Reconstruction of the Extensor Apparatus After Total Patellectomy in Orthopedic Oncology: A Systematic Literature Review
Abstract
1. Introduction
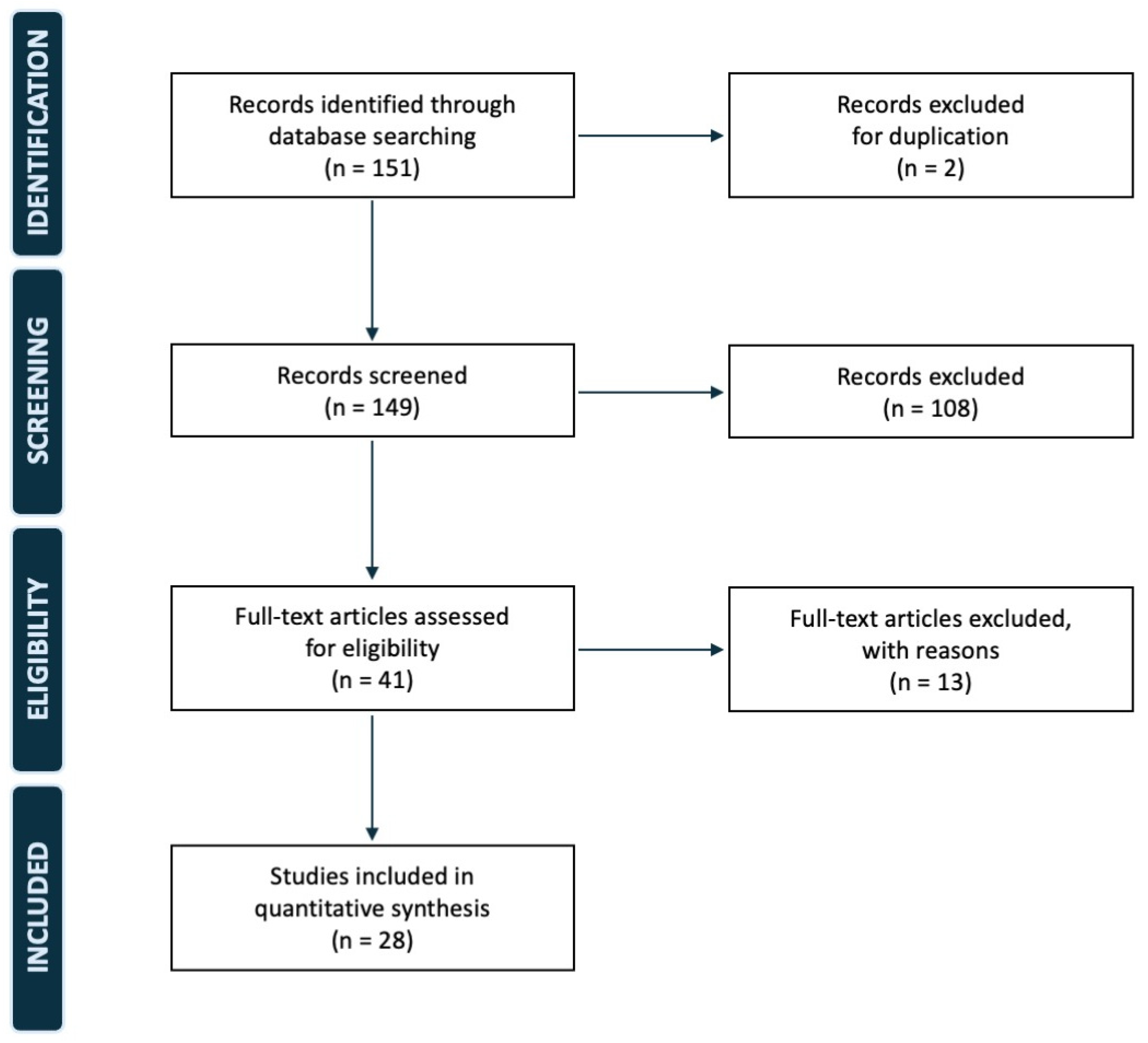
2. Materials and Methods
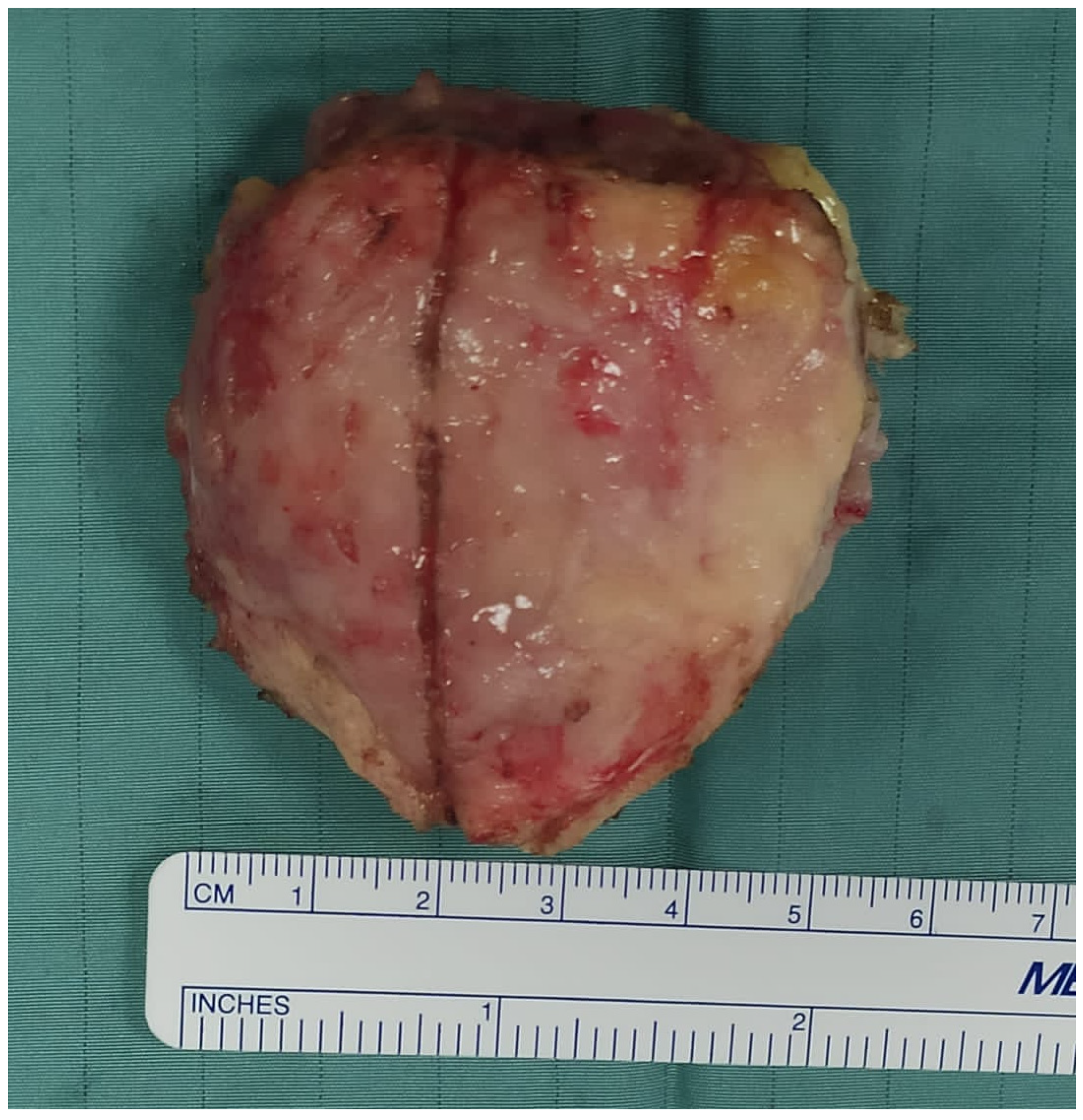
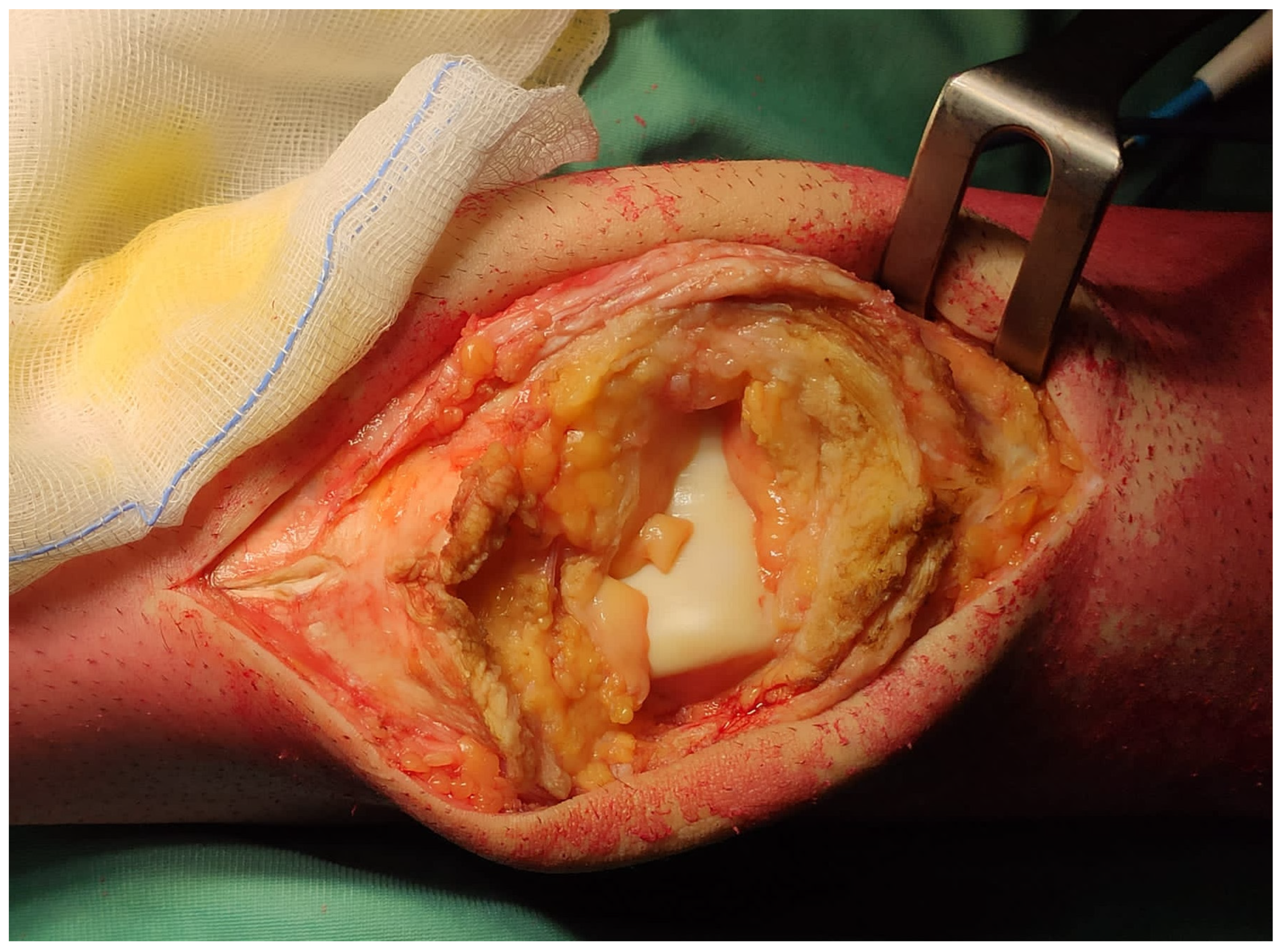
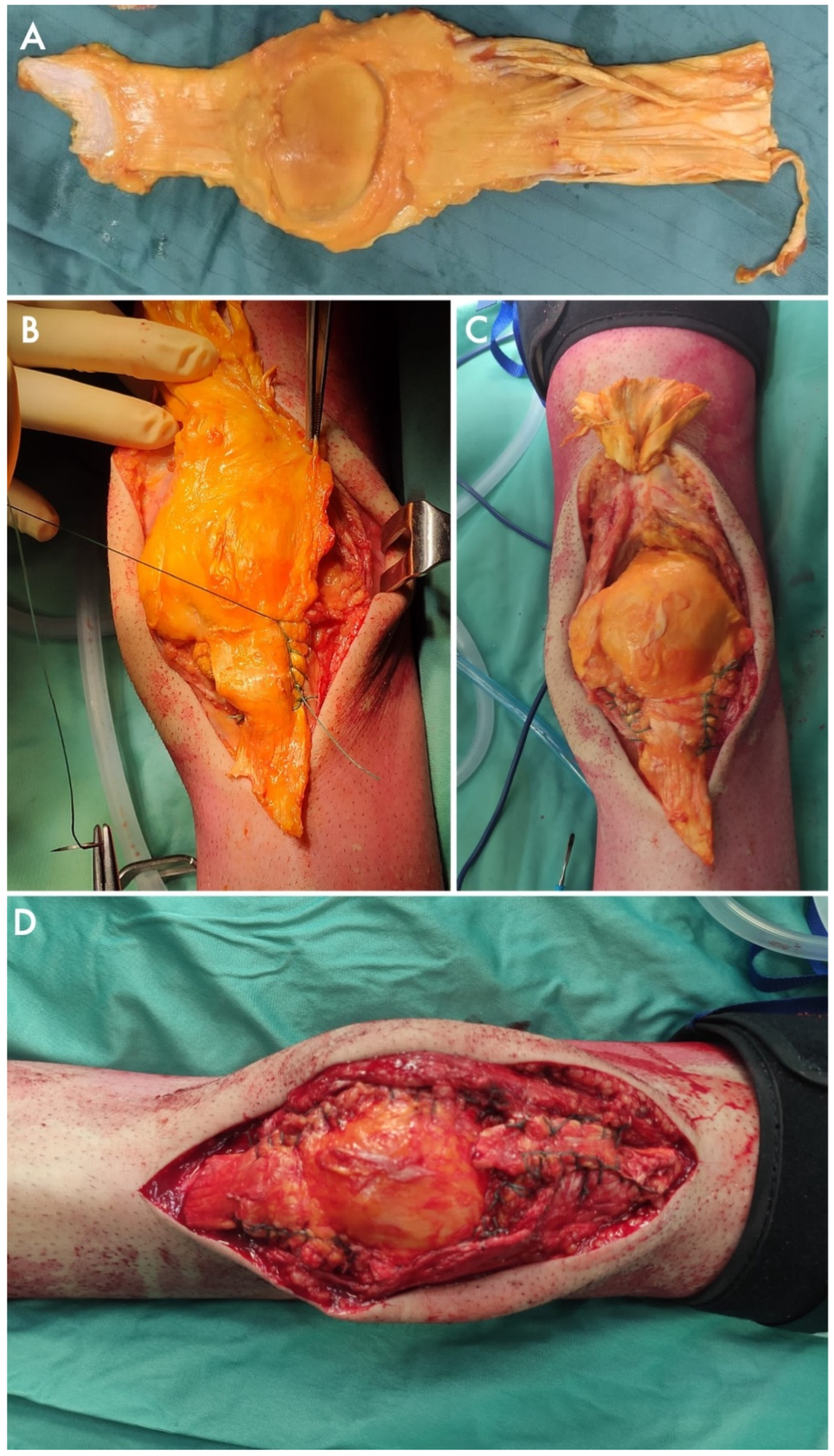
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A
- Search query strings:
- PubMed, Medline, Google Scholar: (((Patella) AND (Replacement)) OR (Patellectomy)) AND ((Bone Tumor) OR (Sarcoma))
- EMBASE, Scopus: (Patellectomy) AND ((Bone Tumor) OR (Sarcoma))
- Data researched on 23 March 2025
References
- Pluot, E.; Davies, A.; James, S. Tumours and tumour-like lesions of the patella. In Imaging of Bone Tumors and Tumor-Like Lesions. Medical Radiology; Davies, A., Sundaram, M., James, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar] [CrossRef]
- Song, M.; Zhang, Z.; Wu, Y.; Ma, K.; Lu, M. Primary tumors of the patella. World J. Surg. Oncol. 2015, 13, 163. [Google Scholar] [CrossRef] [PubMed]
- Casadei, R.; Kreshak, J.; Rinaldi, R.; Rimondi, E.; Bianchi, G.; Alberghini, M.; Ruggieri, P.; Vanel, D. Imaging tumors of the patella. Eur. J. Radiol. 2013, 82, 2140–2148. [Google Scholar] [CrossRef] [PubMed]
- Mercuri, M.; Casadei, R. Patellar tumors. Clin. Orthop. Relat. Res. 2001, 389, 35–46. [Google Scholar] [CrossRef]
- Singh, J.; James, S.L.; Kroon, H.M.; Woertler, K.; Anderson, S.E.; Davies, A.M. Tumour and tumour-like lesions of the patella—A multicentre experience. Eur. Radiol. 2009, 19, 701–712. [Google Scholar] [CrossRef] [PubMed]
- O’Mara, J.W.; Keeling, J.; Montgomery, E.A.; Aaron, A.D. Primary lesions of the patella. Orthopedics 2000, 23, 328–379. [Google Scholar] [CrossRef]
- Li, G.; Shan, C.; Sun, R.; Liu, S.; Chen, S.; Song, M.; Lu, M. Patellar metastasis from primary tumor. Oncol. Lett. 2018, 15, 1389–1396. [Google Scholar] [CrossRef]
