | Exercises: Trapezius stretches (3 × 30 s)/SCOM stretches (3 × 30 s) Neck extensor stretches (3 × 30 s)/Neck flexion RCS: 5 × (3–5 s) TM: Mobilization or HVLA | 2/weeks (TM) Exercises: daily (2 weeks) | / | Home exercises | / | / |
| Home exercises + TM | ||||||||
| Barassi et al. [7] | 6 | 30 min | Muscle pressures: Maintaining pressure of approximately 3 kg and gradually releasing without abruptly stopping contact 5 patients: SCOM + levator scapulae 2 patients: quadratus lumborum + plantar region of the foot 3 patients: trapezius, SCOM, levator scapulae, quadratus lumborum | 1/week (8 weeks) | / | Lumbar massage | / | / |
| Prolonged muscle pressures | ||||||||
| Bialosky et al. (2014) [12] | 8 | / | TM: DL with contralateral lumbar rotation then HVLA; 2 times/side Placebo: Neutral spine push without rotation and no HVLA applied Placebo +: “The manual therapy you will receive has shown a significant reduction in low back pain in many people” + placebo Control: Sitting for 5 min, waiting | 3/week (2 weeks) | / | Manual therapy | / | / |
| Placebo | ||||||||
| Placebo + | ||||||||
| Control | ||||||||
| Fisher et al. (2016) [9] | 7 | Mob: 30 s | Mob: Hold ankle in traction for 30 s TM: Caudal directed ankle HVLA | One time session | / | Mobilization | / | / |
| Manual therapy (HVLA) | ||||||||
| Gay et al. (2014) [10] | 8 | Mob: 5 min TT: 5 min | TM: HVLA type (Grade V), Mob: 2 min at 1 Hz 1 min rest, then 2 min at 1 Hz on the lumbar region TT: light pressure on sacrum for 5 min | One time session | / | Manual therapy (HVLA) | / | / |
| Mobilization (Grade III) | ||||||||
| Therapeutic touch | ||||||||
| Haider et al. [13] | 5 | / | TM: 1 non-thrust mobilization + 3 different thrust techniques on thoracic region + exercises (Maitland type) For both groups: exercises Hot/cold Mobility exercises (flexion and extension with arms in front of the wall; shoulder flexion 90°; exercises with shoulder circles) Strengthening exercises: resistance with elbow flexed at 90°; shoulder elevation with weights between 1–4 kg Deeps with elbow extension | 3/week (2 weeks) | / | Manual therapy (Maitland) + exercises | / | / |
| Exercises | ||||||||
| La Touche et al. (2013) [14] | 8 | 3 × (2 min TM/30 s rest) = 7 min | TM: Patient in supine position, cervical region in neutral. (C0–C3 region); application of posterior force on the patient’s forehead by the therapist with the anterior part of their shoulder, both hands supporting the cervical region. Mobilization at 0.5 Hz Placebo: Same position but no force applied, simple contact maintained | 3/week (2 weeks) | / | Manual therapy | / | ANS measurement immediately after the technique; 5 minutes later: VAS + PPT |
| Placebo | ||||||||
| Peña-Salinas et al. (2017) [15] | 8 | / | TM: Patient lying down, therapist behind. Therapist applies force with their metacarpophalangeal joint of the right index finger towards the 1st rib; right thumb in the supraspinous fossa. The other hand supports the patient’s head with ipsilateral tilt + contralateral rotation. Inferomedial pressure applied to the 1st rib. Placebo: Same position without applying force | One time session | / | Manual therapy (HVLA) | / | / |
| Placebo | ||||||||
