Effects of Virtual Reality-Based Interventions on Pain Catastrophizing in People with Chronic Pain: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registry
2.2. Eligibility Criteria for the Studies
2.2.1. Inclusion Criteria
- Population: Adults with chronic painful musculoskeletal conditions (cause of pain is primary).
- Intervention: Interventions based on VR, both immersive and non-immersive VR. Active or passive interventions in which the participants do or do not do physical activities, respectively. The VR intervention can be applied alone or with another conventional intervention.
- Comparison: Non-intervened control, interventions without VR, standard treatment, usual care, or placebo.
- Outcome: Pain catastrophizing.
- Study design: Two-armed randomized clinical trial (RCT) with parallel groups. Also, pilot RCTs.
2.2.2. Exclusion Criteria
- Adults with locomotor system prostheses.
- Adults with chronic pain associated with non-musculoskeletal conditions (e.g., oncologic or migraine).
- Application of VR with the sole purpose of distracting the patient during another health procedure or intervention.
- Abstracts, posters, or theses
2.3. Sources of Information
2.4. Search Strategy
2.5. Selection of the Studies
2.6. Data Extraction
2.7. Risk of Bias Assessment
2.8. Data Synthesis and Analysis
3. Results
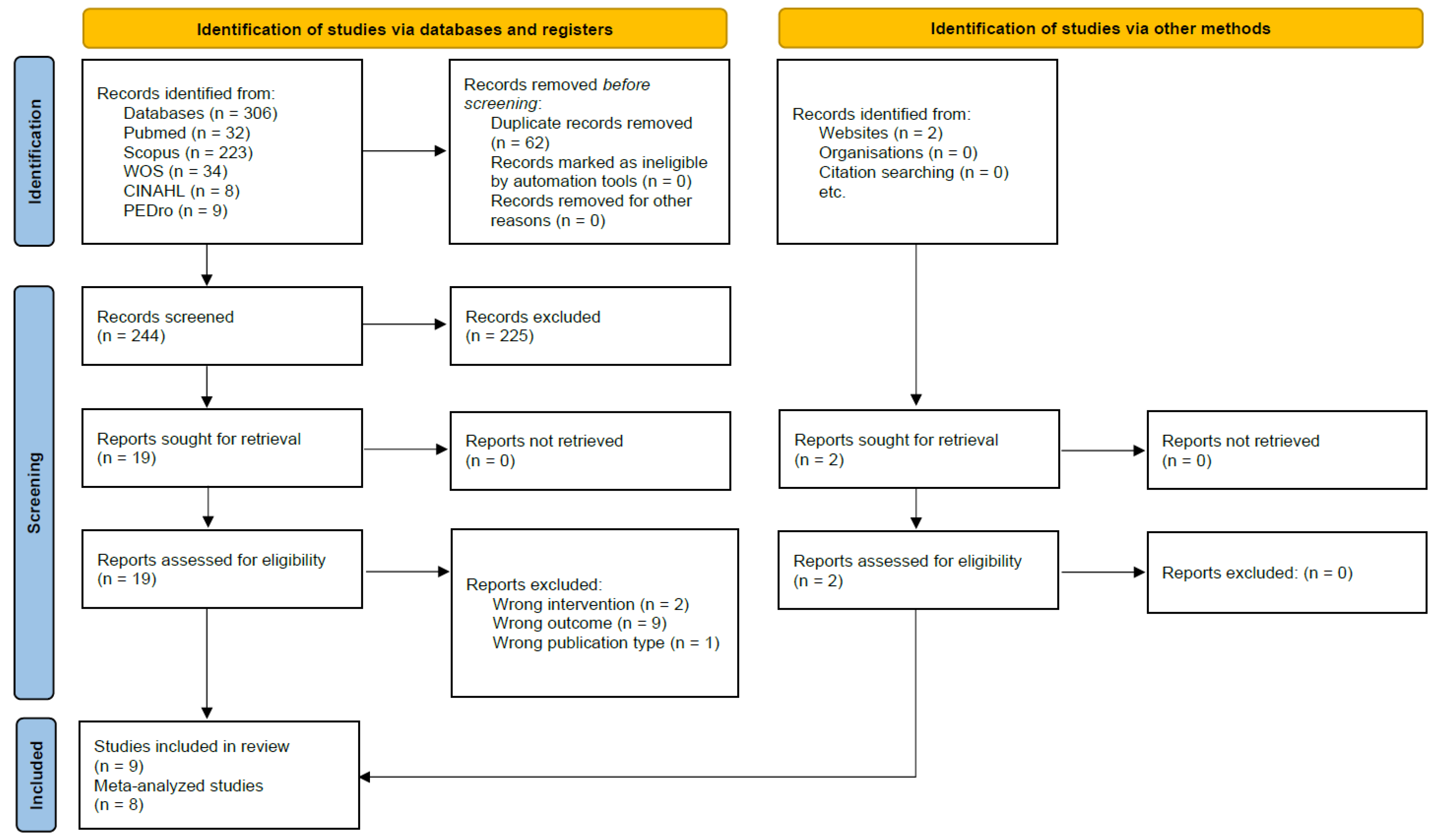
3.1. Search Results
3.2. Characteristics of Included Studies
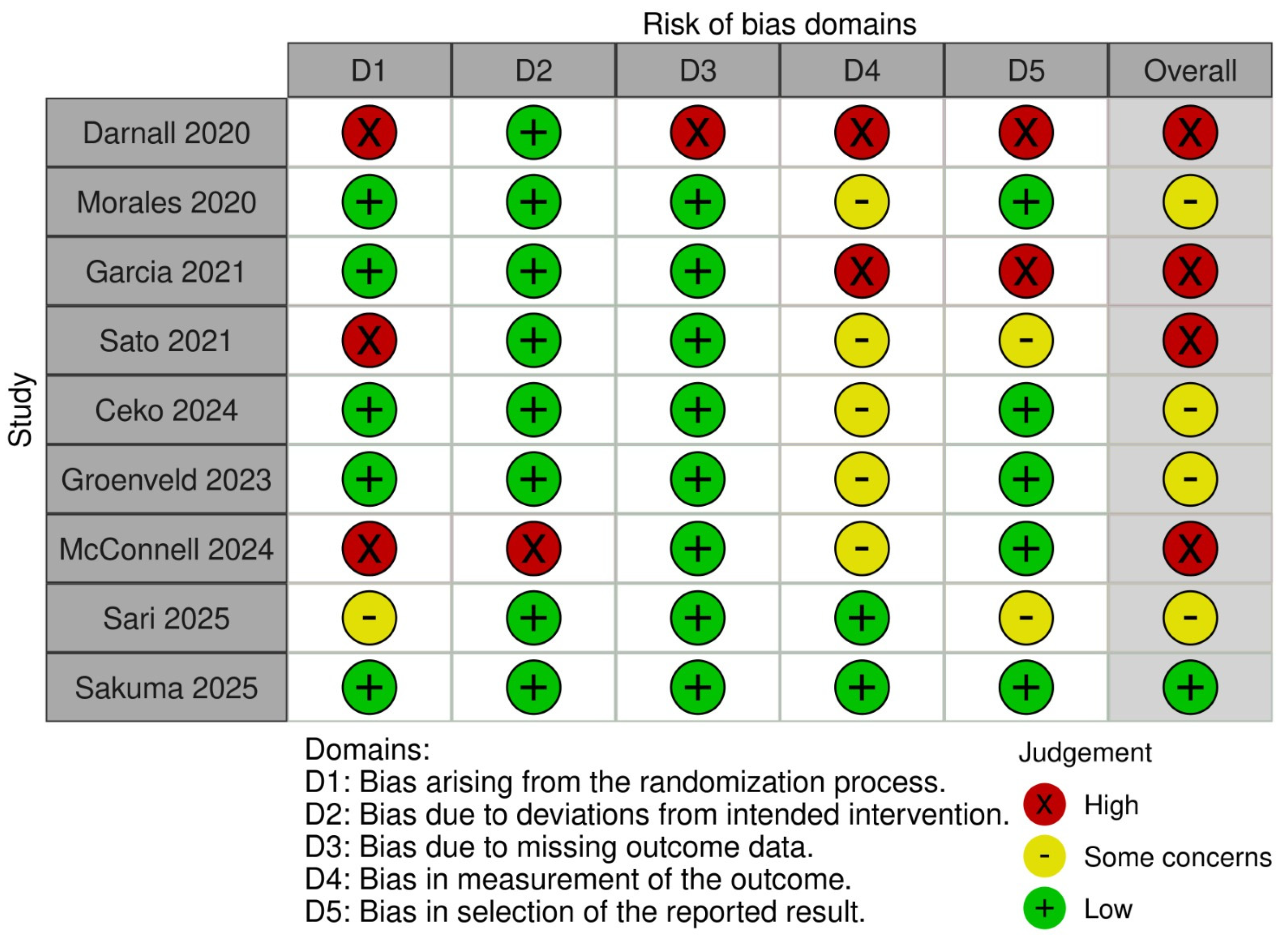
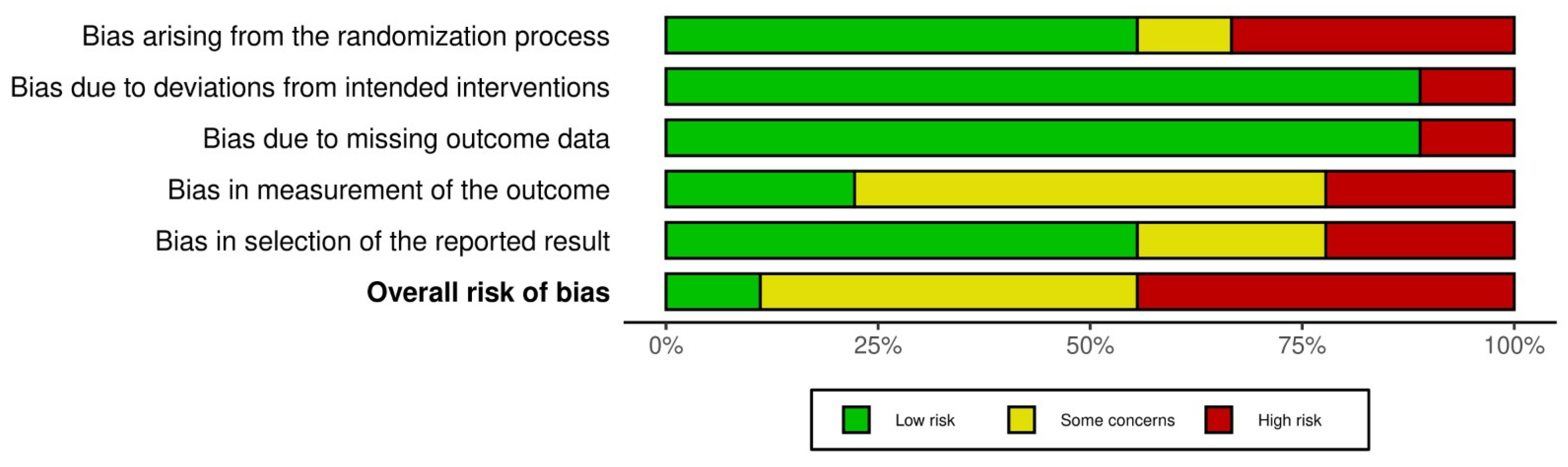
3.3. Risk of Bias
3.4. Characteristics of the Population
3.5. Characteristics of Interventions and Outcome Measures
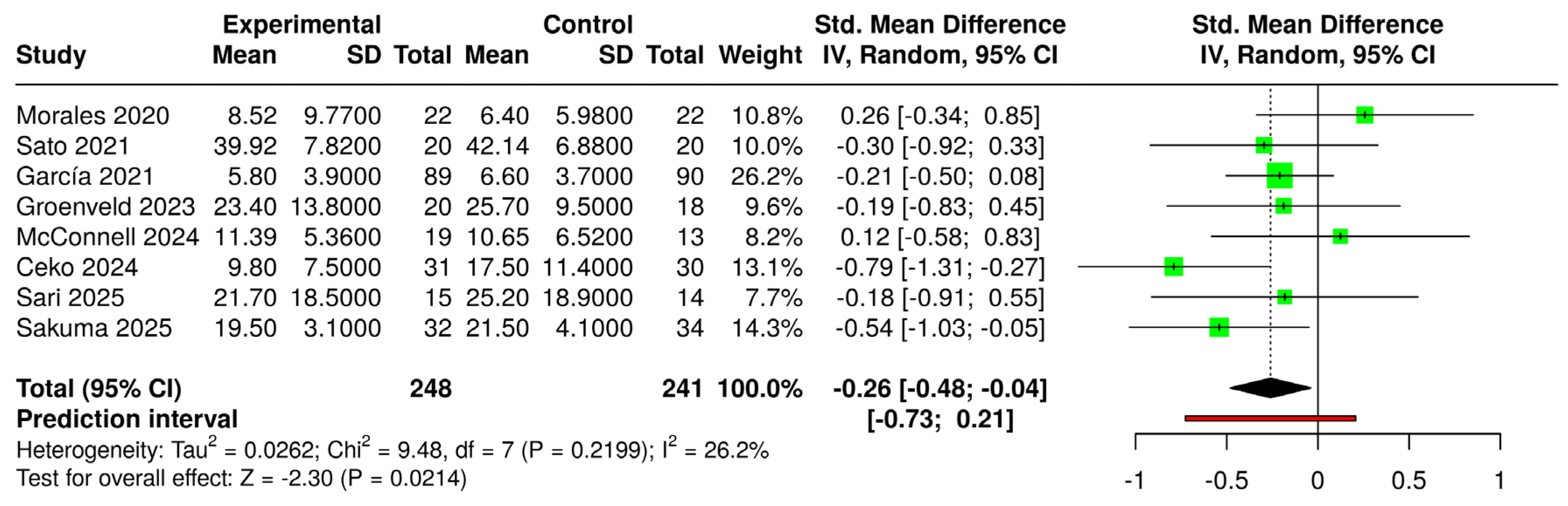
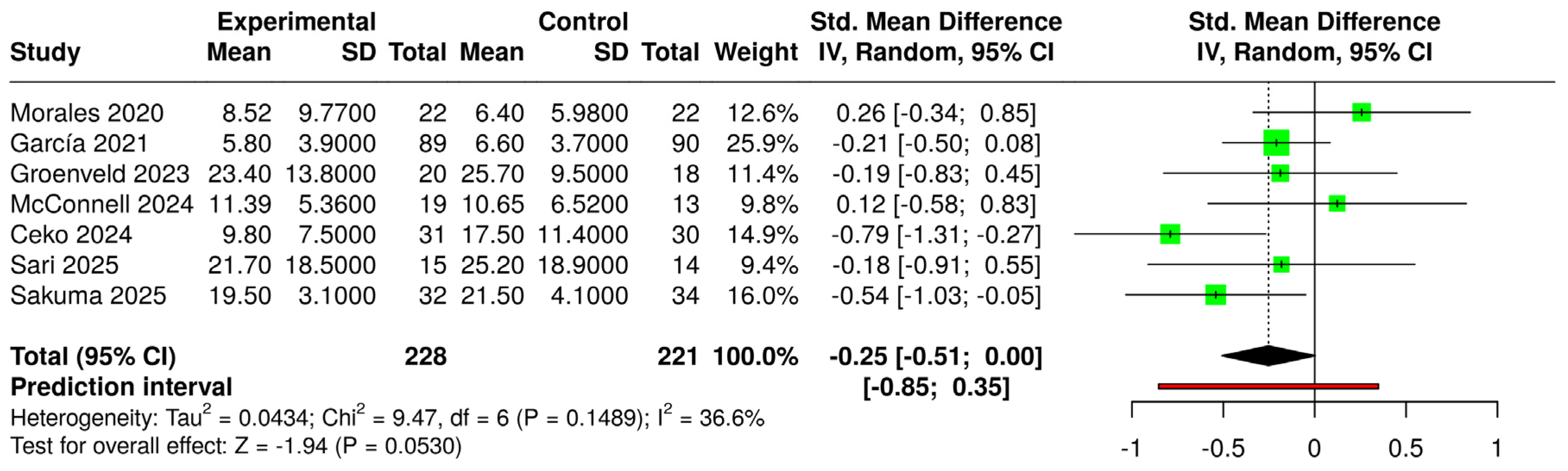
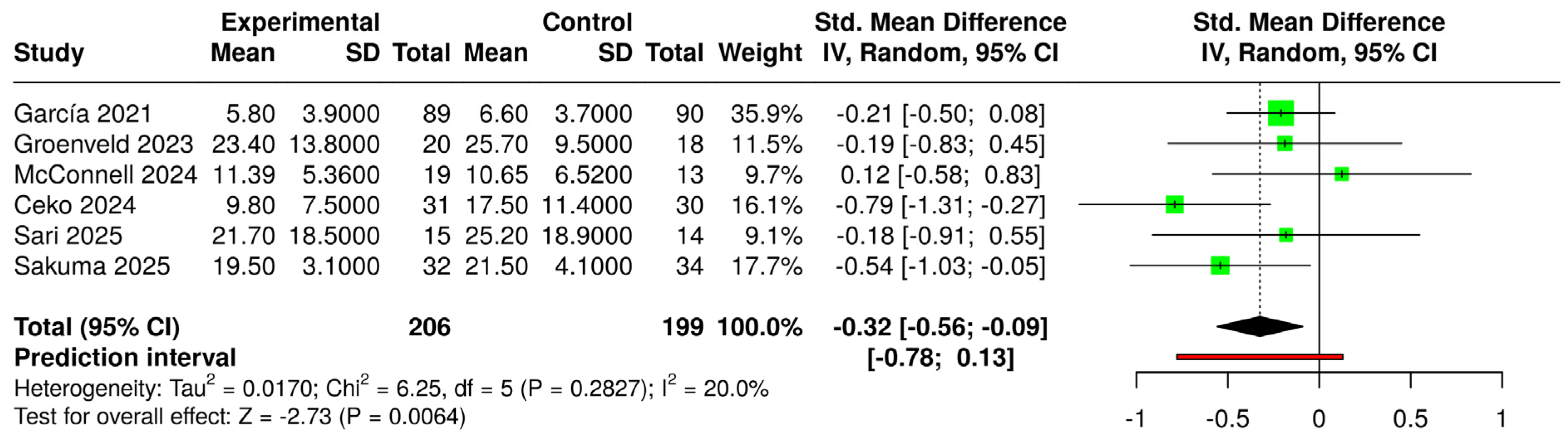
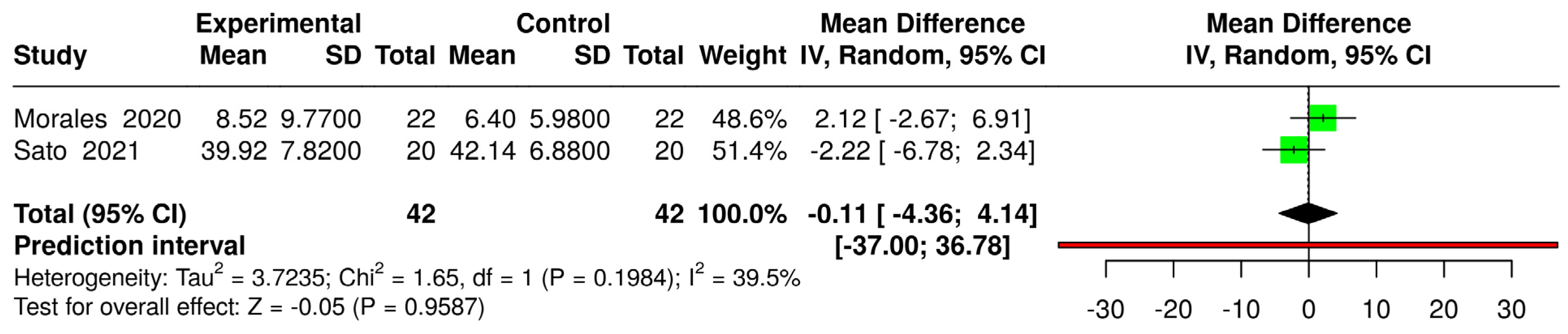
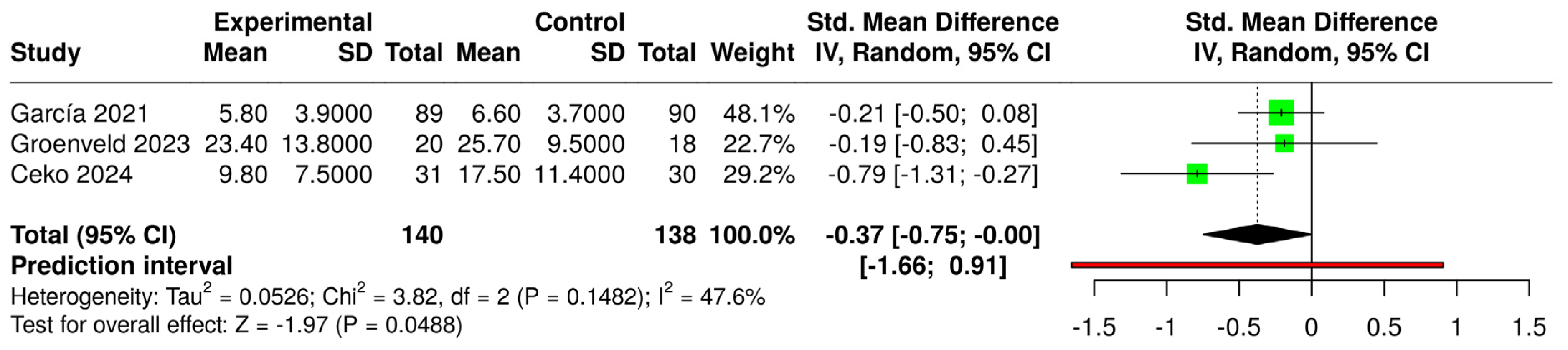
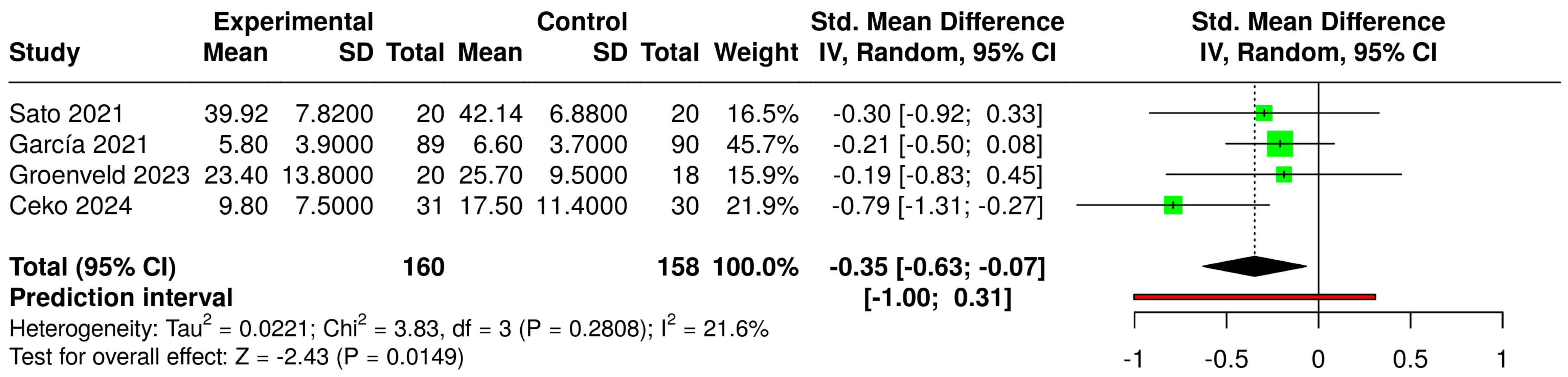
3.6. Effects of Interventions and Heterogeneity
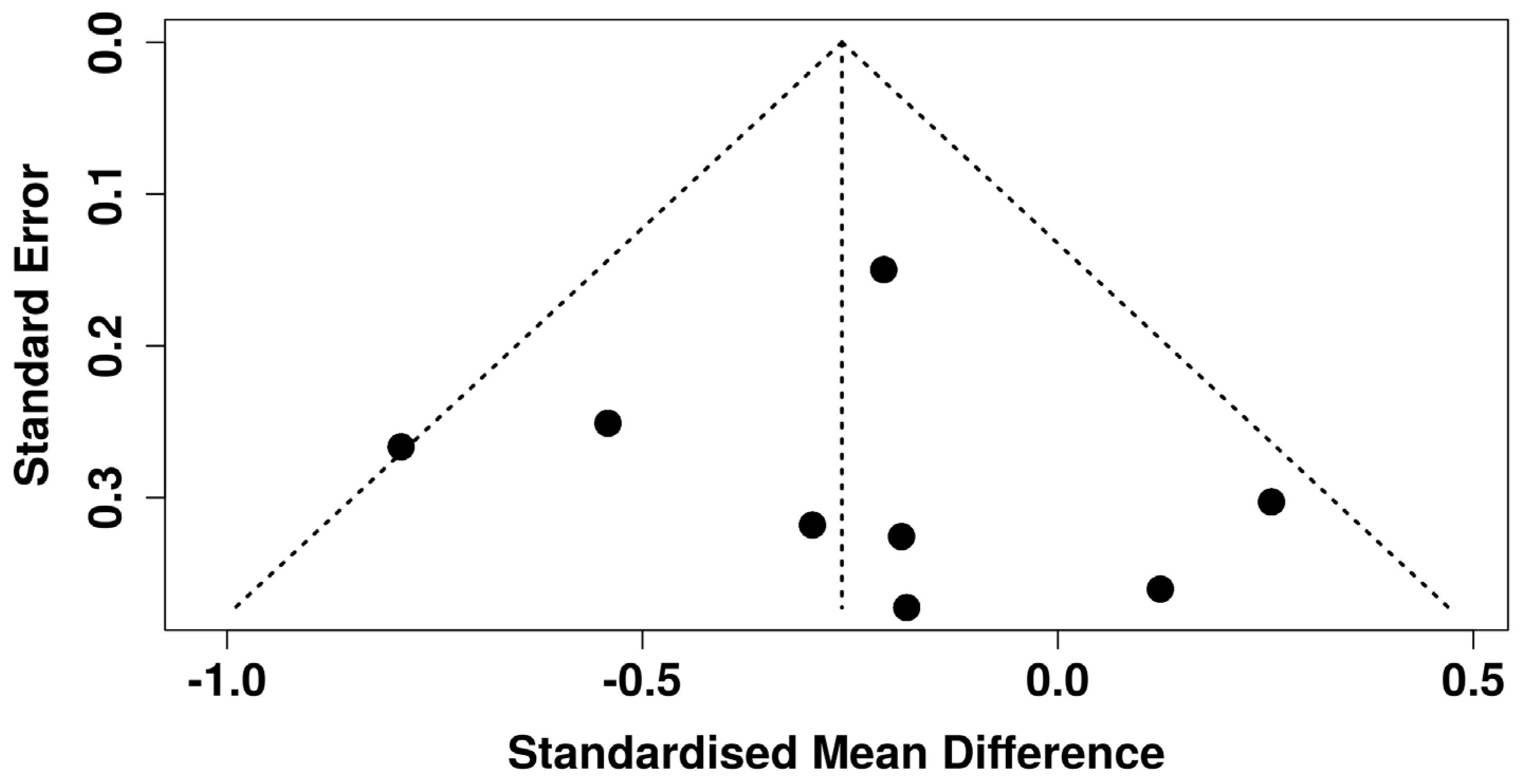
3.7. Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IASP | International Association for the Study of Pain |
| PCS | Pain catastrophizing scale |
| VR | Virtual reality |
| RCT | Randomized clinical trial |
| RCTs | Randomized controlled trials |
| RoB | Risk of bias |
| ES | Effect size |
| SMD | Standardized mean difference |
| CBT | Cognitive behavioral therapy |
| CLBP | Chronic low back pain |
| PNE | Pain neuroscience education |
References
- Zimmer, Z.; Fraser, K.; Grol-Prokopczyk, H.; Zajacova, A. A Global Study of Pain Prevalence across 52 Countries: Examining the Role of Country-Level Contextual Factors. Pain 2022, 163, 1740–1750. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.P.; Vase, L.; Hooten, W.M. Chronic Pain: An Update on Burden, Best Practices, and New Advances. Lancet 2021, 397, 2082–2097. [Google Scholar] [CrossRef] [PubMed]
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.J.; Mogil, J.S.; Ringkamp, M.; Sluka, K.A.; et al. The Revised International Association for the Study of Pain Definition of Pain: Concepts, Challenges, and Compromises. Pain 2020, 161, 1976–1982. [Google Scholar] [CrossRef]
- Treede, R.-D.; Rief, W.; Barke, A.; Aziz, Q.; Bennett, M.I.; Benoliel, R.; Cohen, M.; Evers, S.; Finnerup, N.B.; First, M.B.; et al. Chronic Pain as a Symptom or a Disease: The IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11). Pain 2019, 160, 19–27. [Google Scholar] [CrossRef]
- Vlaeyen, J.W.S.; Linton, S.J. Fear-Avoidance and Its Consequences in Chronic Musculoskeletal Pain: A State of the Art. Pain 2000, 85, 317–332. [Google Scholar] [CrossRef]
