Cervical Dysplasia and Cervical Cancer During Pregnancy: From Pathogenesis to Clinical Management
Abstract
1. Introduction
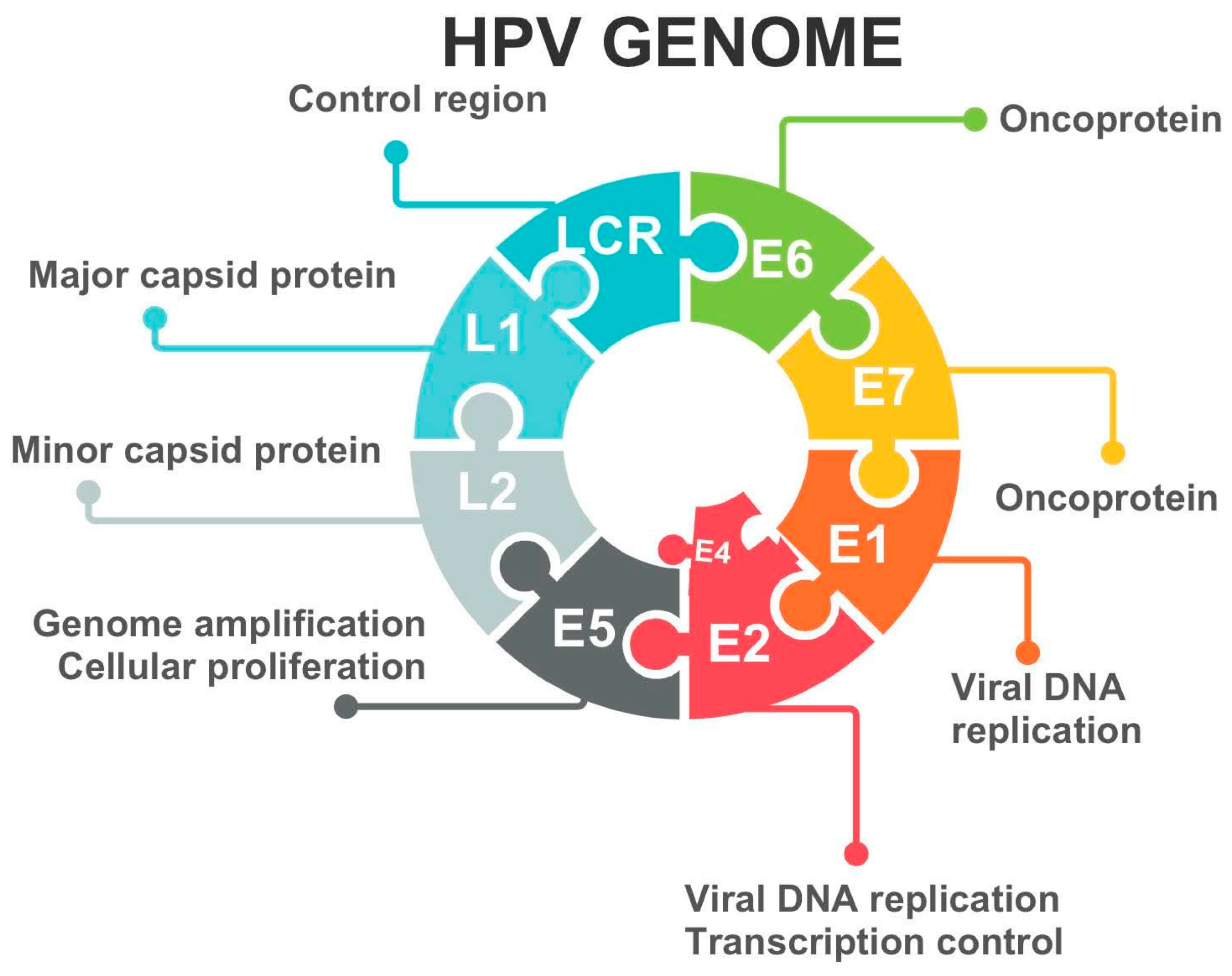
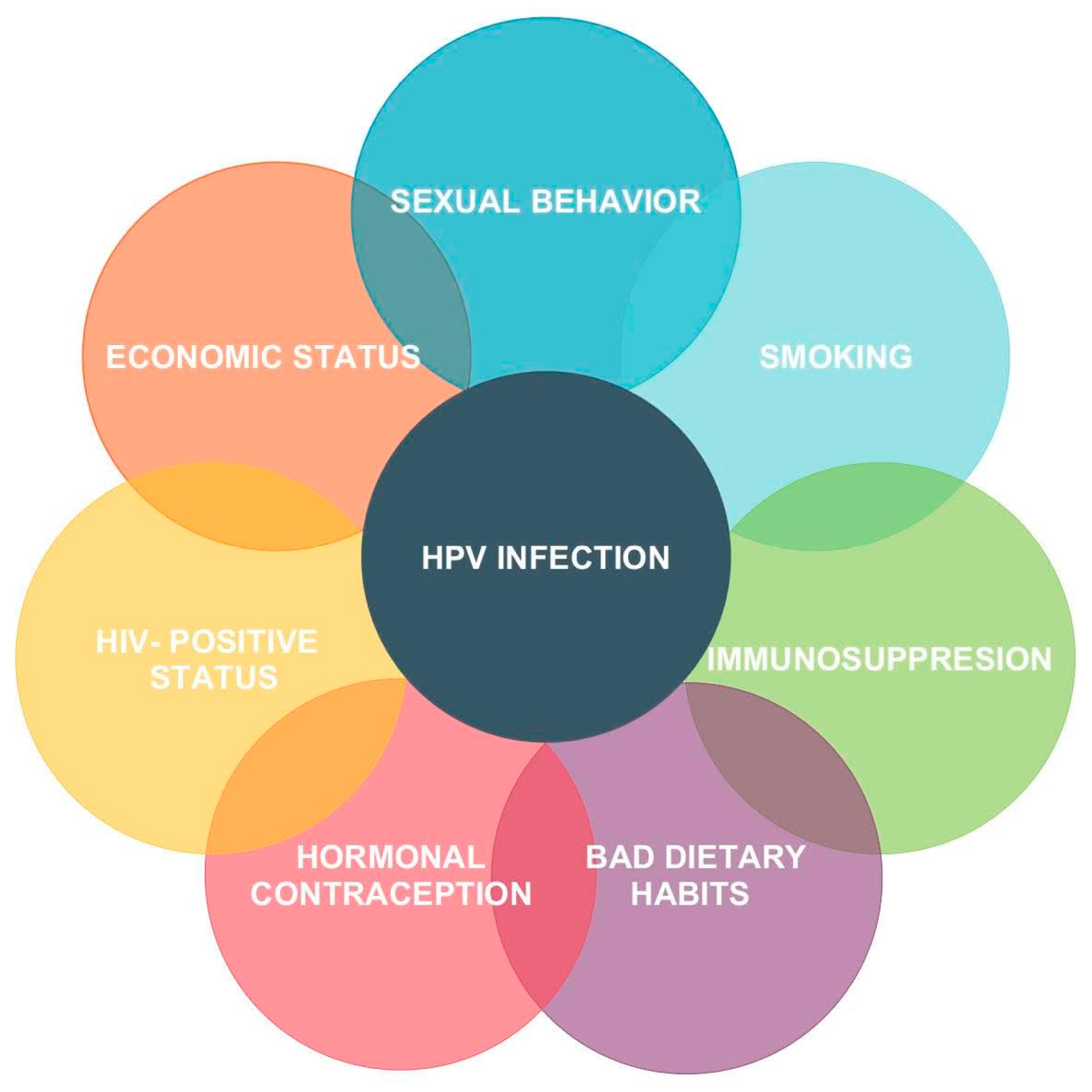
2. Etiology of Cervical Dysplasia
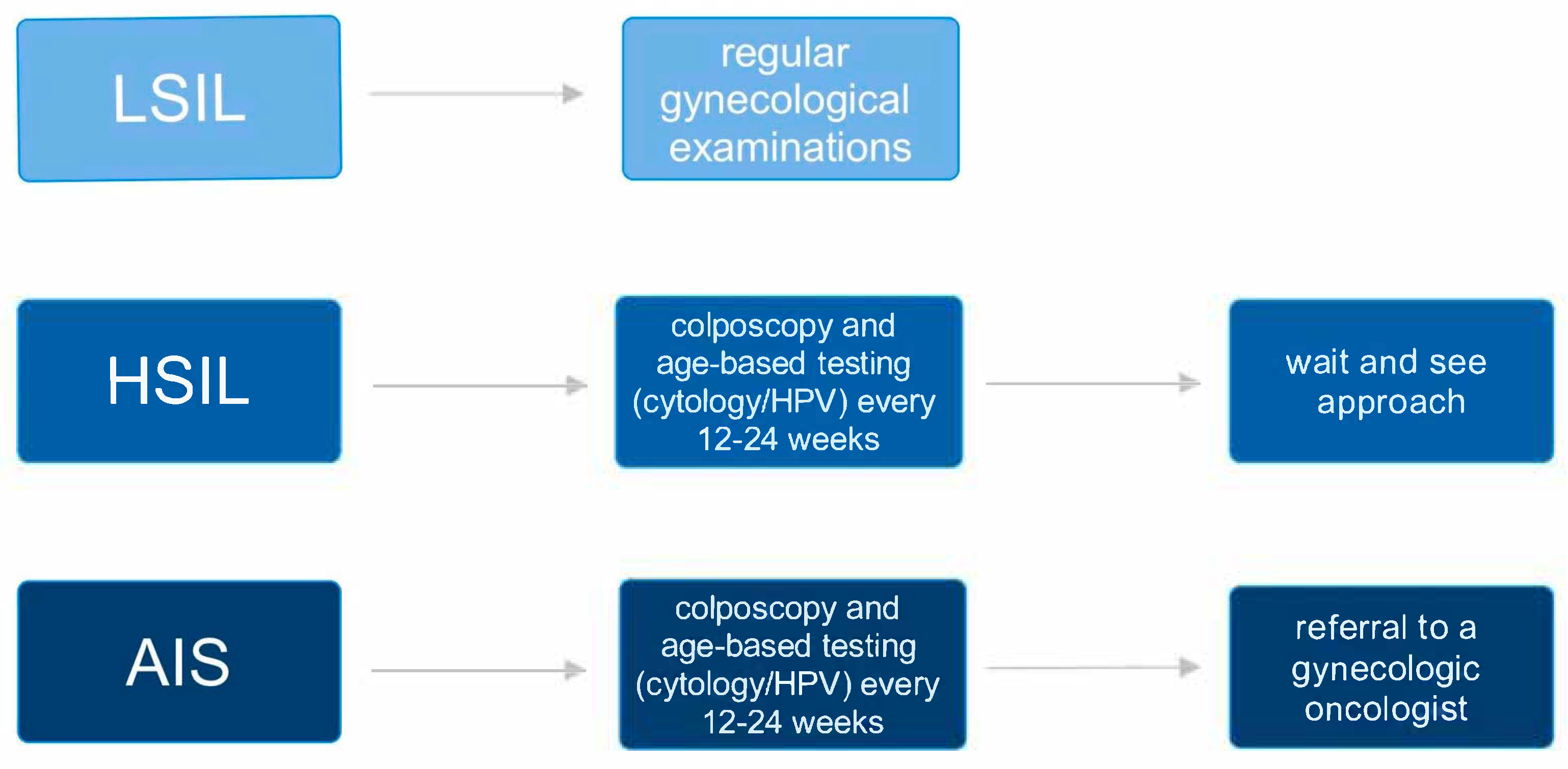
3. Squamous Intraepithelial Lesion in Pregnancy: Diagnostic and Therapeutic Management
4. Cervical Cancer
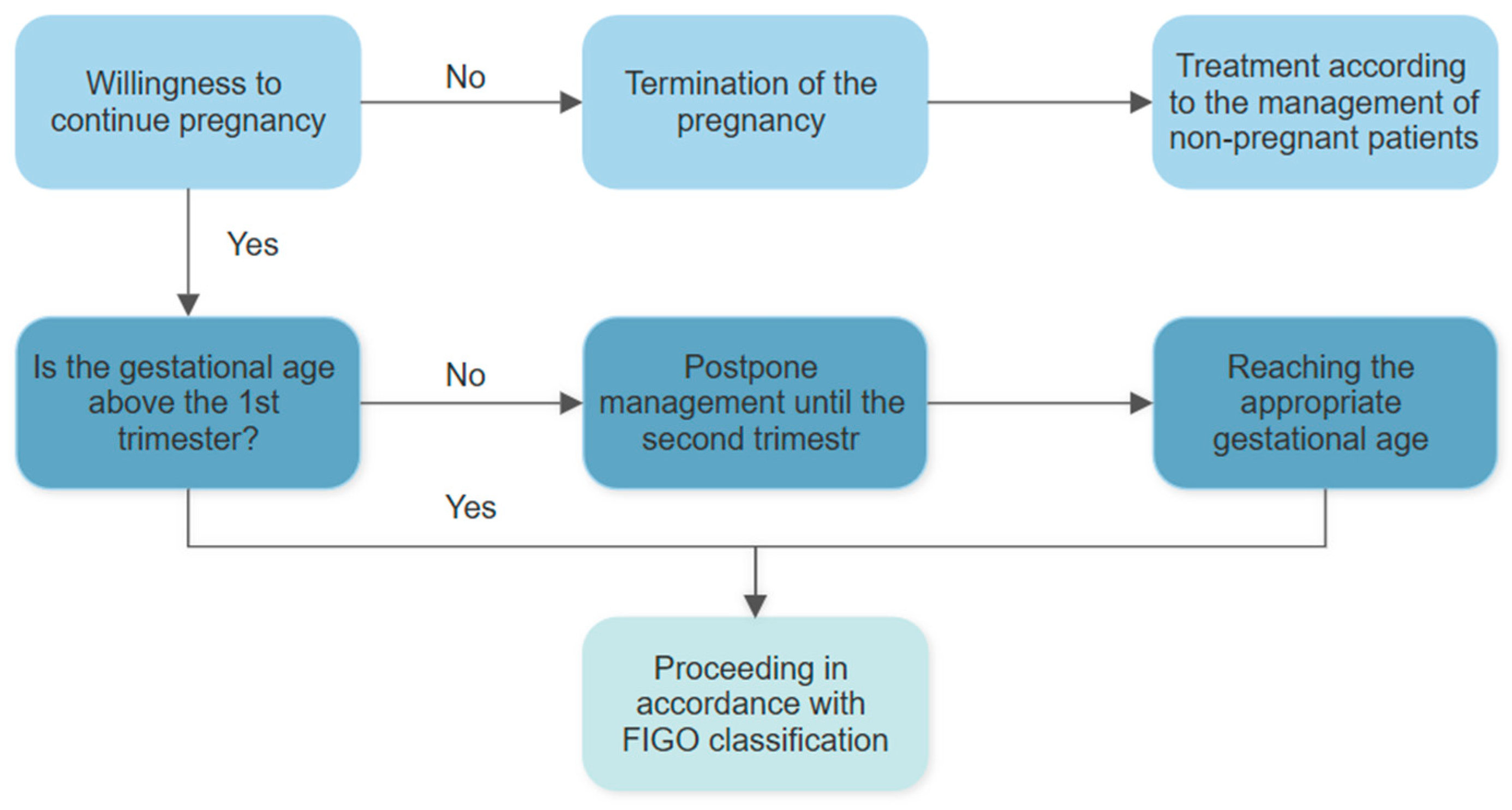
5. Management of Cervical Cancer During Pregnancy
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HPV | human papillomavirus |
| CIN | cervical interepithelial neoplasia |
| LSIL | low-grade squamous intraepithelial lesion |
| HSIL | high-grade squamous intraepithelial lesion |
| AIS | adenocarcinoma in situ |