- Crenn, V.; Vezole, L.; Bouhamama, A.; Meurgey, A.; Karanian, M.; Marec-Bérard, P.; Gouin, F.; Vaz, G. Percutaneous core needle biopsy can efficiently and safely diagnose most primary bone tumors. Diagnostics 2021, 11, 1552. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Zhou, M.; Xu, L.; Zhu, L.; Yan, Z.; Wu, W.; Qiao, Z. Curettage through a wide cortical window for treatment of a primary aneurysmal bone cyst of the patella. J. Int. Med. Res. 2020, 48, 300060520947910. [Google Scholar] [CrossRef]
- Alesawi, A.F.; Abualross, O.H.; Alesawi, N.F.; Samargandi, R.S. Patellar osteoblastoma: A case report and literature review. Radiol. Case Rep. 2024, 19, 2714–2718. [Google Scholar] [CrossRef]
- Kawaguchi, N.; Ahmed, A.R.; Matsumoto, S.; Manabe, J.; Matsushita, Y. The concept of curative margin in surgery for bone and soft tissue sarcoma. Clin. Orthop. Relat. Res. 2004, 419, 165–172. [Google Scholar] [CrossRef]
- Sutton, F.S.; Thompson, C.H.; Lipke, J.; Kettelkamp, D.B. The effect of patellectomy on knee function. J. Bone Jt. Surg. Am. 1976, 58, 537–540. [Google Scholar] [CrossRef]
- Mercuri, M.; Casadei, R.; Ferraro, A.; de Cristofaro, R.; Balladelli, A.; Picci, P. Tumours of the patella. Int. Orthop. 1991, 15, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Bhagat, S.; Sharma, H.; Bansal, M.; Reid, R. Presentation and outcome of primary tumors of the patella. J. Knee Surg. 2008, 21, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Müller, D.A.; Beltrami, G.; Scoccianti, G.; Cuomo, P.; Totti, F.; Capanna, R. Allograft reconstruction of the extensor mechanism after resection of soft tissue sarcoma. Adv. Orthop. 2018, 2018, 6275861. [Google Scholar] [CrossRef] [PubMed]
- Merchán, N.; Yeung, C.M.; Garcia, J.; Schwab, J.H.; Raskin, K.A.; Newman, E.T.; Lozano-Calderon, S.A. Primary and metastatic bone tumors of the patella: Literature review and institutional experience. Arch. Bone Jt. Surg. 2022, 10, 190–203. [Google Scholar] [CrossRef]
- Klenerman, L. A Metastatic deposit in the patella from a carcinoma of the breast. Postgrad. Med. J. 1965, 41, 284–286. [Google Scholar] [CrossRef]
- Jaeger, J.H.; Balliet, J.M.; Schlatterer, B.; Hamdan, M. Surgical treatment of chronic postero-lateral instability of the knee. Techniques, indications, results. Acta Orthop. Belg. 1994, 60 (Suppl. 1), 71–80. [Google Scholar][Green Version]