- Sullivan, M.J.L.; Tripp, D.A. Pain Catastrophizing: Controversies, Misconceptions and Future Directions. J. Pain 2024, 25, 575–587. [Google Scholar] [CrossRef]
- Petrini, L.; Arendt-Nielsen, L. Understanding Pain Catastrophizing: Putting Pieces Together. Front. Psychol. 2020, 11, 603420. [Google Scholar] [CrossRef]
- Lethem, J.; Slade, P.D.; Troup, J.D.G.; Bentley, G. Outline of a Fear-Avoidance Model of Exaggerated Pain Perception—I. Behav. Res. Ther. 1983, 21, 401–408. [Google Scholar] [CrossRef]
- Vlaeyen, J.W.S.; Kole-Snijders, A.M.J.; Boeren, R.G.B.; Van Eek, H. Fear of Movement/(Re)Injury in Chronic Low Back Pain and Its Relation to Behavioral Performance. Pain 1995, 62, 363–372. [Google Scholar] [CrossRef]
- Vlaeyen, J.W.S.; Linton, S.J. Fear-Avoidance Model of Chronic Musculoskeletal Pain: 12 Years On. Pain 2012, 153, 1144–1147. [Google Scholar] [CrossRef]
- Sullivan, M.J.L.; Thorn, B.; Haythornthwaite, J.A.; Keefe, F.; Martin, M.; Bradley, L.A.; Lefebvre, J.C. Theoretical Perspectives on the Relation Between Catastrophizing and Pain. Clin. J. Pain 2001, 17, 52. [Google Scholar] [CrossRef]
- Martinez-Calderon, J.; Jensen, M.P.; Morales-Asencio, J.M.; Luque-Suarez, A. Pain Catastrophizing and Function In Individuals With Chronic Musculoskeletal Pain: A Systematic Review and Meta-Analysis. Clin. J. Pain 2019, 35, 279–293. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, M.J.L.; Bishop, S.R.; Pivik, J. The Pain Catastrophizing Scale: Development and Validation. Psychol. Assess. 1995, 7, 524–532. [Google Scholar] [CrossRef]
- Vlaeyen, J.W.S.; Crombez, G.; Linton, S.J. The Fear-Avoidance Model of Pain. Pain 2016, 157, 1588–1589. [Google Scholar] [CrossRef]
- Alcon, C.; Bergman, E.; Humphrey, J.; Patel, R.M.; Wang-Price, S. The Relationship between Pain Catastrophizing and Cognitive Function in Chronic Musculoskeletal Pain: A Scoping Review. Pain. Res. Manag. 2023, 2023, 5851450. [Google Scholar] [CrossRef]
- Carvalho, S.; Martins, C.P.; Almeida, H.S.; Silva, F. The Evolution of Cognitive Behavioural Therapy—The Third Generation and Its Effectiveness. Eur. Psychiatry 2017, 41, S773–S774. [Google Scholar] [CrossRef]
- Schütze, R.; Rees, C.; Smith, A.; Slater, H.; Campbell, J.M.; O’Sullivan, P. How Can We Best Reduce Pain Catastrophizing in Adults With Chronic Noncancer Pain? A Systematic Review and Meta-Analysis. J. Pain 2018, 19, 233–256. [Google Scholar] [CrossRef]
- Ahmed, H.; Mushahid, H.; Shuja, M.H. Virtual Reality Therapy: A Promising Solution to Chronic Pain Management amidst an Opioid Crisis. J. Glob. Health 2023, 13, 03033. [Google Scholar] [CrossRef]
- Wong, K.P.; Tse, M.M.Y.; Qin, J. Effectiveness of Virtual Reality-Based Interventions for Managing Chronic Pain on Pain Reduction, Anxiety, Depression and Mood: A Systematic Review. Healthcare 2022, 10, 2047. [Google Scholar] [CrossRef]
- Goudman, L.; Jansen, J.; Billot, M.; Vets, N.; De Smedt, A.; Roulaud, M.; Rigoard, P.; Moens, M. Virtual Reality Applications in Chronic Pain Management: Systematic Review and Meta-Analysis. JMIR Serious Games 2022, 10, e34402. [Google Scholar] [CrossRef] [PubMed]
- Bilika, P.; Karampatsou, N.; Stavrakakis, G.; Paliouras, A.; Theodorakis, Y.; Strimpakos, N.; Kapreli, E. Virtual Reality-Based Exercise Therapy for Patients with Chronic Musculoskeletal Pain: A Scoping Review. Healthcare 2023, 11, 2412. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Shimizu, K.; Shiko, Y.; Kawasaki, Y.; Orita, S.; Inage, K.; Shiga, Y.; Suzuki, M.; Sato, M.; Enomoto, K.; et al. Effects of Nintendo Ring Fit Adventure Exergame on Pain and Psychological Factors in Patients with Chronic Low Back Pain. Games Health J. 2021, 10, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Čeko, M.; Baeuerle, T.; Webster, L.; Wager, T.D.; Lumley, M.A. The Effects of Virtual Reality Neuroscience-Based Therapy on Clinical and Neuroimaging Outcomes in Patients with Chronic Back Pain: A Randomized Clinical Trial. Pain 2024, 165, 1860–1874. [Google Scholar] [CrossRef]