| Rodrigues et al. (2021) [16] | 7 | 3 min | TM: Patient lying on their back, arms across the chest. HVLA on the anteroposterior thoracic region. If no audible sound: maneuver repeated (max: 2/patients) Mob: Patient lying on their back, therapist’s pressure on the sternum, other hand supporting the patient’s neck. Longitudinal pressure directed caudally on the sternum. Once tissue barrier felt: 2 min at 0.3–0.4 Hz. Placebo: 2 min of ultrasound without current on the thoracic region | One time session | / | Manual therapy (HVLA) | / | / |
| Mobilization | ||||||||
| Placebo | ||||||||
| Sillevis et al. (2011) [17] | 5 | / | TM: HVLA T3–T4, anterior-posterior manipulation on the back, arms across the chest. Mob: Same position, but instructed to associate breathing; therapist’s 3-s compression during expiration | One time session | / | Manual therapy (HVLA) | / | Diameter measurement after intervention + measurement 4 min later |
| Mobilization | ||||||||
| Weber et al. (2019) [18] | 8 | <5 min | TM: HVLA anterior-posterior T4–T5, patient lying on their back, arms crossed on the chest. Placebo: Same position but minimal pressure | One time session | / | Manual therapy (HVLA) | / | / |
| Placebo |
| Studies | Pedro Scale | Population «Baseline» | Samples | ||
|---|---|---|---|---|---|
| Inclusion Criteria | Population Data | ||||
| Bakken et al. [6] | 8 | >18 years with recurrent (at least 1 previous episode) or persistent (>6 months) neck pain No chiropractic treatment in the last 3 months | Average age 57; 55% women; start pain (4/10); +80% neck pain > many years; 80% low risk of chronicity (Starback); 65% working people | Group: MT (HVLA or mobilization) + home exercises | N = 62 |
| Group: home exercises | N = 61 | ||||
| Barassi et al. [7] | 6 | Spinal pain between the ages of 20–29 | Average age 25 | Group: MT (prolonged muscular pressure) | N = 10 |
| Group: lumbar massage | N = 10 | ||||
| Bialosky et al. (2014) [12] | 8 | Between 18 and 60 years of age with low back pain > 4/10 in the last 24 h. | 70% women, Average age 31, SMT: 12 weeks of pain Placebo: 24 weeks of pain Placebo +: 36 weeks of pain Control: 4 weeks of pain | Group: MT | N = 28 |
| Group: MT placebo | N = 27 | ||||
| Group: MT placebo + positive message | N = 27 | ||||
| Group: no interventions | N = 28 | ||||
| Fisher et al. (2016) [9] | 7 | Between 18 and 60 years of age with a history of ankle sprains (>2 weeks), and an ankle functional score below 24/48. | Average score: 18.7 ± 5.6 | Group: TM type HVLA | N = 15 |
| Group: Ankle mobilization | N = 15 | ||||
| Gay et al. (2014) [10] | 8 | 21 years old on average with no current experience of low back pain, but performing a protocol prior to the study inducing low back pain. Return 48 h later to have an intervention according to the group. | 70% women | Group: MT (HVLA) | N = 6 |
| Group: MT (grade III) | N = 8 | ||||
| Group: therapeutic touch | N = 10 | ||||
| Haider et al. [13] | 5 | Subacromial pain for 2–3 months, aged 25–60 years. | Average age 50, 55% women, 50% right side; 45% left side; 5% both side; pain > 3 months for 40% of participants | Group: MT Maitland + exercises | N = 20 |
| Group: exercises | N = 20 | ||||
| La Touche et al. (2013) [14] | 8 | Craniocervical pain of myofascial origin localized to the cervical and masticatory muscles. 1st diagnosis of myofascial pain; bilateral pain (masseter, temporal, upper trapezius, suboccipital muscles), pain > 3 months and > 30/100; neck or shoulder pain provoked by cervical | / | Group: antero posterior cervical MT | N = 11 |
