Preliminary Results of Surgical Treatment for Enchondroma Using a Novel Bioactive and Osseoconductive HAP/β–Glucan Bone Substitute FlexiOss®—Case Series
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Salvo, S.; Pavone, V.; Coco, S.; Dell’Agli, E.; Blatti, C.; Testa, G. Benign Bone Tumors: An Overview of What We Know Today. J. Clin. Med. 2022, 11, 699. [Google Scholar] [CrossRef]
- Yenigül, A.E.; Sofulu, Ö.; Erol, B. Treatment of locally aggressive benign bone tumors by means of extended intralesional curettage without chemical adjuvants. SAGE Open Med. 2022, 10, 20503121221094199. [Google Scholar] [CrossRef] [PubMed]
- Smolle, M.A.; Roessl, V.; Leithner, A. Effect of Local Adjuvants Following Curettage of Benign and Intermediate Tumours of Bone: A Systematic Review of the Literature. Cancers 2023, 15, 4258. [Google Scholar] [CrossRef]
- Myeroff, C.; Archdeacon, M. Autogenous bone graft: Donor sites and techniques. J. Bone Joint Surg. Am. 2011, 93, 2227–2236. [Google Scholar] [CrossRef] [PubMed]
- Bohatyrewicz, A.; Bohatyrewicz, R.; Dobiecki, K.; Kotrych, D.; Kamiński, A.; Zienkiewicz, M.; Zietek, P.; Dziedzic-Gocławska, A. Is retrieval of bone material from multiorgan donors effective enough to cover demand for biostatic bone tissue grafts in Poland? Transpl. Proc. 2006, 38, 297–300. [Google Scholar] [CrossRef]
- Baldwin, P.; Li, D.J.; Auston, D.A.; Mir, H.S.; Yoon, R.S.; Koval, K.J. Autograft, Allograft, and Bone Graft Substitutes: Clinical Evidence and Indications for Use in the Setting of Orthopaedic Trauma Surgery. J. Orthop. Trauma. 2019, 33, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Sallent, I.; Capella-Monsonís, H.; Procter, P.; Bozo, I.Y.; Deev, R.V.; Zubov, D.; Vasyliev, R.; Perale, G.; Pertici, G.; Baker, J.; et al. The Few Who Made It: Commercially and Clinically Successful Innovative Bone Grafts. Front. Bioeng. Biotechnol. 2020, 8, 952. [Google Scholar] [CrossRef]
- Spurrier, E.; Payton, O.; Latimer, M. Bone temperature during cementation with a heatsink: A bovine model pilot study. BMC Res Notes 2014, 7, 494. [Google Scholar] [CrossRef][Green Version]