| MRI | magnetic resonance imaging |
| CT | computed tomography |
| PET | position-emitting tomography |
| CK | cold knife |
| CO2 | carbon dioxide |
| NACT | neoadjuvant chemotherapy |
References
- Pawlus, B.; Zwolinski, J.; Koneczna, U.; Pawlus, G.; Kordek, A. Neonatal Outcomes for Women Diagnosed with Cancer during Pregnancy—Single-Center Study. Ginekol. Pol. 2023. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Petca, A.; Niculae, L.E.; Tocariu, R.; Nodiți, A.-R.; Petca, R.-C.; Rotar, I.C. Evaluating Offspring After Pregnancy-Associated Cancer: A Systematic Review of Neonatal Outcomes. Cancers 2025, 17, 299. [Google Scholar] [CrossRef] [PubMed]
- Schwab, R.; Anic, K.; Hasenburg, A. Cancer and Pregnancy: A Comprehensive Review. Cancers 2021, 13, 3048. [Google Scholar] [CrossRef] [PubMed]
- Dalmartello, M.; Negri, E.; La Vecchia, C.; Scarfone, G.; Buonomo, B.; Peccatori, F.A.; Parazzini, F. Frequency of Pregnancy-Associated Cancer: A Systematic Review of Population-Based Studies. Cancers 2020, 12, 1356. [Google Scholar] [CrossRef]
- Osterman, M.J.K.; Hamilton, B.E.; Martin, J.A.; Driscoll, A.K.; Valenzuela, C.P. Births: Final Data for 2023. Natl. Vital Stat. Rep. 2025, 74, 1. [Google Scholar]
- Mathews, T.J.; Hamilton, B.E. Mean Age of Mother, 1970–2000. Natl. Vital Stat. Rep. 2002, 51, 1–13. [Google Scholar]
- Eastwood-Wilshere, N.; Turner, J.; Oliveira, N.; Morton, A. Cancer in Pregnancy. Asia Pac. J. Clin. Oncol. 2019, 15, 296–308. [Google Scholar] [CrossRef]
- Dinu, M.-D.; Sima, R.-M.; Diaconescu, A.-S.; Poenaru, M.-O.; Gorecki, G.-P.; Amza, M.; Popescu, M.; Georgescu, M.-T.; Constantin, A.-A.; Mihai, M.-M.; et al. Diagnosis and Management of Cancers in Pregnancy: The Results of a Dual Battle Between Oncological Condition and Maternal Environment—Literature Review. Cancers 2025, 17, 389. [Google Scholar] [CrossRef]
- Kurniadi, A.; Setiawan, D.; Kireina, J.; Suardi, D.; Salima, S.; Erfiandi, F.; Andarini, M.Y. Clinical and Management Dilemmas Concerning Early-Stage Cervical Cancer in Pregnancy—A Case Report. Int. J. Womens Health 2023, 15, 1213–1218. [Google Scholar] [CrossRef]
- Cervical Cancer. Available online: https://www.who.int/health-topics/cervical-cancer (accessed on 8 April 2025).