- Okada, K.; Sato, K.; Abe, E.; Kataoka, Y.; Miyakoshi, N.; Ishikawa, N.; Sageshima, M. Case report 858: Postradiation osteosarcoma of the patella. Skelet. Radiol. 1994, 23, 471–474. [Google Scholar] [CrossRef]
- Connell, D.; Munk, P.L.; Lee, M.J.; O’COnnell, J.X.; Janzen, D.; Vu, M.; Masri, B.A. Giant cell tumor of bone with selective metastases to mediastinal lymph nodes. Skelet. Radiol. 1998, 27, 341–345. [Google Scholar] [CrossRef]
- Aktas, S.; Demiral, H.; Bilgi, S.; Caglar, T.; Calpur, O.U. Patellar metastasis from a lung epidermoid carcinoma. Yonsei Med. J. 1998, 39, 474–477. [Google Scholar] [CrossRef]
- Macdonald, D.; Fornasier, V.; Cameron, J. Multicentric giant cell tumour involving the patella. Can. J. Surg. 2001, 44, 222–223. [Google Scholar] [PubMed]
- Gudi, N.; Venkatesh, R.; Chidanand, K.J. Chondroblastoma patella presenting as a pathological fracture. Indian. J. Orthop. 2008, 42, 100–101. [Google Scholar] [CrossRef]
- Osanai, T.; Tsuchiya, T.; Ogino, T. Gastrocnemius muscle flap including Achilles tendon after extensive patellectomy for soft tissue sarcoma. Scand. J. Plast. Reconstr. Surg. Hand Surg. 2008, 42, 161–163. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Kim, J.D.; Chung, S.H. Osteosarcoma of the patella: Biologic reconstruction with allograft. Orthopedics 2009, 32, 43783. [Google Scholar] [CrossRef] [PubMed]
- Burk, T.F.; Horvai, A.E.; Gottschalk, A.R.; Leong, S.P.L.; Kashani-Sabet, M.; E Goldsby, R.; Law, J.; O’Donnell, R.J. Patellar metastatic melanoma in a 13-year-old boy. Am. J. Orthop. (Belle Mead NJ) 2010, 39, 582–586. [Google Scholar]
- Muramatsu, K.; Yoshida, K.; Fujii, K.; Okazaki, T.; Moriya, A.; Taguchi, T. Anatomical reconstruction of the knee extensor apparatus for prepatellar myxofibrosarcoma. Orthopedics 2010, 33, 773. [Google Scholar] [CrossRef]
- Çetinkaya, M.; Özer, H.; Selek, H.Y.; Erekul, S. Total patellectomy for patellar aneurysmal bone cyst. Eklem Hastalik. Cerrahisi. 2016, 27, 175–178. [Google Scholar] [CrossRef]