- Amorim, P. Virtual Reality in Chronic Pain Rehabilitation: A Systematic Review. J. Phys. Med. Rehabil. 2025, 7, 14–78. [Google Scholar] [CrossRef]
- Brea-Gómez, B.; Laguna-González, A.; Pérez-Gisbert, L.; Valenza, M.C.; Torres-Sánchez, I. Virtual Reality Based Rehabilitation in Adults with Chronic Neck Pain: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Virtual Real. 2024, 28, 86. [Google Scholar] [CrossRef]
- Brea-Gómez, B.; Torres-Sánchez, I.; Ortiz-Rubio, A.; Calvache-Mateo, A.; Cabrera-Martos, I.; López-López, L.; Valenza, M.C. Virtual Reality in the Treatment of Adults with Chronic Low Back Pain: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Int. J. Environ. Res. Public Health 2021, 18, 11806. [Google Scholar] [CrossRef]
- Gava, V.; Fialho, H.R.F.; Calixtre, L.B.; Barbosa, G.M.; Kamonseki, D.H. Effects of Gaming on Pain-Related Fear, Pain Catastrophizing, Anxiety, and Depression in Patients with Chronic Musculoskeletal Pain: A Systematic Review and Meta-Analysis. Games Health J. 2022, 11, 369–384. [Google Scholar] [CrossRef]
- Henríquez-Jurado, J.M.; Osuna-Pérez, M.C.; García-López, H.; Lomas-Vega, R.; López-Ruiz, M.d.C.; Obrero-Gaitán, E.; Cortés-Pérez, I. Virtual Reality-Based Therapy for Chronic Low Back and Neck Pain: A Systematic Review with Meta-Analysis. EFORT Open Rev. 2024, 9, 685–699. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Methley, A.M.; Campbell, S.; Chew-Graham, C.; McNally, R.; Cheraghi-Sohi, S. PICO, PICOS and SPIDER: A Comparison Study of Specificity and Sensitivity in Three Search Tools for Qualitative Systematic Reviews. BMC Health Serv. Res. 2014, 14, 579. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A Web and Mobile App for Systematic Reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- Landis, J.R.; Koch, G.G. The Measurement of Observer Agreement for Categorical Data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Savović, J.; Page, M.J.; Elbers, R.G.; Sterne, J.A. Assessing Risk of Bias in a Randomized Trial. In Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2019; pp. 205–228. ISBN 978-1-119-53660-4. [Google Scholar]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-Bias VISualization (Robvis): An R Package and Shiny Web App for Visualizing Risk-of-Bias Assessments. Res. Synth. Methods 2021, 12, 55–61. [Google Scholar] [CrossRef]
- Fekete, J.T.; Győrffy, B. MetaAnalysisOnline.Com: Web-Based Tool for the Rapid Meta-Analysis of Clinical and Epidemiological Studies. J. Med. Internet Res. 2025, 27, e64016. [Google Scholar] [CrossRef]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the Sample Mean and Standard Deviation from the Sample Size, Median, Range and/or Interquartile Range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef]
- Luo, D.; Wan, X.; Liu, J.; Tong, T. Optimally Estimating the Sample Mean from the Sample Size, Median, Mid-Range, and/or Mid-Quartile Range. Stat. Methods Med. Res. 2018, 27, 1785–1805. [Google Scholar] [CrossRef]
- Higgins, J.P.; Li, T.; Deeks, J.J. Choosing Effect Measures and Computing Estimates of Effect. In Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2019; pp. 143–176. ISBN 978-1-119-53660-4. [Google Scholar]