- Suchonska, B.; Gajewska, M.; Madej, A.; Wielgoś, M. Cervical Intraepithelial Neoplasia during Pregnancy. Indian J. Cancer 2020, 57, 31–35. [Google Scholar] [CrossRef]
- Al-Halal, H.; Kezouh, A.; Abenhaim, H.A. Incidence and Obstetrical Outcomes of Cervical Intraepithelial Neoplasia and Cervical Cancer in Pregnancy: A Population-Based Study on 8.8 Million Births. Arch. Gynecol. Obs. 2013, 287, 245–250. [Google Scholar] [CrossRef] [PubMed]
- La Russa, M.; Jeyarajah, A.R. Invasive Cervical Cancer in Pregnancy. Best Pract. Res. Clin. Obs. Gynaecol. 2016, 33, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Balan, T.A.; Balan, R.A.; Socolov, D.; Gheorghiță, V.R.; Buțureanu, T.A.; Păvăleanu, I.; Coșovanu, E.T.; Căruntu, I.-D. Pregnancy-Related Precancerous Cervical Lesions: Pathogenesis, Diagnosis, Evolution, and Impact upon Gestation and Fertility. J. Clin. Med. 2024, 13, 6718. [Google Scholar] [CrossRef]
- Cooper, D.B.; McCathran, C.E. Cervical Dysplasia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Stumbar, S.E.; Stevens, M.; Feld, Z. Cervical Cancer and Its Precursors: A Preventative Approach to Screening, Diagnosis, and Management. Prim. Care 2019, 46, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Castle, P.E.; Pierz, A. (At Least) Once in Her Lifetime: Global Cervical Cancer Prevention. Obs. Gynecol. Clin. N. Am. 2019, 46, 107–123. [Google Scholar] [CrossRef]
- Brusselaers, N.; Shrestha, S.; van de Wijgert, J.; Verstraelen, H. Vaginal Dysbiosis and the Risk of Human Papillomavirus and Cervical Cancer: Systematic Review and Meta-Analysis. Am. J. Obs. Gynecol. 2019, 221, 9–18.e8. [Google Scholar] [CrossRef]
- Kornovski, Y.; Slavchev, S.; Kostov, S.; Ivanova, Y.; Yordanov, A. Etiologia, klasyfikacja, diagnostyka i profilaktyka stanów przedrakowych szyjki macicy. Onkol. Prakt. Klin.—Eduk. 2021, 7, 387–393. [Google Scholar]
- Asiaf, A.; Ahmad, S.T.; Mohammad, S.O.; Zargar, M.A. Review of the Current Knowledge on the Epidemiology, Pathogenesis, and Prevention of Human Papillomavirus Infection. Eur. J. Cancer Prev. 2014, 23, 206–224. [Google Scholar] [CrossRef]
- Nasierowska-Guttmejer, A.; Kędzia, W.; Rokita, W.; Wojtylak, S.; Lange, D.; Jach, R.; Wielgoś, M. Rekomendacje dotyczące diagnostyki i leczenia płaskonabłonkowych zmian śródnabłonkowych szyjki macicy na podstawie wytycznych CAP/ASCCP. Ginekol. Perinatol. Prakt. 2016, 1, 130–138. [Google Scholar]
- Zespół ekspertów Standardy postępowania w przypadkach choroby nowotworowej u kobiety w ciąży Część II. Rak szyjki macicy, guzy jajnika. Ginekol. Perinatol. Prakt. 2017, 2, 28–39.
- Fader, A.N.; Alward, E.K.; Niederhauser, A.; Chirico, C.; Lesnock, J.L.; Zwiesler, D.J.; Guido, R.S.; Lofgren, D.J.; Gold, M.A.; Moore, K.N. Cervical Dysplasia in Pregnancy: A Multi-Institutional Evaluation. Am. J. Obstet. Gynecol. 2010, 203, e1–e113. [Google Scholar] [CrossRef] [PubMed]
- Hannigan, E.V. Cervical Cancer in Pregnancy. Clin. Obs. Gynecol. 1990, 33, 837–845. [Google Scholar] [CrossRef]
- Yost, N.P.; Santoso, J.T.; McIntire, D.D.; Iliya, F.A. Postpartum Regression Rates of Antepartum Cervical Intraepithelial Neoplasia II and III Lesions. Obs. Gynecol. 1999, 93, 359–362. [Google Scholar] [CrossRef]
- Updated Cervical Cancer Screening Guidelines. Available online: https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2021/04/updated-cervical-cancer-screening-guidelines (accessed on 4 May 2025).