- Gomes, A.R.; Campos, F.N.; Becker, N.M.; Tamanini, J.G. Aneurysmal patellar bone cyst: Case report. Rev. Bras. Ortop. 2019, 54, 609–616. [Google Scholar] [CrossRef]
- Gómez-Palomo, J.M.; Jiménez-Garrido, C.; Martínez-Crespo, A.; García-Vera, J.J.; Pérez-Cardeña, J. Montañez-Heredia E. Extensor mechanism allograft in osteosarcoma of the patella: A case report. JBJS Case Connect 2019, 9, e0277. [Google Scholar] [CrossRef]
- Konchada, S.; Mishra, D.; Sinha, V.K.; Pradhan, P.; Salunke, A.A. “Banana patella”, fibrous dysplasia of patella: A rare case report. J. Clin. Orthop. Trauma 2019, 10, 418–421. [Google Scholar] [CrossRef]
- Kumar Arora, M.; Madaan, E.; Kumar, R. Giant cell tumor of patella: A case report and review of literature. J. Emerg. Pract. Trauma 2020, 6, 47–49. [Google Scholar] [CrossRef]
- Cao, X.; Chen, H.J. Successful outcome of patellectomy plus chemotherapy for primary bone lymphoma of the patella: A case report and literature review. Front. Oncol. 2021, 11, 786495. [Google Scholar] [CrossRef]
- Andreani, L.; Ipponi, E.; Nulvesu, G.; Di Sacco, F.; Fusari, N.; Capanna, R. Reconstruction of the extensor apparatus using a hybrid augment with olyethylene Mesmh and fascia lata allograft: Report of a case with patellar giant cell tumor of the Bone. J. Orthop. Case Rep. 2022, 12, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Furuta, T.; Sakuda, T.; Yoshioka, K.; Arihiro, K.; Adachi, N. Metastatic patellar bone tumor due to gastric cancer resembling a primary or secondary aneurysmal bone cyst: A case report. Int. J. Surg. Case Rep. 2023, 108, 108379. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, N.; Yanagawa, T.; Saito, K.; Chikuda, H. A new procedure for reconstructing the knee extension mechanism after resection of the knee joint and patella: A case report. JBJS Case Connect 2023, 13, e22.00341. [Google Scholar] [CrossRef]
- Chhajed, B.B.; Mehta, M.A.; Prajapati, A. A novel and effective surgical technique for reconstruction of extensor mechanism with iliotibial band tendon graft after patellectomy for primary patella tumor. Indian. J. Orthop. 2024, 58, 1310–1315. [Google Scholar] [CrossRef] [PubMed]