| Group: placebo | N = 11 | ||||
| Peña-Salinas et al. (2017) [15] | 8 | Cervical or cervico-brachial pain following MVA within 3 months. Positive lateral rotation flexion test | Average age 34, 55% women | Group: TM (1st rib) | N = 27 |
| Group: placebo | N = 26 | ||||
| Rodrigues et al. (2021) [16] | 7 | Musculoskeletal pain > 18 years old | Average age 45, 55% women, >50% make exercises; chronic pain for >80%; 15% chest pain; 45% lumbar pain Significant difference between groups for blood pressure. | Group: TM spinal HVLA | N = 19 |
| Group: TM myofascial | N = 20 | ||||
| Group: ultrasound placebo | N = 20 | ||||
| Sillevis et al. (2011) [17] | 5 | Patients with chronic neck pain below T4, provoked by cervical movements. Age range 18–65. | +70% women, average age 44 | Group: TM type HVLA | N = 50 |
| Group: mobilization | N = 50 | ||||
| Weber et al. (2019) [18] | 8 | Participants with acute or sub-acute neck pain (<6 weeks). | Average age 37, 65% women | Group: TM type HVLA | N = 12 |
| Group: placebo | N = 12 | ||||
References
- Pettman, E. A History of Manipulative Therapy. J. Man. Manip. Ther. 2007, 15, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Cookson, J.C.; Kent, B.E. Orthopedic Manual Therapy—An Overview: Part I: The Extremities. Phys. Ther. 1979, 59, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Maitland, G.D. Maitland’s Vertebral Manipulation, 6th ed.; Butterworth-Heinemann: Oxford, UK, 2001; ISBN 978-0-7506-2447-3. [Google Scholar]
- Farley, A.; Johnstone, C.; Hendry, C.; McLafferty, E. Nervous System: Part 1. Nurs. Stand. 2014, 28, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Vachon-Presseau, E.; Centeno, M.V.; Ren, W.; Berger, S.E.; Tétreault, P.; Ghantous, M.; Baria, A.; Farmer, M.; Baliki, M.N.; Schnitzer, T.J.; et al. The Emotional Brain as a Predictor and Amplifier of Chronic Pain. J. Dent. Res. 2016, 95, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Galaasen Bakken, A.; Eklund, A.; Hallman, D.M.; Axén, I. The Effect of Spinal Manipulative Therapy and Home Stretching Exercises on Heart Rate Variability in Patients with Persistent or Recurrent Neck Pain: A Randomized Controlled Trial. Chiropr. Man. Ther. 2021, 29, 48. [Google Scholar] [CrossRef] [PubMed]
- Barassi, G.; Bellomo, R.G.; Di Giulio, C.; Giannuzzo, G.; Irace, G.; Barbato, C.; Saggini, R. Effects of Manual Somatic Stimulation on the Autonomic Nervous System and Posture. Adv. Exp. Med. Biol. 2018, 1070, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Apkarian, A.V.; Bushnell, M.C.; Treede, R.-D.; Zubieta, J.-K. Human Brain Mechanisms of Pain Perception and Regulation in Health and Disease. Eur. J. Pain Lond. Engl. 2005, 9, 463–484. [Google Scholar] [CrossRef] [PubMed]
- Fisher, B.E.; Piraino, A.; Lee, Y.-Y.; Smith, J.A.; Johnson, S.; Davenport, T.E.; Kulig, K. The Effect of Velocity of Joint Mobilization on Corticospinal Excitability in Individuals With a History of Ankle Sprain. J. Orthop. Sports Phys. Ther. 2016, 46, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Gay, C.W.; Robinson, M.E.; George, S.Z.; Perlstein, W.M.; Bishop, M.D. Immediate Changes after Manual Therapy in Resting-State Functional Connectivity as Measured by Functional Magnetic Resonance Imaging in Participants with Induced Low Back Pain. J. Manip. Physiol. Ther. 2014, 37, 614–627. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Bialosky, J.E.; George, S.Z.; Horn, M.E.; Price, D.D.; Staud, R.; Robinson, M.E. Spinal Manipulative Therapy-Specific Changes in Pain Sensitivity in Individuals with Low Back Pain (NCT01168999). J. Pain 2014, 15, 136–148. [Google Scholar] [CrossRef] [PubMed]