- Pearl—Management During Pregnancy—ASCCP. Available online: https://www.asccp.org/practice-pearls/management-during-pregnancy (accessed on 9 April 2025).
- Perkins, R.B.; Guido, R.S.; Castle, P.E.; Chelmow, D.; Einstein, M.H.; Garcia, F.; Huh, W.K.; Kim, J.J.; Moscicki, A.-B.; Nayar, R.; et al. 2019 ASCCP Risk-Based Management Consensus Guidelines for Abnormal Cervical Cancer Screening Tests and Cancer Precursors. J. Low. Genit. Tract Dis. 2020, 24, 102–131. [Google Scholar] [CrossRef]
- Rubach, E.M. Cancer in Pregnant Women. Oncol. Clin. Pract. 2018, 14, 62–78. [Google Scholar] [CrossRef]
- Mailath-Pokorny, M.; Schwameis, R.; Grimm, C.; Reinthaller, A.; Polterauer, S. Natural History of Cervical Intraepithelial Neoplasia in Pregnancy: Postpartum Histo-Pathologic Outcome and Review of the Literature. BMC Pregnancy Childbirth 2016, 16, 74. [Google Scholar] [CrossRef]
- Lantsman, T.; Seagle, B.-L.; Yang, J.; Margul, D.J.; Thorne-Spencer, J.; Miller, E.S.; Kocherginsky, M.; Shahabi, S. Association between Cervical Dysplasia and Adverse Pregnancy Outcomes. Am. J. Perinatol. 2020, 37, 947–954. [Google Scholar] [CrossRef] [PubMed]
- Morice, P.; Uzan, C.; Gouy, S.; Verschraegen, C.; Haie-Meder, C. Gynaecological Cancers in Pregnancy. Lancet 2012, 379, 558–569. [Google Scholar] [CrossRef]
- Kalliala, I.; Anttila, A.; Nieminen, P.; Halttunen, M.; Dyba, T. Pregnancy Incidence and Outcome before and after Cervical Intraepithelial Neoplasia: A Retrospective Cohort Study. Cancer Med. 2014, 3, 1512–1516. [Google Scholar] [CrossRef]
- Dicu-Andreescu, I.-G.; Marincaș, A.-M.; Ungureanu, V.-G.; Ionescu, S.-O.; Prunoiu, V.-M.; Brătucu, E.; Simion, L. Current Therapeutic Approaches in Cervical Cancer Based on the Stage of the Disease: Is There Room for Improvement? Medicina 2023, 59, 1229. [Google Scholar] [CrossRef]
- Cohen, P.A.; Jhingran, A.; Oaknin, A.; Denny, L. Cervical Cancer. Lancet 2019, 393, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.A.; James, D.; Marzan, A.; Armaos, M. Cervical Cancer: An Overview of Pathophysiology and Management. Semin. Oncol. Nurs. 2019, 35, 166–174. [Google Scholar] [CrossRef]
- Mruzek, H.; Kacperczyk-Bartnik, J.; Dańska-Bidzińska, A.; Ciebiera, M.; Grabowska-Derlatka, L.; Derlatka, P. Early-Stage and Locally Advanced Cervical Cancer during Pregnancy: Clinical Presentation, Diagnosis and Treatment. Medicina 2024, 60, 1700. [Google Scholar] [CrossRef] [PubMed]
- Beharee, N.; Shi, Z.; Wu, D.; Wang, J. Diagnosis and Treatment of Cervical Cancer in Pregnant Women. Cancer Med. 2019, 8, 5425–5430. [Google Scholar] [CrossRef]
- He, Z.; Xie, C.; Qi, X.; Hu, Z.; He, Y. The Effect of Preserving Pregnancy in Cervical Cancer Diagnosed during Pregnancy: A Retrospective Study. BMC Womens Health 2022, 22, 314. [Google Scholar] [CrossRef]
- Cintra, G.F.; Derchain, S.F.M.; Bicalho, D.S.; Filho, A.L.d.S.; Primo, W.Q.S.P. Cervical Cancer in Pregnancy. Rev. Bras. Ginecol. Obs. 2023, 45, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Zemlickis, D.; Lishner, M.; Degendorfer, P.; Panzarella, T.; Sutcliffe, S.B.; Koren, G. Maternal and Fetal Outcome after Invasive Cervical Cancer in Pregnancy. J. Clin. Oncol. 1991, 9, 1956–1961. [Google Scholar] [CrossRef]