- Chen, C.J.; Brien, E.W. Early postoperative compilations of bone filling in curettage defects. J. Orthop. Surg. Res. 2019, 14, 261. [Google Scholar] [CrossRef]
- Kotrych, D.; Korecki, S.; Ziętek, P.; Kruk, B.; Kruk, A.; Wechmann, M.; Kamiński, A.; Kotrych, K.; Bohatyrewicz, A. Preliminary Results of Highly Injectable Bi-Phasic Bone Substitute (CERAMENT) in the Treatment of Benign Bone Tumors and Tumor-like Lesions. Open Med. 2018, 13, 487–492. [Google Scholar] [CrossRef]
- Zrodowski, M.; Ciechanowicz, D.; Dembski, M.; Piotrowicz, M.; Napora, J.; Mazurek, T. The use of bone substitute (CERAMENT) in the treatment of benign bone tumors and tumor-like lesions. Chir. Narzadow Ruchu Ortop. Pol. 2022, 7, 1–7. [Google Scholar] [CrossRef]
- Borkowski, L.; Lübek, T.; Jojczuk, M.; Nogalski, A.; Belcarz, A.; Palka, K.; Hajnos, M.; Ginalska, G. Behavior of new hydroxyapatite/glucan composite in human serum. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 2653–2664. [Google Scholar] [CrossRef] [PubMed]
- Belcarz, A.; Zima, A.; Ginalska, G. Biphasic mode of antibacterial action of aminoglycoside antibiotics-loaded elastic hydroxyapatite-glucan composite. Int. J. Pharm. 2013, 454, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Belcarz, A.; Ginalska, G.; Polkowska, I.; Przekora, A.; Ślósarczyk, A.; Zima, A.; Paszkiewicz, Z. Pilot clinical study of efficacy of flexible HAp-based composite for bone defects replacement. Eng. Biomater. 2010, 99–101, 16–18. [Google Scholar]
- West, T.P. Production of the Polysaccharide Curdlan by Agrobacterium species on Processing Coproducts and Plant Lignocellulosic Hydrolysates. Fermentation 2020, 6, 16. [Google Scholar] [CrossRef]
- Yuan, M.; Fu, G.; Sun, Y.; Zhang, D. Biosynthesis and applications of curdlan. Carbohydr. Polym. 2021, 273, 118597. [Google Scholar] [CrossRef]
