Myopericytoma Masquerading as Dupuytren’s Disease: A Case Report and Systematic Literature Review
Abstract
1. Introduction
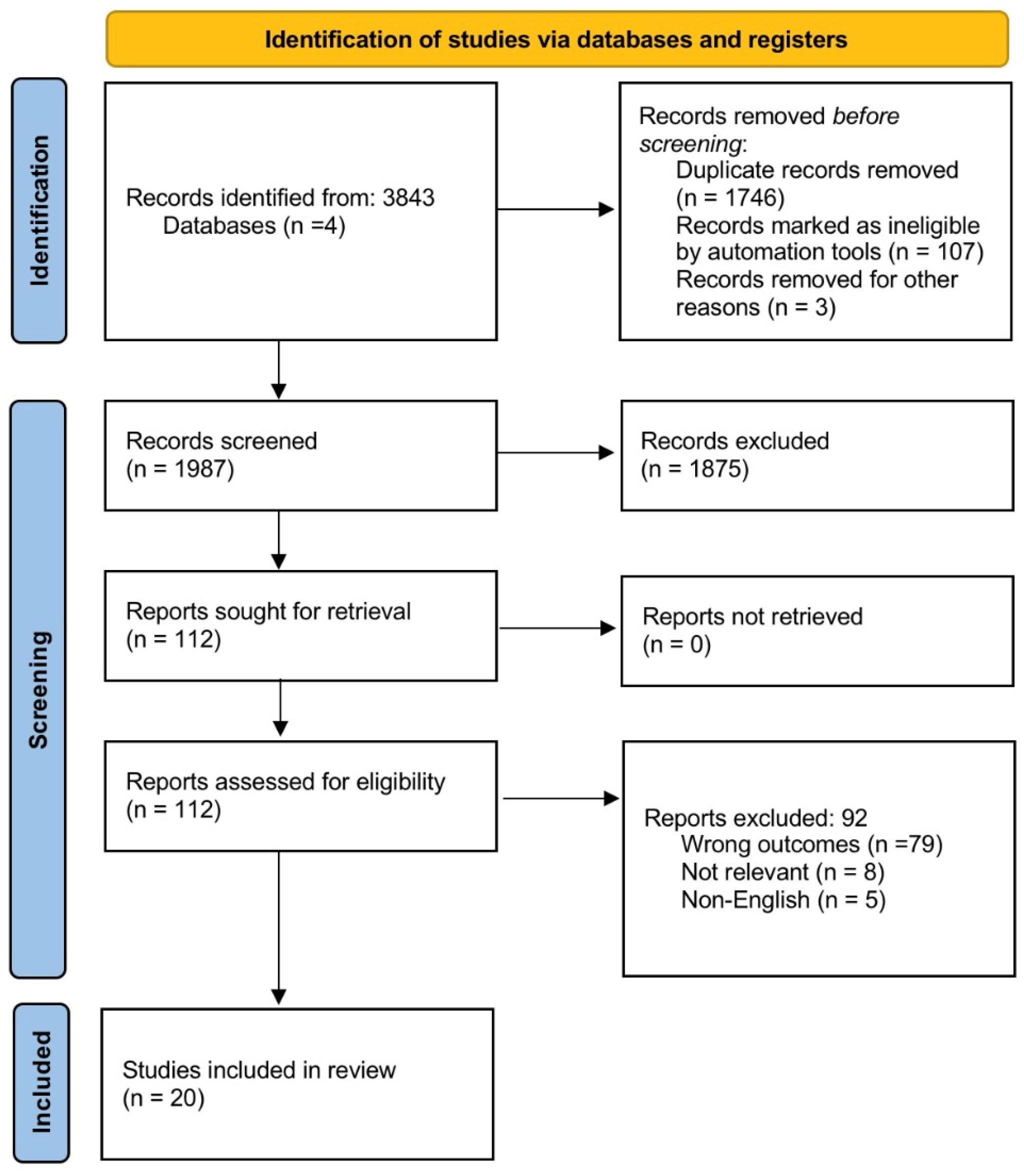