- Morales, D.; Beltran-Alacreu, H.; Cano-de-la-Cuerda, R.; Leon Hernández, J.V.; Martín-Pintado-Zugasti, A.; Calvo-Lobo, C.; Gil-Martínez, A.; Fernández-Carnero, J. Effects of Virtual Reality versus Exercise on Pain, Functional, Somatosensory and Psychosocial Outcomes in Patients with Non-Specific Chronic Neck Pain: A Randomized Clinical Trial. Int. J. Environ. Res. Public Health 2020, 17, 5950. [Google Scholar] [CrossRef]
- Garcia, L.M.; Birckhead, B.J.; Krishnamurthy, P.; Sackman, J.; Mackey, I.G.; Louis, R.G.; Salmasi, V.; Maddox, T.; Darnall, B.D. An 8-Week Self-Administered At-Home Behavioral Skills-Based Virtual Reality Program for Chronic Low Back Pain: Double-Blind, Randomized, Placebo-Controlled Trial Conducted During COVID-19. J. Med. Internet Res. 2021, 23, e26292. [Google Scholar] [CrossRef]
- Darnall, B.D.; Krishnamurthy, P.; Tsuei, J.; Minor, J.D. Self-Administered Skills-Based Virtual Reality Intervention for Chronic Pain: Randomized Controlled Pilot Study. JMIR Form. Res. 2020, 4, e17293. [Google Scholar] [CrossRef]
- Sakuma, S.; Kimpara, K.; Kawai, Y.; Yanagita, Y.; Tanaka, N.; Tawara, Y.; Matsui, G.; Terada, K.; Arizono, S. Integrating Physical Therapy and Virtual Reality to Manage Pain-Related Fear of Movement in Patients With Chronic Pain: A Randomized Controlled Trial. Cureus 2025, 17, e79551. [Google Scholar] [CrossRef]
- Sari, F.; Sudan Aran, A.; Alp, G. The Psychological and Physiological Effects of a Virtual Reality-Based Treatment Program in Female Patients with Fibromyalgia Syndrome: A Randomized Controlled Trial. Assist. Technol. 2025, 37, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Groenveld, T.D.; Smits, M.L.M.; Knoop, J.; Kallewaard, J.W.; Staal, J.B.; de Vries, M.; van Goor, H. Effect of a Behavioral Therapy-Based Virtual Reality Application on Quality of Life in Chronic Low Back Pain. Clin. J. Pain 2023, 39, 278–285. [Google Scholar] [CrossRef] [PubMed]
- McConnell, R.; Lane, E.; Webb, G.; LaPeze, D.; Grillo, H.; Fritz, J. A Multicenter Feasibility Randomized Controlled Trial Using a Virtual Reality Application of Pain Neuroscience Education for Adults with Chronic Low Back Pain. Ann. Med. 2024, 56, 2311846. [Google Scholar] [CrossRef] [PubMed]
- Haddaway, N.R.; Page, M.J.; Pritchard, C.C.; McGuinness, L.A. PRISMA2020: An R Package and Shiny App for Producing PRISMA 2020-Compliant Flow Diagrams, with Interactivity for Optimised Digital Transparency and Open Synthesis. Campbell Syst. Rev. 2022, 18, e1230. [Google Scholar] [CrossRef]
- Bot, A.G.J.; Becker, S.J.E.; Bruijnzeel, H.; Mulders, M.A.M.; Ring, D.; Vranceanu, A.-M. Creation of the Abbreviated Measures of the Pain Catastrophizing Scale and the Short Health Anxiety Inventory: The PCS-4 and SHAI-5. J. Musculoskelet. Pain 2014, 22, 145–151. [Google Scholar] [CrossRef]
- Rose, T.; Nam, C.S.; Chen, K.B. Immersion of Virtual Reality for Rehabilitation—Review. Appl. Ergon. 2018, 69, 153–161. [Google Scholar] [CrossRef]
- Brady, N.; McVeigh, J.G.; McCreesh, K.; Rio, E.; Dekkers, T.; Lewis, J.S. Exploring the Effectiveness of Immersive Virtual Reality Interventions in the Management of Musculoskeletal Pain: A State-of-the-Art Review. Phys. Ther. Rev. 2021, 26, 262–275. [Google Scholar] [CrossRef]
- Asadzadeh, A.; Samad-Soltani, T.; Salahzadeh, Z.; Rezaei-Hachesu, P. Effectiveness of Virtual Reality-Based Exercise Therapy in Rehabilitation: A Scoping Review. Inform. Med. Unlocked 2021, 24, 100562. [Google Scholar] [CrossRef]
- Wiederhold, B.K.; Gao, K.; Sulea, C.; Wiederhold, M.D. Virtual Reality as a Distraction Technique in Chronic Pain Patients. Cyberpsychol. Behav. Soc. Netw. 2014, 17, 346–352. [Google Scholar] [CrossRef]