- Cosseddu, F.; Cordoni, M.; Bechini, E.; Ipponi, E.; Campo, F.R.; D’Arienzo, A.; Andreani, L. Knee extensor apparatus reconstruction with allograft after patellar resection: A case report. Acta Med. Litu. 2024, 31, 187–193. [Google Scholar] [CrossRef]
- Mukherjee, K.; Ghorai, T.K.; Kumar, A.; Basak, I. Giant cell tumor of patella with secondary aneurysmal bone cyst—A rare case report of anterior knee pain in young adults. J. Orthop. Case Rep. 2024, 14, 65–69. [Google Scholar] [CrossRef]
- Sakuda, T.; Furuta, T.; Adachi, N. Myopericytoma of the patella with local recurrence and patellectomy: A case report. Int. J. Surg. Case Rep. 2024, 115, 109263. [Google Scholar] [CrossRef]
- Kakazu, R.; Archdeacon, M.T. Surgical management of patellar fractures. Orthop. Clin. N. Am. 2016, 47, 77–83. [Google Scholar] [CrossRef]
- Markowitz, M.I.; Donato, Z.; Constantinescu, D.S.; Al-Hardan, W.; Baron, M.; Crawford, B. Orthopedic approaches for bone sarcoma: A bibliometric review of the 50 most cited papers. J. Orthop. 2023, 38, 53–61. [Google Scholar] [CrossRef]
- Ipponi, E.; Bechini, E.; Bettarini, V.; Cordoni, M.; Gentili, F.; D’arienzo, A.; Parchi, P.D.; Andreani, L. Fixation with carbon fiber plates after curettage in benign and locally aggressive bone tumors: Clinical and radiographic outcomes. J. Clin. Med. 2025, 14, 2371. [Google Scholar] [CrossRef] [PubMed]
- Andreani, L.; Ipponi, E.; Serrano, E.; De Franco, S.; Cordoni, M.; Bechini, E.; D’arienzo, A.; Parchi, P.D. Aneurysmal bone cyst of the pelvis in children and adolescents: Effectiveness of surgical treatment with curettage, cryotherapy and bone grafting. Healthcare 2023, 11, 2658. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.N.; Cammisa, F.P.; Sandhu, H.S.; Diwan, A.D.; Girardi, F.P.; Lane, J.M. The biology of bone grafting. J. Am. Acad. Orthop. Surg. 2005, 13, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Lucattelli, E.; Delcroix, L.; Baldrighi, C.; Tanini, S.; Innocenti, M. Quadriceps tendon reconstruction using a fascia lata included in a reverse-flow anterolateral thigh flap. Microsurgery 2019, 39, 642–646. [Google Scholar] [CrossRef]