- Amant, F.; Berveiller, P.; Boere, I.A.; Cardonick, E.; Fruscio, R.; Fumagalli, M.; Halaska, M.J.; Hasenburg, A.; Johansson, A.L.V.; Lambertini, M.; et al. Gynecologic Cancers in Pregnancy: Guidelines Based on a Third International Consensus Meeting. Ann. Oncol. 2019, 30, 1601–1612. [Google Scholar] [CrossRef]
- Dąbrowska, A.; Perdyan, A.; Sobocki, B.K.; Rutkowski, J. Management of Cervical Cancer during Pregnancy—A Systematic Review. Nowotwory J. Oncol. 2024, 74, 27–33. [Google Scholar] [CrossRef]
- Webb, J.A.W.; Thomsen, H.S.; Morcos, S.K.; Members of Contrast Media Safety Committee of European Society of Urogenital Radiology (ESUR). The Use of Iodinated and Gadolinium Contrast Media during Pregnancy and Lactation. Eur. Radiol. 2005, 15, 1234–1240. [Google Scholar] [CrossRef]
- Korenaga, T.-R.K.; Tewari, K.S. Gynecologic Cancer in Pregnancy. Gynecol. Oncol. 2020, 157, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Bhatla, N.; Aoki, D.; Sharma, D.N.; Sankaranarayanan, R. Cancer of the Cervix Uteri: 2021 Update. Int. J. Gynecol. Obstet. 2021, 155, 28–44. [Google Scholar] [CrossRef] [PubMed]
- Amant, F.; Van Calsteren, K.; Halaska, M.J.; Gziri, M.M.; Hui, W.; Lagae, L.; Willemsen, M.A.; Kapusta, L.; Van Calster, B.; Wouters, H.; et al. Long-Term Cognitive and Cardiac Outcomes after Prenatal Exposure to Chemotherapy in Children Aged 18 Months or Older: An Observational Study. Lancet Oncol. 2012, 13, 256–264. [Google Scholar] [CrossRef] [PubMed]
- D’Augè, T.G.; Donato, V.D.; Giannini, A. Strategic Approaches in Management of Early-Stage Cervical Cancer: A Comprehensive Editorial. Clin. Exp. Obstet. Gynecol. 2024, 51, 235. [Google Scholar] [CrossRef]
- Plante, M.; Kwon, J.S.; Ferguson, S.; Samouëlian, V.; Ferron, G.; Maulard, A.; De Kroon, C.; Van Driel, W.; Tidy, J.; Williamson, K.; et al. Simple versus Radical Hysterectomy in Women with Low-Risk Cervical Cancer. N. Engl. J. Med. 2024, 390, 819–829. [Google Scholar] [CrossRef]
- Ferrari, F.; Bonetti, E.; Oliveri, G.; Giannini, A.; Gozzini, E.; Conforti, J.; Ferrari, F.A.; Salinaro, F.; Tisi, G.; Ciravolo, G.; et al. Cold Knife Versus Carbon Dioxide for the Treatment of Preinvasive Cervical Lesion. Medicina 2024, 60, 1056. [Google Scholar] [CrossRef]
- Botha, M.H.; Rajaram, S.; Karunaratne, K. Cancer in Pregnancy. Int. J. Gynaecol. Obs. 2018, 143 (Suppl. 2), 137–142. [Google Scholar] [CrossRef]
- Le Guévelou, J.; Selleret, L.; Laas, E.; Lecuru, F.; Kissel, M. Cervical Cancer Associated with Pregnancy: Current Challenges and Future Strategies. Cancers 2024, 16, 1341. [Google Scholar] [CrossRef]
- Cibula, D.; Raspollini, M.R.; Planchamp, F.; Centeno, C.; Chargari, C.; Felix, A.; Fischerová, D.; Jahnn-Kuch, D.; Joly, F.; Kohler, C.; et al. ESGO/ESTRO/ESP Guidelines for the Management of Patients with Cervical Cancer—Update 2023. Int. J. Gynecol. Cancer 2023, 33, 649–666. [Google Scholar] [CrossRef]
- Lee, B. Cervical Cancer: Pharmacologic Management. Cancer Therapy Advisor 2023. Available online: https://www.cancertherapyadvisor.com/ddi/cervical-cancer-pharmacologic-treatment/ (accessed on 24 May 2025).