- Trost, Z.; France, C.; Anam, M.; Shum, C. Virtual Reality Approaches to Pain: Toward a State of the Science. Pain 2021, 162, 325. [Google Scholar] [CrossRef]
- Ahern, M.M.; Dean, L.V.; Stoddard, C.C.; Agrawal, A.; Kim, K.; Cook, C.E.; Narciso Garcia, A. The Effectiveness of Virtual Reality in Patients With Spinal Pain: A Systematic Review and Meta-Analysis. Pain Pract. 2020, 20, 656–675. [Google Scholar] [CrossRef] [PubMed]
- Viderman, D.; Tapinova, K.; Dossov, M.; Seitenov, S.; Abdildin, Y.G. Virtual Reality for Pain Management: An Umbrella Review. Front. Med. 2023, 10, 1203670. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Scott, K.; Dukewich, M. Innovative Technology Using Virtual Reality in the Treatment of Pain: Does It Reduce Pain via Distraction, or Is There More to It? Pain Med. 2018, 19, 151–159. [Google Scholar] [CrossRef]
- Tack, C. Virtual Reality and Chronic Low Back Pain. Disabil. Rehabil. Assist. Technol. 2021, 16, 637–645. [Google Scholar] [CrossRef]
- Li, R.; Li, Y.; Kong, Y.; Li, H.; Hu, D.; Fu, C.; Wei, Q. Virtual Reality–Based Training in Chronic Low Back Pain: Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Med. Internet Res. 2024, 26, e45406. [Google Scholar] [CrossRef]
- Dy, M.; Olazo, K.; Lisker, S.; Brown, E.; Saha, A.; Weinberg, J.; Sarkar, U. Virtual Reality for Chronic Pain Management Among Historically Marginalized Populations: Systematic Review of Usability Studies. J. Med. Internet Res. 2023, 25, e40044. [Google Scholar] [CrossRef]
- Matthie, N.S.; Giordano, N.A.; Jenerette, C.M.; Magwood, G.S.; Leslie, S.L.; Northey, E.E.; Webster, C.I.; Sil, S. Use and Efficacy of Virtual, Augmented, or Mixed Reality Technology for Chronic Pain: A Systematic Review. Pain Manag. 2022, 12, 859–878. [Google Scholar] [CrossRef]
- Alemanno, F.; Houdayer, E.; Emedoli, D.; Locatelli, M.; Mortini, P.; Mandelli, C.; Raggi, A.; Iannaccone, S. Efficacy of Virtual Reality to Reduce Chronic Low Back Pain: Proof-of-Concept of a Non-Pharmacological Approach on Pain, Quality of Life, Neuropsychological and Functional Outcome. PLoS ONE 2019, 14, e0216858. [Google Scholar] [CrossRef]
- Igna, R.; Stefan, S.; Onac, I.; Onac, I.; Ungur, R.-A.; Tatar, A.S. Mindfulness-Based Cognitive-Behavior Therapy (Mcbt) Versus Virtual Reality (vr) Enhanced Cbt, Versus Treatment as Usual for Chronic Back Pain. a Clinical Trial. J. Evid. Based Psychother. 2014, 14, 229–247. [Google Scholar]
- Georgescu, R.; Fodor, L.A.; Dobrean, A.; Cristea, I.A. Psychological Interventions Using Virtual Reality for Pain Associated with Medical Procedures: A Systematic Review and Meta-Analysis. Psychol. Med. 2020, 50, 1795–1807. [Google Scholar] [CrossRef]
- Arnow, B.A.; Blasey, C.M.; Constantino, M.J.; Robinson, R.; Hunkeler, E.; Lee, J.; Fireman, B.; Khaylis, A.; Feiner, L.; Hayward, C. Catastrophizing, Depression and Pain-Related Disability. Gen. Hosp. Psychiatry 2011, 33, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Vowles, K.E.; McCracken, L.M.; Eccleston, C. Patient Functioning and Catastrophizing in Chronic Pain: The Mediating Effects of Acceptance. Health Psychol. Off. J. Div. Health Psychol. Am. Psychol. Assoc. 2008, 27, S136–S143. [Google Scholar] [CrossRef] [PubMed]
- Lazaridou, A.; Franceschelli, O.; Buliteanu, A.; Cornelius, M.; Edwards, R.R.; Jamison, R.N. Influence of Catastrophizing on Pain Intensity, Disability, Side Effects, and Opioid Misuse among Pain Patients in Primary Care. J. Appl. Biobehav. Res. 2017, 22, e12081. [Google Scholar] [CrossRef]