- Rovere, G.; Smakaj, A.; Calori, S.; Barbaliscia, M.; Ziranu, A.; Pataia, E.; Maccauro, G.; De Mauro, D.; Liuzza, F. Use of muscular flaps for the treatment of knee prosthetic joint infection: A systematic review. Orthop. Rev. 2022, 14, 33943. [Google Scholar] [CrossRef]
- Rovere, G.; Smakaj, A.; De Mauro, D.; Marino, S.; Vitiello, R.; Meschini, C.; Ziranu, A.; Liuzza, F.; Maccauro, G.; Pataia, E. Medial gastrocnemius flap for the treatment of infected knee prostheses. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 60–65. [Google Scholar] [CrossRef]

| Article (Year) | N | Age | Diagnosis | D | S. T. I. | Fr. | Reconstruction | LR | C | C Type | Functional Comments | MSTS | ROM | FU |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Klenerman (1965) [17] | 1 | 48 | MTS Breast Carc. | - | No | No | NA | No | No | - | Walked with sticks before aggravation | NA | NA | 3 |
| Mercuri et al. (1991) [13] | 7 | 33.7 | 1 ABC 5 GCT bone 1 Plasmocyt. | - | NA | 7 No | NA | 1 Yes 6 No | NA | - | - | NA | NA | 53.7 |
| Jaeger et al. (1992) [18] | 1 | 65 | MTS Melanoma | - | No | No | Type A | NA | Yes | Died 2 weeks after surgery | - | NA | NA | 0.4 |
| Okada et al. (1994) [19] | 1 | 54 | OS | 5.0 | No | Yes | Type A | No | NA | - | - | NA | NA | 24 |
| Connel et al. (1997) [20] | 1 | 44 | GCT bone | - | No | No | Type B | No | No | - | - | NA | NA | 10 |
| Aktas et al. (1998) [21] | 1 | 55 | MTS Lung Carc. | 4.0 | No | No | NA | No | No | - | Returned to daily activities | NA | NA | 8 |
| MacDonald et al. (2001) [22] | 1 | 36 | GCT bone | 5.0 | No | No | None | No | No | - | - | NA | NA | 15 |
| Bhagat et al. (2008) [14] | 2 | 63.5 | 2 GCT bone | No | 1 Yes 1 No | NA | No | 1 Yes 1 No | 1 Post-op Stiffness | 1 Post-op Stiffness 1 Ok | NA | 1 Limited range 1 Full range | 60 | |
| Gudi et al. (2008) [23] | 1 | 24 | CB | - | No | Yes | None | No | No | - | - | NA | Full range | 24 |
| Osanai et al. (2008) [24] | 1 | 42 | MFH | 6.5 | Yes | No | Type B | No | No | - | - | 28 | 5/85 | 12 |
| Cho et al. (2009) [25] | 1 | 53 | OS | No | No | Type C | No | No | - | - | NA | 10/140 | 26 | |