- Mandic, A.; Maricic, S.; Malenkovic, G.; Stojic, I.; Gutic, B. Neoadjuvant Chemotherapy in Locally Advanced Cervical Cancer in Pregnancy-Review of the Literature. J. BUON 2020, 25, 597–604. [Google Scholar]
- Rydzewska, L.; Tierney, J.; Vale, C.L.; Symonds, P.R. Neoadjuvant Chemotherapy plus Surgery versus Surgery for Cervical Cancer. Cochrane Database Syst. Rev. 2012, 12, CD007406. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Liu, Y.; Lin, M.; Sheng, B.; Zhu, X. Efficacy of Neoadjuvant Platinum-Based Chemotherapy during the Second and Third Trimester of Pregnancy in Women with Cervical Cancer: An Updated Systematic Review and Meta-Analysis. Drug Des. Dev. Ther. 2019, 13, 79–102. [Google Scholar] [CrossRef] [PubMed]
- Halaska, M.J.; Komar, M.; Vlk, R.; Tomek, V.; Skultety, J.; Robova, H.; Rob, L. A Pilot Study on Peak Systolic Velocity Monitoring of Fetal Anemia after Administration of Chemotherapy during Pregnancy. Eur. J. Obs. Gynecol. Reprod. Biol. 2014, 174, 76–79. [Google Scholar] [CrossRef] [PubMed]

| Stage | Description |
|---|---|
| I | Carcinoma confined to the cervix |
| IA | Diagnosed only by microscopy, and the maximal invasional depth ≤ 5 mm |
| IA1 | Measured stromal invasion ≤ 3 mm in depth |
| IA2 | Measured stromal invasion > 3 mm and ≤5 mm in depth |
| IB | The deepest invasion measurement of >5 mm (deeper than stage IA) only cervical lesion |
| IB1 | Clinically visible lesion >5 mm deep and ≤2 cm in greatest dimension |
| IB2 | Clinically visible lesion > 2 and ≤4 cm in greatest dimension |
| IB3 | Lesion > 4 cm in greatest dimension |
| II | Carcinoma invasion beyond the uterus, without the lower 1/3 part of the vagina or pelvic wall |
| IIA | The tumor involves only the upper 2/3 of the vagina |
| IIA1 | The tumor involves only the upper 2/3 of the vagina and is ≤4 cm in greatest dimension |
| IIA2 | The tumor involves only the upper 2/3 of the vagina and is >4 cm in greatest dimension |
| IIB | With parametrial invasion |
| III | Carcinoma extends to the pelvic wall and/or involves the lower third of the vagina and/or causes hydronephrosis or renal failure and/or involves pelvic and/or para-aortic lymph nodes |
| IIIA | The tumor involves a third of the vagina without reaching the pelvic wall |
| IIIB | The tumor involves the pelvic wall and/or causes hydronephrosis or renal failure |
| IIIC | Pelvic and/or para-aortic lymph node involvement |
| IIIC1 | Pelvic lymph node metastasis only |
| IIIC2 | Para-aortic lymph node metastasis |
| IV | The tumor has extended beyond the true pelvis or has involved bladder or rectal mucosa |
| IVA | The tumor spread to adjacent pelvic organs |
| IVB | The tumor spread to distant organs |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piórecka, A.; Marcinkowska, W.; Gągorowski, F.; Gąsior, M.; Kazimierczuk, K.; Żalińska, A.; Oszukowski, P.; Pięta-Dolińska, A. Cervical Dysplasia and Cervical Cancer During Pregnancy: From Pathogenesis to Clinical Management. J. Clin. Med. 2025, 14, 3784. https://doi.org/10.3390/jcm14113784
Piórecka A, Marcinkowska W, Gągorowski F, Gąsior M, Kazimierczuk K, Żalińska A, Oszukowski P, Pięta-Dolińska A. Cervical Dysplasia and Cervical Cancer During Pregnancy: From Pathogenesis to Clinical Management. Journal of Clinical Medicine. 2025; 14(11):3784. https://doi.org/10.3390/jcm14113784
Chicago/Turabian StylePiórecka, Aleksandra, Weronika Marcinkowska, Filip Gągorowski, Magdalena Gąsior, Katarzyna Kazimierczuk, Agnieszka Żalińska, Przemysław Oszukowski, and Agnieszka Pięta-Dolińska. 2025. "Cervical Dysplasia and Cervical Cancer During Pregnancy: From Pathogenesis to Clinical Management" Journal of Clinical Medicine 14, no. 11: 3784. https://doi.org/10.3390/jcm14113784
APA StylePiórecka, A., Marcinkowska, W., Gągorowski, F., Gąsior, M., Kazimierczuk, K., Żalińska, A., Oszukowski, P., & Pięta-Dolińska, A. (2025). Cervical Dysplasia and Cervical Cancer During Pregnancy: From Pathogenesis to Clinical Management. Journal of Clinical Medicine, 14(11), 3784. https://doi.org/10.3390/jcm14113784