- Racine, M.; Moulin, D.E.; Nielson, W.R.; Morley-Forster, P.K.; Lynch, M.; Clark, A.J.; Stitt, L.; Gordon, A.; Nathan, H.; Smyth, C.; et al. The Reciprocal Associations between Catastrophizing and Pain Outcomes in Patients Being Treated for Neuropathic Pain: A Cross-Lagged Panel Analysis Study. Pain 2016, 157, 1946–1953. [Google Scholar] [CrossRef]
- Simic, K.; Savic, B.; Knezevic, N.N. Pain Catastrophizing: How Far Have We Come. Neurol. Int. 2024, 16, 483–501. [Google Scholar] [CrossRef]
- Mouatt, B.; Smith, A.E.; Mellow, M.L.; Parfitt, G.; Smith, R.T.; Stanton, T.R. The Use of Virtual Reality to Influence Motivation, Affect, Enjoyment, and Engagement During Exercise: A Scoping Review. Front. Virtual Real. 2020, 1, 564664. [Google Scholar] [CrossRef]
- Osman, A.; Barrios, F.X.; Kopper, B.A.; Hauptmann, W.; Jones, J.; O’Neill, E. Factor Structure, Reliability, and Validity of the Pain Catastrophizing Scale. J. Behav. Med. 1997, 20, 589–605. [Google Scholar] [CrossRef]
- Ikemoto, T.; Hayashi, K.; Shiro, Y.; Arai, Y.C.; Marcuzzi, A.; Costa, D.; Wrigley, P. A Systematic Review of Cross-Cultural Validation of the Pain Catastrophizing Scale. Eur. J. Pain Lond. Engl. 2020, 24, 1228–1241. [Google Scholar] [CrossRef]
- Cook, K.F.; Mackey, S.; Jung, C.; Darnall, B.D. The Factor Structure and Subscale Properties of the Pain Catastrophizing Scale: Are There Differences in the Distinctions? Pain Rep. 2021, 6, e909. [Google Scholar] [CrossRef]
- Walton, D.M.; Mehta, S.; Seo, W.; MacDermid, J.C. Creation and Validation of the 4-Item BriefPCS-Chronic through Methodological Triangulation. Health Qual. Life Outcomes 2020, 18, 124. [Google Scholar] [CrossRef]
- Franchignoni, F.; Giordano, A.; Ferriero, G.; Monticone, M. Measurement Precision of the Pain Catastrophizing Scale and Its Short Forms in Chronic Low Back Pain. Sci. Rep. 2022, 12, 12042. [Google Scholar] [CrossRef]

| Author | Clinical Characteristics | Age (Years), Mean (SD) | Sample (n); M/F/O | Type of Intervention | Duration; Frequency; Exercise Session Time/Repetitions | Scale | Intragroup Result (Pre-Test vs. Post-Test) | Intergroup Post-Test Result |
|---|---|---|---|---|---|---|---|---|
| Darnall et al., 2020 [41] | Males and females, aged 18–75 years, self-reported chronic nonmalignant low back pain or fibromyalgia, with an average pain intensity >4 over the past month and chronic pain duration >6 months. | G1: NR G2: NR | G1: n = 35; 26/9 G2: n = 39; 26/13 | G1: VR intervention group. Received 4–8 treatment sessions from each content category (CBT, Relaxation, Mindfulness) using a VR system (Oculus Go). G2: Audio group. Received 4–8 treatment sessions from each content category (CBT, Relaxation, Mindfulness) via audio. | G1: 21 days, 1 daily session, 1–15 min/session G2: 21 days, 1 daily session, 1–15 min/session | 4-item PCS (adapted) | Significant main effect of time for both groups (p < 0.001) (ANOVA). G1: Significant improvement (p < 0.001) G2: Significant improvement (p < 0.001) | No significant group effect (p = 0.61) (ANOVA). G1 did not differ from G2 (p = 0.61). |
| Morales et al., 2020 [39] | Males and females, aged 18–65 years, chronic non-specific neck pain (criteria NR) | G1: 32.72 (11.63) G2: 26.68 (9.21) | G1: n = 22; 11/11 G2: n = 22; 10/12 | G1: VR treatment group. Cervical exercises (flexion-extension, lateral bending, axial rotation) performed using “Fulldive VR” and “VR Ocean Aquarium 3D” (VR Vox Play system and LGQ6 smartphone). G2: Neck Exercises group. Conventional cervical exercises (flexion-extension, lateral bending, axial rotation). | G1: 4 weeks; 2 sessions/week; 3 sets/10 reps/exercise G2: 4 weeks; 2 sessions/week; 3 sets/10 reps/exercise | PCS-13 | G1: Significant improvement (p < 0.05) (d = 0.77) G2: Significant improvement (p < 0.05) (d = 0.7) | G1 did not differ from G2 (p > 0.05) |