| Burk et al. (2010) [26] | 1 | 13 | MTS Melanoma | No | No | NA | No | No | - | - | 30 | Full range | 42 | |
| Muramatsu et al. (2010) [27] | 1 | 69 | MFS | 3.5 | Yes | No | Type B | No | No | - | Extensor Apparatus MRC 4/5 | NA | 10/110 | 18 |
| Cetinkaya et al. (2016) [28] | 1 | 32 | ABC | 8.0 | No | No | Type B | No | No | - | - | NA | 5/105 | 22 |
| Muller et al. (2018) [15] | 6 | - | 2 MFS 2 PS 2 SS | Yes | No | Type B Type C | 2 Yes 4 No | 3 Yes 2 No | 1 Tendon graft rupture 1 Flap neurosis 1 Bone graft fracture | - | 24.7 | 10/82.5 | 80.3 | |
| Gomes et al. (2019) [29] | 1 | 23 | ABC | 12.3 | No | No | Type B | No | No | - | - | 30 | 0/110 | 2 |
| Gomez Palomo et al. (2019) [30] | 1 | 24 | MFS | - | No | No | Type C | No | No | - | - | NA | 0/120 | 60 |
| Srikant et al. (2019) [31] | 1 | 23 | FD | 13 | No | No | NA | No | No | - | - | NA | NA | NA |
| Kumar Arora et al. (2020) [32] | 1 | 16 | GCT bone | - | No | No | Type A | No | No | - | 30/30 | Full range | 22 | |
| Cao et al. (2021) [33] | 1 | 50 | Lymphoma | No | Yes | NA | No | No | - | NA | 0/135 | 12 | ||
| Merchan et al. (2021) [16] | 8 | 41.9 | 3 GCT bone 1 CB 1 CS 1 SS 1 MTS Lung Carc. 1 Lymphoma | - | NA | 1 Yes 7 No | NA | 8 No | NA | - | - | NA | NA | NA |
| Andreani et al. (2022) [34] | 1 | 67 | GCT bone | - | No | No | Type C | No | No | Extensor Apparatus MRC 5/5 | 30 | 0/120 | 14 | |
| Furuta et al. (2023) [35] | 1 | 65 | MTS Gastric Carc. | - | No | No | None | No | No | - | 26 | NA | 10 | |
| Yokoyama et al. (2023) [36] | 1 | 16 | OS | - | Yes | No | Type C | No | No | Walks with orthosis, but no crutches | NA | NA | 12 | |
| Chhajed et al. (2024) [37] | 1 | 33 | GCT bone | - | Yes | No | Type C | No | No | Gradual return to complete extension | 30 | Full range | 10 | |
| Cosseddu et al. (2024) [38] | 1 | 39 | MTS Lung Carc. | - | No | No | Type C | No | No | - | Extensor Apparatus MRC 5/5 | 30 | 0/120 | 12 |
| Mukherjee et al. (2024) [39] | 1 | 20 | GCT bone + ABC | - | No | No | Type A | No | No | - | Good range of motion, pain-free, no supports | NA | NA | 12 |