- Haider, R.; Bashir, M.S.; Adeel, M.; Ijaz, M.J.; Ayub, A. Comparison of Conservative Exercise Therapy with and without Maitland Thoracic Manipulative Therapy in Patients with Subacromial Pain: Clinical Trial. J. Pak. Med. Assoc. 2018, 68I, 381–387. [Google Scholar]
- La Touche, R.; París-Alemany, A.; Mannheimer, J.S.; Angulo-Díaz-Parreño, S.; Bishop, M.D.; Lopéz-Valverde-Centeno, A.; von Piekartz, H.; Fernández-Carnero, J. Does Mobilization of the Upper Cervical Spine Affect Pain Sensitivity and Autonomic Nervous System Function in Patients with Cervico-Craniofacial Pain?: A Randomized-Controlled Trial. Clin. J. Pain 2013, 29, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Peña-Salinas, M.; Oliva-Pascual-Vaca, J.; Heredia-Rizo, A.M.; Rodriguez-Blanco, C.; Ricard, F.; Oliva-Pascual-Vaca, Á. No Immediate Changes on Neural and Muscular Mechanosensitivity after First Rib Manipulation in Subjects with Cervical Whiplash: A Randomized Controlled Trial. J. Back Musculoskelet. Rehabil. 2017, 30, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, P.T.V.; Corrêa, L.A.; Reis, F.J.J.; Meziat-Filho, N.A.; Silva, B.M.; Nogueira, L.A.C. One Session of Spinal Manipulation Improves the Cardiac Autonomic Control in Patients with Musculoskeletal Pain: A Randomized Placebo-Controlled Trial. Spine 2021, 46, 915–922. [Google Scholar] [CrossRef] [PubMed]
- Sillevis, R.; Cleland, J. Immediate Effects of the Audible Pop From a Thoracic Spine Thrust Manipulation on the Autonomic Nervous System and Pain: A Secondary Analysis of a Randomized Clinical Trial. J. Manip. Physiol. Ther. 2011, 34, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Weber Ii, K.A.; Wager, T.D.; Mackey, S.; Elliott, J.M.; Liu, W.-C.; Sparks, C.L. Evidence for Decreased Neurologic Pain Signature Activation Following Thoracic Spinal Manipulation in Healthy Volunteers and Participants with Neck Pain. NeuroImage Clin. 2019, 24, 102042. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.P.; Karoly, P.; Braver, S. The Measurement of Clinical Pain Intensity: A Comparison of Six Methods. Pain 1986, 27, 117–126. [Google Scholar] [CrossRef]
- Fazeli, M.S.; Pourrahmat, M.-M.; Massah, G.; Lee, K.; Lavoie, P.M.; Fazeli, M.; Esser, A.; Collet, J.-P. The Effect of Massage on the Cardiac Autonomic Nervous System and Markers of Inflammation in Night Shift Workers: A Pilot Randomized Crossover Trial. Int. J. Ther. Massage Bodyw. 2020, 13, 6–17. [Google Scholar]
- George, S.Z.; Fritz, J.M.; Silfies, S.P.; Schneider, M.J.; Beneciuk, J.M.; Lentz, T.A.; Gilliam, J.R.; Hendren, S.; Norman, K.S.; Beattie, P.F.; et al. Interventions for the Management of Acute and Chronic Low Back Pain: Revision 2021. J. Orthop. Sports Phys. Ther. 2021, 51, CPG1–CPG60. [Google Scholar] [CrossRef] [PubMed]

| Study | Population/Sample | Intervention | Frequency/Duration | Control/Comparator | Outcome Measure |
|---|---|---|---|---|---|