| García et al., 2021 [40] | Males and females aged 18–85 with self-reported CLBP without radicular symptoms, pain duration ≥6 months, and pain intensity ≥4 on the DVPRS scale | G1: 51.5 (13.5) G2: 51.4 (12.9) | G1: n = 89; 22/67 G2: n = 90; 19/70/1 | G1: Therapeutic VR. CBT, mindfulness, PNE, BPS education, breathing-relaxation exercises, executive function exercises (EaseVRx/Applied VR). G2: Sham VR. Non-immersive, non-interactive 2D VR with 20 nature scenes and neutral music. | G1: 56 days; 1 daily session; 2–16 min/session G2: 56 days; 1 daily session; 2–16 min/session | 4-item PCS (adapted) | G1: No significant improvement (p > 0.05); G2: No significant improvement (p > 0.05) | G1 did not differ from G2 (p > 0.05) |
| Sato et al., 2021 [22] | Males and females with CLBP (≥3 months), referred to the hospital without response to previous conservative treatment | G1: 49.31 (12.59) G2: 55.61 (10.96) | G1: n = 20; 9/11 G2: n = 20; 12/8 | G1: Ring Fit Adventure (RFA) group. Performed dynamic physical exercises using RFA games on a non-immersive VR system (NIVR, Nintendo Switch) while maintaining prescribed medication. G2: Control group. Continued prescribed medication plus NSAIDs, Tramadol, and Duloxetine (dose controlled bi-weekly based on interview results). | G1: 8 weeks; 1 session/week; 40 min/session G2: 8 weeks; medication + NSAIDs, Tramadol, Duloxetine | PCS-13 | G1: No significant improvement (p > 0.05); G2: No significant improvement (p > 0.05) | G1 did not differ from G2 (p > 0.05) |
| Groenveld et al., 2023 [44] | Males and females aged 18+ years with non-specific CLBP of intensity ≥4 on an 11-point Likert scale, without radicular pain worse than CLBP, and no treatment other than analgesics or physical therapy | G1: 51.0 (2.9) G2: 52.0 (2.5) | G1: n = 20; 3/17 G2: n = 20; 4/16 | G1: Cognitive-behavioral therapy via the Reducept VR app (Oculus Go). Therapy included 5 psychological treatment games (acceptance and commitment, mindfulness, hypnotherapy, eye movement desensitization and reprocessing) with educational content on maladaptive CNS changes. G2: Control group. Waitlisted for advanced pain treatment, no intervention. | G1: 4 weeks; up to 3 daily sessions; at least 10 min/day, maximum 30 min/session G2: NA | PCS-13 | NR | G1 did not differ from G2 (p > 0.05) |
| Čeko et al., 2024 [23] | Males and females aged 21–70 years, CLBP (present for at least 50% of the past 6 months) with an average pain intensity ≥4/10 | G1: 34.8 (9.9) G2: 33.5 (9.2) | G1: n = 31; 16/15 G2: n = 30; 15/15 | G1: Neuroscience-based VR therapy group (VRNT). Received pain neuroscience education plus cognitive, behavioral, and affective exercises via an immersive VR system (Samsung Gear VR + Samsung Galaxy S-9 phone). No physical exercise included. G2: Control group. Waitlisted or usual care. | G1: 8 weeks; 10 weekly sessions (2/day, 5 days); 20 min/session (7–27 min) G2: NA | PCS-13 | Intragroup result not specified, likely ANOVA | “Compared to the Control condition, the VRNT group showed significant improvements in pain catastrophizing at post-treatment (condition by time interaction controlled for age and sex: g = 0.86, p = 0.002)” |