- Jeys, L.M.; Morris, G.V.; Kurisunkal, V.J.; Botello, E.; Boyle, R.A.; Ebeid, W.; Houdek, M.T.; Puri, A.; Ruggieri, P.; Brennan, B.; et al. Identifying consensus and areas for future research in chondrosarcoma: A report from the Birmingham Orthopaedic Oncology Meeting. Bone Joint J 2025, 107-B, 246–252. [Google Scholar] [CrossRef]
- Wu, P.K.; Chen, C.F.; Chen, C.M.; Tsai, S.W.; Cheng, Y.C.; Chang, M.C.; Chen, W.M. Grafting for bone defects after curettage of benign bone tumor—Analysis of factors influencing the bone healing. J. Chin. Med. Assoc. 2018, 81, 643–648. [Google Scholar] [CrossRef]
- Sroka-Bartnicka, A.; Kimber, J.A.; Borkowski, L.; Pawlowska, M.; Polkowska, I.; Kalisz, G.; Belcarz, A.; Jozwiak, K.; Ginalska, G.; Kazarian, S.G. The biocompatibility of carbon hydroxyapatite/β-glucan composite for bone tissue engineering studied with Raman and FTIR spectroscopic imaging. Anal. Bioanal. Chem. 2015, 407, 7775–7785. [Google Scholar] [CrossRef]
- Borkowski, L.; Pawłowska, M.; Radzki, R.P.; Bieńko, M.; Polkowska, I.; Belcarz, A.; Karpiński, M.; Słowik, T.; Matuszewski, Ł.; Ślósarczyk, A.; et al. Effect of a carbonated HAP/β-glucan composite bone substitute on healing of drilled bone voids in the proximal tibial metaphysis of rabbits. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 53, 60–67. [Google Scholar] [CrossRef]
- Hu, Y.C.; Lun, D.X.; Zhao, S.K. Combined anterior and lateral approaches for bone tumors of the femoral neck and head. Orthopedics 2012, 35, e628–e634. [Google Scholar] [CrossRef] [PubMed]
- Ismail, T.B.; Mahmoud, M.M.; Ahmed, O.E.; Bola, A.H.; Bahaa, Z.H. Filling of the resultant cavity after curettage of benign bone tumours is still controversial. Med. J. Malaysia. 2023, 78, 163–170. [Google Scholar] [PubMed]
- Noordin, S.; Allana, S.; Umer, M.; Jamil, M.; Hilal, K.; Uddin, N. Unicameral bone cysts: Current concepts. Ann. Med. Surg. 2018, 34, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Fillingham, Y.A.; Lenart, B.A.; Gitelis, S. Function after injection of benign bone lesions with a bioceramic. Clin. Orthop. Relat. Res. 2012, 470, 2014–2020. [Google Scholar] [CrossRef]