| Bakken et al. (2021) [6] | Patients with neck pain/n = 50 | Spinal manipulation + stretching | 3×/week for 2 weeks | Stretching only | Heart rate variability (HRV) |
| Barassi et al. (2018) [7] | Healthy adults/n = 20 | Manual therapy on lumbar spine | 1×/week for 8 weeks | Massage | Pain (NRS), postural control, HR, RR, SpO2, CO2 |
| Bialosky et al. (2014) [12] | Lower back pain/n = 80 | Spinal manipulation + messages | 3×/week for 2 weeks | Placebo + message/No treatment | Pain sensitivity (PPT), thermal threshold, satisfaction |
| Fisher et al. (2016) [9] | Ankle sprain history/n = 24 | Joint mobilization (varied velocities) | Single session | None | Motor-evoked potentials (MEPs) |
| Gay et al. (2014) [10] | Induced lower back pain/n = 24 | Manual therapy session | Single session | Sham MT | fMRI connectivity, pain (NRS), PPT |
| Haider et al. (2018) [13] | Subacromial pain/n = 22 | Maitland thoracic manipulation | 3×/week for 2 weeks | Conservative exercise therapy | Pain (NRS), Shoulder Function Score |
| La Touche et al. (2013) [14] | Cervico-craniofacial pain/n = 22 | Upper cervical mobilization | 3×/week for 2 weeks | Placebo mobilization | PPT, pain (NRS), skin conductance, HR, RR, temperature |
| Peña-Salinas et al. (2017) [15] | Cervical whiplash/n = 30 | First rib manipulation | Single session | None | PPT (nerve and muscle) |
| Rodrigues et al. (2021) [16] | Musculoskeletal pain/n = 30 | Spinal manipulation | Single session | Sham MT | HRV, blood pressure, HR |
| Sillevis et al. (2011) [17] | Neck pain/n = 20 | Thoracic manipulation | Single session | Sham manipulation | Pupil diameter, pain (NRS) |
| Weber et al. (2019) [18] | Neck pain/n = 24 | Thoracic manipulation | Single session | Sham manipulation | Neurological activation zones, Pain (NRS) |
| Study | Key Outcomes | Statistical Significance | Domains Assessed | Effect Size |
|---|---|---|---|---|
| Bakken et al. (2021) [6] | No significant difference in HRV | Not significant | SNA (HRV) | Not reported |
| Barassi et al. (2018) [7] | Significant ↓ in pain and RR; improved postural control | p < 0.05 | Pain, SNA (HR, RR), postural control | Pain: d = 2.75; RR: d = 3.00; Postural control: d = 0.47 |
| Bialosky et al. (2014) [12] | ↓ in pain (all groups); greater effect with contextual verbal suggestion (“positive message”) | p < 0.05 (positive message condition) | Pain, Pain sensitivity (PPT), Placebo effect | η2 = 0.17 (message effect on pain) |
| Fisher et al. (2016) [9] | ↑ in passive & active MEPs (tibialis anterior) | p < 0.05 | CNS (MEPs) | d = 0.90 (MEPs amplitude) |
| Gay et al. (2014) [10] | ↑ in functional connectivity (HVLA group); ↓ pain | p < 0.05 (group × time interaction) | Pain, CNS (fMRI connectivity) | η = 0.24 (connectivity change) |
| Haider et al. (2018) [13] | ↓ in pain and ↑ shoulder function (Maitland group) | p < 0.05 | Pain, Function | Pain: d = 0.34–1.29; function: d = 0.34–1.66 |
| La Touche et al. (2013) [14] | ↑ PPT, ↓ pain, ↑ HR and RR, ↑ skin conductance | p < 0.05 | Pain, PPT, ANS (HR, RR, skin conductance), CNS | Pain: d = 4.26; RR: d = 3.79; PPT: d = 3.34; HR: d = 2.68; skin conductance: d = 3.78 |
| Peña-Salinas et al. (2017) [15] | No significant difference in PPT | Not significant | Pain sensitivity (PPT) | Not reported |
| Rodrigues et al. (2021) [16] | ↓ sympathetic activity; ↑ parasympathetic (HRV); ↓ SBP | p < 0.05 | ANS (HRV, HR, BP) | d = 0.40 (HRV, BP) |