| Sakuda et al. (2024) [40] | 1 | 74 | Myopericyt. | 2.0 | No | No | Type A | No | No | - | Extensor Apparatus MRC 3/5 | NA | 10/110 | 36 |
| Reconstruction | Advantages | Disadvantages | Indications |
|---|---|---|---|
| TYPE A Direct suture | Less surgical time No augments needed | Soft tissues of the extensor apparatus must be preserved Limited extensor apparatus’ strength and clinical performances | Preserved soft tissues of the extensor apparatus and
|
| TYPE B Tendon plasty/Transfers/ Muscle flaps | Autologous tissue and active blood supply May include skin coverage | Longer surgical times Involvement of a donor site | Gaps in the extensor apparatus that could not be sutured, and
|
| TYPE C Allografts/ Synthetic augments | Mechanical strength and functional performances Can fulfill large gaps without a donor site | Require bone and tissue banks Risk of mechanical complications and failures |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ipponi, E.; Gentili, F.; Cosseddu, F.; D’Arienzo, A.; Parchi, P.D.; Andreani, L. Reconstruction of the Extensor Apparatus After Total Patellectomy in Orthopedic Oncology: A Systematic Literature Review. J. Clin. Med. 2025, 14, 4818. https://doi.org/10.3390/jcm14144818
Ipponi E, Gentili F, Cosseddu F, D’Arienzo A, Parchi PD, Andreani L. Reconstruction of the Extensor Apparatus After Total Patellectomy in Orthopedic Oncology: A Systematic Literature Review. Journal of Clinical Medicine. 2025; 14(14):4818. https://doi.org/10.3390/jcm14144818
Chicago/Turabian StyleIpponi, Edoardo, Fabrizia Gentili, Fabio Cosseddu, Antonio D’Arienzo, Paolo Domenico Parchi, and Lorenzo Andreani. 2025. "Reconstruction of the Extensor Apparatus After Total Patellectomy in Orthopedic Oncology: A Systematic Literature Review" Journal of Clinical Medicine 14, no. 14: 4818. https://doi.org/10.3390/jcm14144818
APA StyleIpponi, E., Gentili, F., Cosseddu, F., D’Arienzo, A., Parchi, P. D., & Andreani, L. (2025). Reconstruction of the Extensor Apparatus After Total Patellectomy in Orthopedic Oncology: A Systematic Literature Review. Journal of Clinical Medicine, 14(14), 4818. https://doi.org/10.3390/jcm14144818