- Coetzee, A.S. Regeneration of bone in the presence of calcium sulfate. Arch. Otolaryngol. 1980, 106, 405–409. [Google Scholar] [CrossRef]
- Dong, C.; Klimek, P.; Abächerli, C.; De Rosa, V.; Krieg, A.H. Percutaneous cyst aspiration with injection of two different bioresorbable bone cements in treatment of simple bone cyst. J. Child. Orthop. 2020, 14, 76–84. [Google Scholar] [CrossRef]
- Rauschmann, M.A.; Wichelhaus, T.A.; Stirnal, V.; Dingeldein, E.; Zichner, L.; Schnettler, R.; Alt, V. Nanocrystalline hydroxyapatite and calcium sulphate as biodegradable composite carrier material for local delivery of antibiotics in bone infections. Biomaterials 2005, 26, 2677–2684. [Google Scholar] [CrossRef]
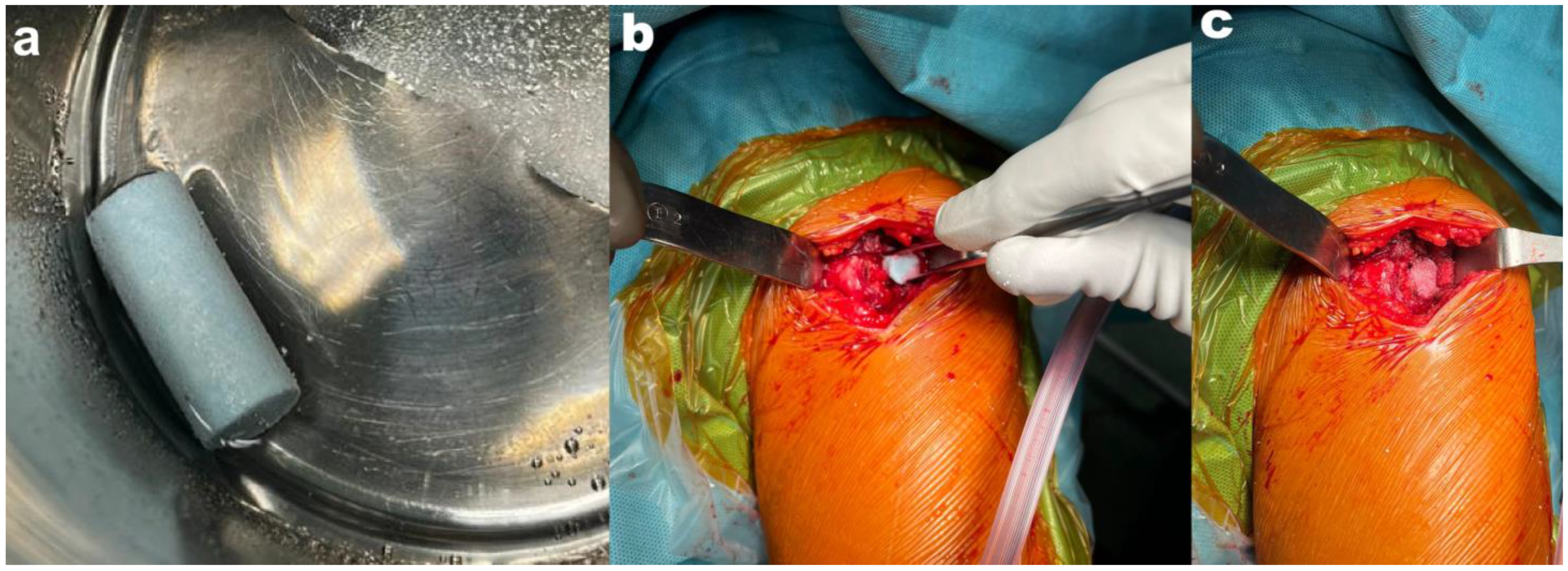

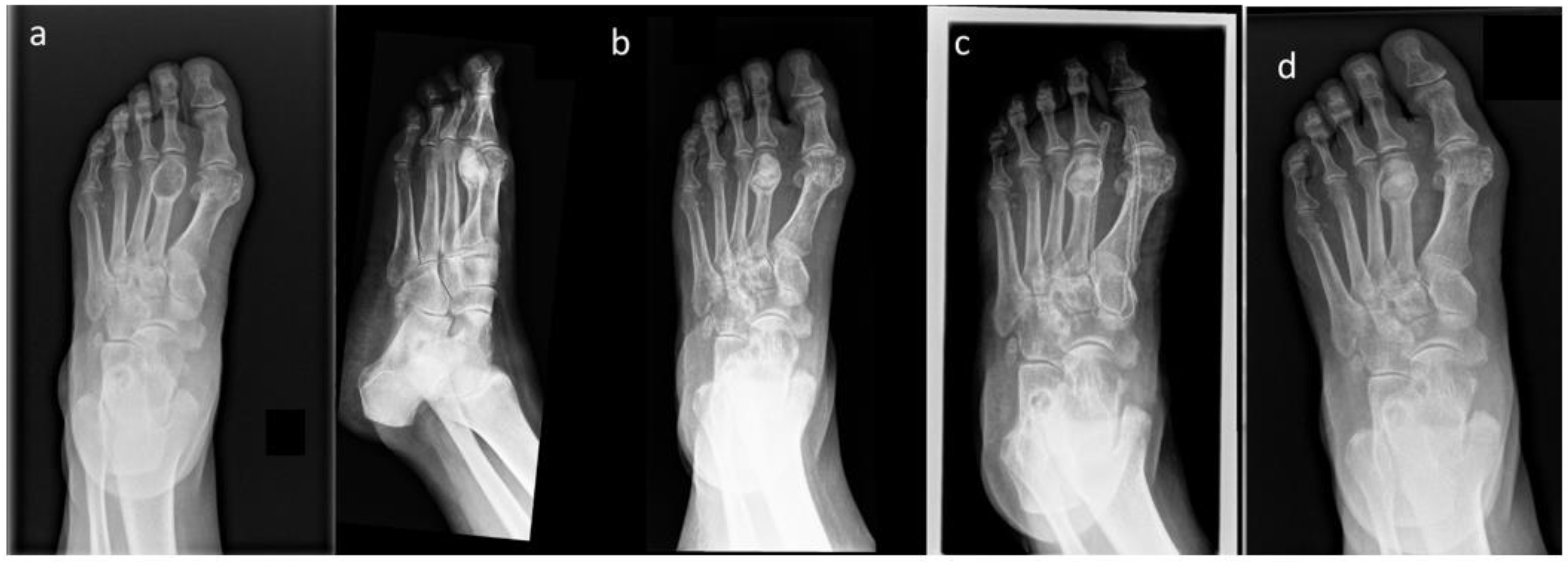
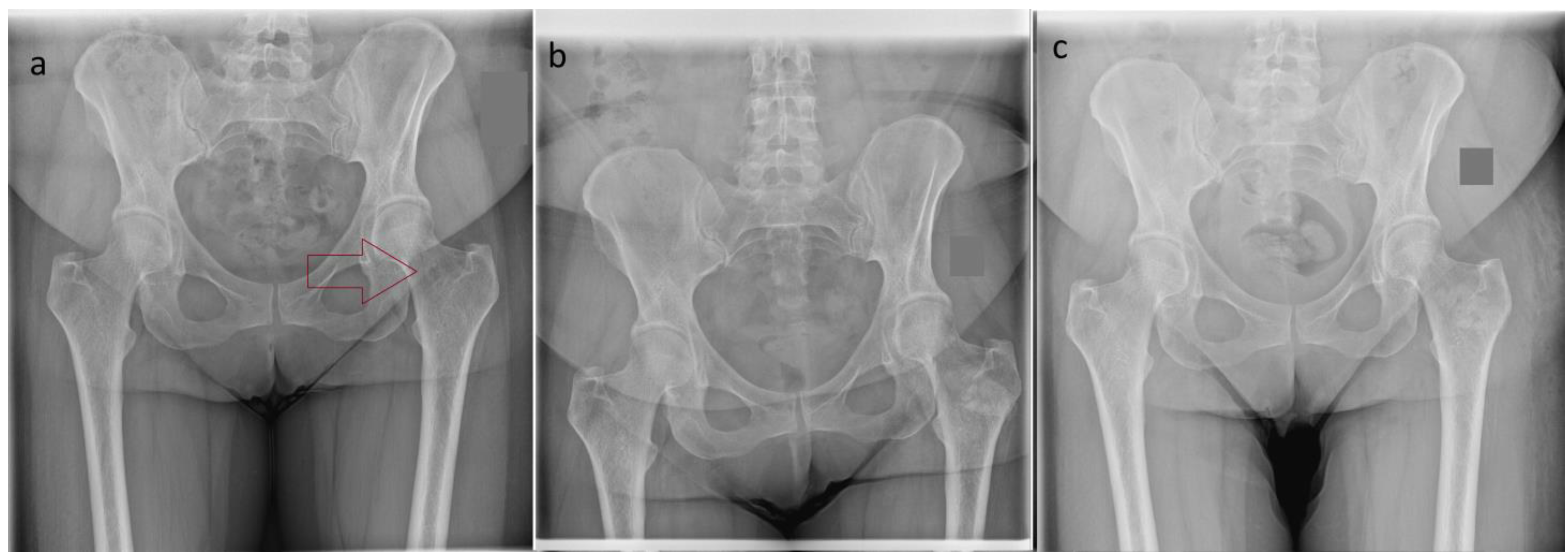
| Case Number | Gender | Age at Surgery (Years) | Tumor Localization | Tumor Size [mm] | Tumor Characteristic |
|---|---|---|---|---|---|
| 1 | F | 42 | Femur (neck) | 11 | Enchondorma |
| 2 | F | 66 | Foot (metatarsus) | 30 | Enchodnorma |
| 3 | F | 20 | Fibula | 18 | Endchondroma |
| 4 | F | 48 | Ilium | 25 | Enchindroma |
| 5 | M | 55 | Tibia | 33 | Enchondroma |
| 6 | F | 18 | Fibula | 40 | Enchondroma |
| 7 | F | 42 | Fibula | 10 | Enchondroma |
| 8 | F | 44 | Femur (shaft) | 50 | Enchondroma |
| Case Number | Pain | Function | Emotional | Supports | Walking | Gait | MSTS Result |
|---|---|---|---|---|---|---|---|
| 1 | Intermediate (4) | Intermediate (4) | Intermediate (4) | None (5) | Unlimited (5) | Normal (5) | 27 |
| 2 | No pain (5) | No restriction (5) | Enthused (5) | None (5) | Unlimited (5) | Normal (5) | 30 |
| 3 | No pain (5) | No restriction (5) | Enthused (5) | None (5) | Unlimited (5) | Normal (5) | 30 |
| 4 | No pain (5) | No restriction (5) | Enthused (5) | None (5) | Unlimited (5) | Normal (5) | 30 |
| 5 | No pain (5) | No restriction (5) | Enthused (5) | None (5) | Unlimited (5) | Normal (5) | 30 |
| 6 | No pain (5) | No restriction (5) | Enthused (5) | None (5) | Unlimited (5) | Normal (5) | 30 |