| McConnell et al., 2024 [45] | Males and females aged 18–75 years with low back pain lasting ≥12 weeks, no relevant comorbidities, no spine surgery in the past 12 months | G1: 48.2 (12.7) G2: 43.3 (17.4) | G1: n = 19; 8/11 G2: n = 13; 4/9 | G1: VR pain neuroscience education (VR-PNE) group. Received standard PT plus VR-based pain neuroscience education via the “PNE 2.0” software administered through PICO G2 4K. Therapy included education, patient testimonials, emotional regulation exercises, breathing, and meditation. G2: PT group. Standard PT only. | G1: 6 weeks; variable frequency; 21 min/session on average G2: 6 weeks; frequency and session duration as needed | PCS-13 | NR | G1 did not differ from G2 (p > 0.05) |
| Sari et al., 2025 [43] | Females diagnosed with fibromyalgia for at least 1 year, aged 18–65 years, with stable health status over the previous 6 months. Patients with physical, neurological, or psychiatric conditions interfering with treatment were excluded. | G1: 38.8 (5.63) G2: 39.21 (8.42) | G1: n = 15 G2: n = 14 | G1: Exposure to a relaxing virtual environment using Oculus Quest 2. The session included video with relaxing sounds simulating a walk through an animated natural forest. G2: Progressive muscle relaxation and breathing exercises without VR. | G1: 4 weeks; 1 session per week; 30 min per session G2: 4 weeks; 1 session per week; 30 min per session | PCS-13 | G1: Significant improvement (p < 0.05, d = 0.59). G2: No significant improvement (p > 0.05, d = 0.04) | Significant difference in favor of G1 (p = 0.002, d = 1.271) |
| Sakuma et al., 2025 [42] | Males and females aged 18–75 years, employed, with chronic pain (≥3 months) and average pain intensity ≥ 4/10. Exclusion criteria included acute pain, cognitive impairment, or physical incompatibility with VR. | G1: 52.6 (10.9) G2: 56.8 (14.1) | G1: n = 32; 18/14 G2: n = 34; 14/20 | G1: Simulated forest walk using Oculus Quest 2 followed by a supervised exercise program (stretching, strengthening, aerobic activity). Load was progressive and individualized. Home-based exercises were also prescribed. G2: Same exercise protocol without VR exposure. | G1: 12 weeks; 1 weekly VR session (10 min) + 1–2 weekly supervised exercise sessions (40 min/session: 20 min of strengthening/flexibility and 20 min of treadmill or ergometer). G2: 1–2 weekly supervised exercise sessions (40 min/session: 20 min of strengthening/flexibility and 20 min of treadmill or ergometer). | PCS-13 | G1: Significant improvement (p < 0.001, d = 1.13). G2: No significant improvement (p > 0.05, d = 0.04) | G1 did not differ from G2 (p > 0.05) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvajal-Parodi, C.; Rossel, P.O.; Rodríguez-Alvarado, A.; Guede-Rojas, F.; Ponce-González, J.G. Effects of Virtual Reality-Based Interventions on Pain Catastrophizing in People with Chronic Pain: A Systematic Review and Meta-Analysis. J. Clin. Med. 2025, 14, 3782. https://doi.org/10.3390/jcm14113782
Carvajal-Parodi C, Rossel PO, Rodríguez-Alvarado A, Guede-Rojas F, Ponce-González JG. Effects of Virtual Reality-Based Interventions on Pain Catastrophizing in People with Chronic Pain: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2025; 14(11):3782. https://doi.org/10.3390/jcm14113782
Chicago/Turabian StyleCarvajal-Parodi, Claudio, Pedro O. Rossel, Alejandra Rodríguez-Alvarado, Francisco Guede-Rojas, and Jesús G. Ponce-González. 2025. "Effects of Virtual Reality-Based Interventions on Pain Catastrophizing in People with Chronic Pain: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 14, no. 11: 3782. https://doi.org/10.3390/jcm14113782
APA StyleCarvajal-Parodi, C., Rossel, P. O., Rodríguez-Alvarado, A., Guede-Rojas, F., & Ponce-González, J. G. (2025). Effects of Virtual Reality-Based Interventions on Pain Catastrophizing in People with Chronic Pain: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 14(11), 3782. https://doi.org/10.3390/jcm14113782