| Sillevis et al. (2011) [17] | Pupil response differed by noise; ↓ pain observed | Mixed, trend only | Pain, ANS (pupil diameter) | d = 0.50 (pupil response to noise) |
| Weber et al. (2019) [18] | ↓ in pain and in neurological activation (ACC) | p < 0.05 | Pain, CNS (neurological activation) | d = 0.32 (pain and CNS activation) |
| Study | PEDro Score (/10) | Quality Level | Blinding (Participants/Therapists/Assessors) |
|---|---|---|---|
| Bakken et al. (2021) [6] | 8 | High | No/No/Yes |
| Barassi et al. (2018) [7] | 6 | Moderate | No/No/Yes |
| Bialosky et al. (2014) [12] | 8 | High | Yes/No/Yes |
| Fisher et al. (2016) [9] | 7 | High | No/No/Yes |
| Gay et al. (2014) [10] | 8 | High | No/Yes/Yes |
| Haider et al. (2018) [13] | 5 | Low | No/No/Yes |
| La Touche et al. (2013) [14] | 8 | High | Yes/No/Yes |
| Peña-Salinas et al. (2017) [15] | 8 | High | No/No/Yes |
| Rodrigues et al. (2021) [16] | 7 | High | No/No/Yes |
| Sillevis et al. (2011) [17] | 5 | Low | Yes/No/No |
| Weber et al. (2019) [18] | 8 | High | Yes/Yes/No |
| Study | Sample Size (<30) | Randomization Reported | Allocation Concealed | Blinding Limitations | Overall Risk of Bias |
|---|---|---|---|---|---|
| Bakken et al. (2021) [6] | No | Yes | Yes | No blinding of participants or therapists | Low |
| Barassi et al. (2018) [7] | Yes | Yes | No | No blinding of participants or therapists | Moderate |
| Bialosky et al. (2014) [12] | No | Yes | Yes | No therapist blinding | Low |
| Fisher et al. (2016) [9] | Yes | Yes | No | No blinding of participants or therapists | Moderate |
| Gay et al. (2014) [10] | Yes | Yes | Yes | No participant blinding | Moderate |
| Haider et al. (2018) [13] | Yes | Yes | No | No blinding of participants or therapists | High |
| La Touche et al. (2013) [14] | Yes | Yes | Yes | No therapist blinding | Low |
| Peña-Salinas et al. (2017) [15] | No | Yes | Yes | No blinding of participants or therapists | Low |
| Rodrigues et al. (2021) [16] | No | Yes | Yes | No blinding of participants or therapists | Moderate |
| Sillevis et al. (2011) [17] | Yes | Yes | No | Only participant blinding | High |
| Weber et al. (2019) [18] | Yes | Yes | No | No assessor blinding | Moderate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jupin, C.; Beltran Aibar, V.; Sarhan, F.-R. Short-Term Effects of Spinal Manual Therapy on the Nervous System in Managing Musculoskeletal Pain: A Systematic Review. J. Clin. Med. 2025, 14, 3830. https://doi.org/10.3390/jcm14113830
Jupin C, Beltran Aibar V, Sarhan F-R. Short-Term Effects of Spinal Manual Therapy on the Nervous System in Managing Musculoskeletal Pain: A Systematic Review. Journal of Clinical Medicine. 2025; 14(11):3830. https://doi.org/10.3390/jcm14113830
Chicago/Turabian StyleJupin, Chloé, Vicente Beltran Aibar, and François-Régis Sarhan. 2025. "Short-Term Effects of Spinal Manual Therapy on the Nervous System in Managing Musculoskeletal Pain: A Systematic Review" Journal of Clinical Medicine 14, no. 11: 3830. https://doi.org/10.3390/jcm14113830
APA StyleJupin, C., Beltran Aibar, V., & Sarhan, F.-R. (2025). Short-Term Effects of Spinal Manual Therapy on the Nervous System in Managing Musculoskeletal Pain: A Systematic Review. Journal of Clinical Medicine, 14(11), 3830. https://doi.org/10.3390/jcm14113830