| 7 | Intermediate (4) | Intermediate (4) | Enthused (5) | None (5) | Unlimited (5) | Normal (5) | 28 |
| 8 | No pain (5) | No restriction (5) | Enthused (5) | None (5) | Unlimited (5) | Normal (5) | 30 |
| Case Number | MNC | VAS Score Pre-Operative | VAS Score Postoperative | Complications |
|---|---|---|---|---|
| 1 | Score II | 6 | 3–4 | Serum leakage from the wound—2 weeks after surgery |
| 2 | Score I | 5 | 0 | None |
| 3 | Score I | 4 | 0–1 | Periodic foot swelling |
| 4 | Score I | 7 | 4 | None |
| 5 | Score I | 6 | 3 | None |
| 6 | Score I | 5 | 2 | None |
| 7 | Score II | 3 | 2 | Serum leakage from the wound—2 weeks after surgery |
| 8 | Score I | 5 | 0 | None |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotrych, D.; Ciechanowicz, D.; Bielewicz, F.; Baryluk, A.; Podsiadło, S.; Ziętek, P. Preliminary Results of Surgical Treatment for Enchondroma Using a Novel Bioactive and Osseoconductive HAP/β–Glucan Bone Substitute FlexiOss®—Case Series. J. Clin. Med. 2025, 14, 3738. https://doi.org/10.3390/jcm14113738
Kotrych D, Ciechanowicz D, Bielewicz F, Baryluk A, Podsiadło S, Ziętek P. Preliminary Results of Surgical Treatment for Enchondroma Using a Novel Bioactive and Osseoconductive HAP/β–Glucan Bone Substitute FlexiOss®—Case Series. Journal of Clinical Medicine. 2025; 14(11):3738. https://doi.org/10.3390/jcm14113738
Chicago/Turabian StyleKotrych, Daniel, Dawid Ciechanowicz, Filip Bielewicz, Andrzej Baryluk, Sebastian Podsiadło, and Paweł Ziętek. 2025. "Preliminary Results of Surgical Treatment for Enchondroma Using a Novel Bioactive and Osseoconductive HAP/β–Glucan Bone Substitute FlexiOss®—Case Series" Journal of Clinical Medicine 14, no. 11: 3738. https://doi.org/10.3390/jcm14113738
APA StyleKotrych, D., Ciechanowicz, D., Bielewicz, F., Baryluk, A., Podsiadło, S., & Ziętek, P. (2025). Preliminary Results of Surgical Treatment for Enchondroma Using a Novel Bioactive and Osseoconductive HAP/β–Glucan Bone Substitute FlexiOss®—Case Series. Journal of Clinical Medicine, 14(11), 3738. https://doi.org/10.3390/jcm14113738