2. Materials and Methods
2.1. Search Strategy
2.2. Literature Search
2.3. Data Extraction
2.4. Quality Assessment
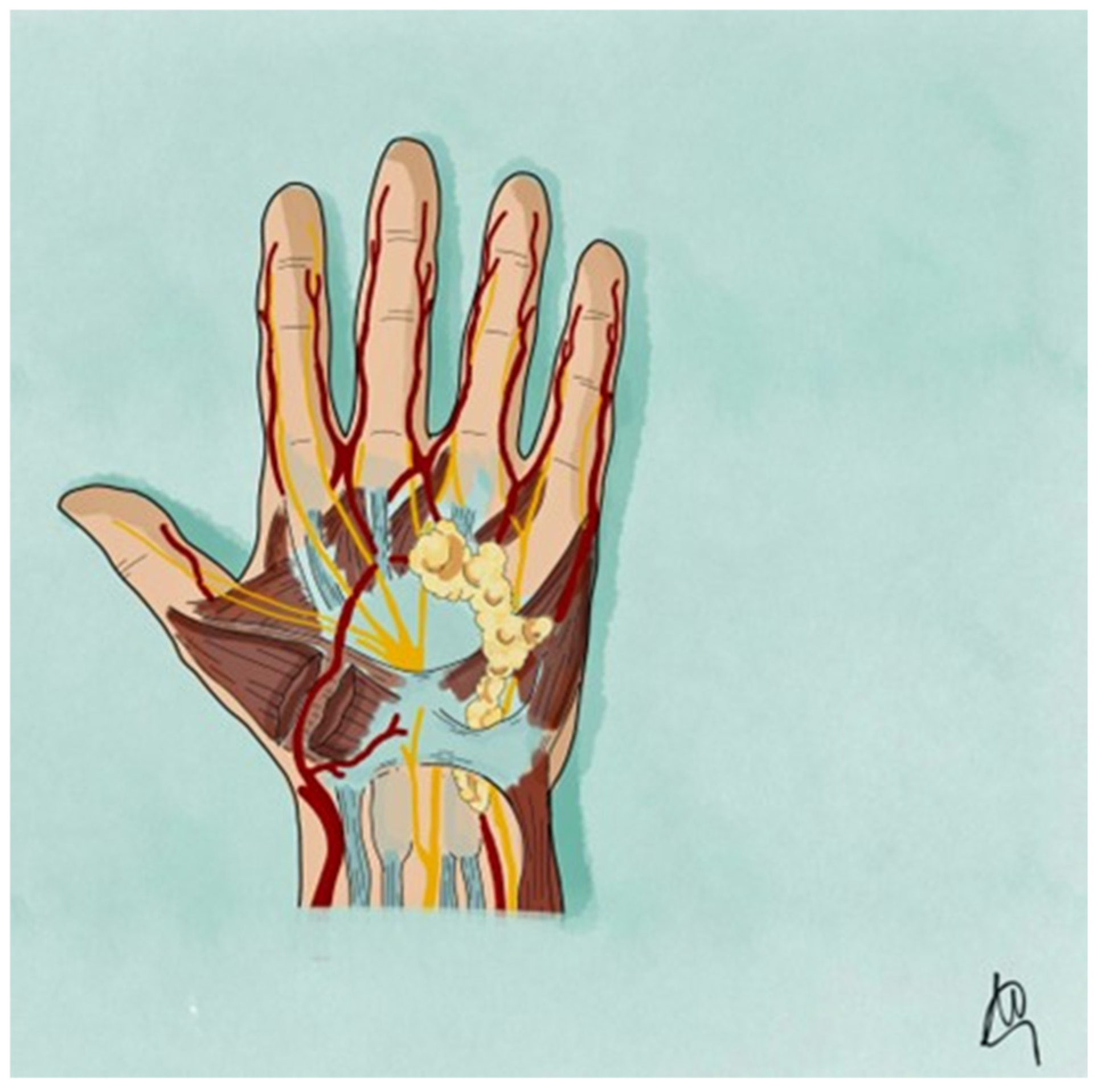
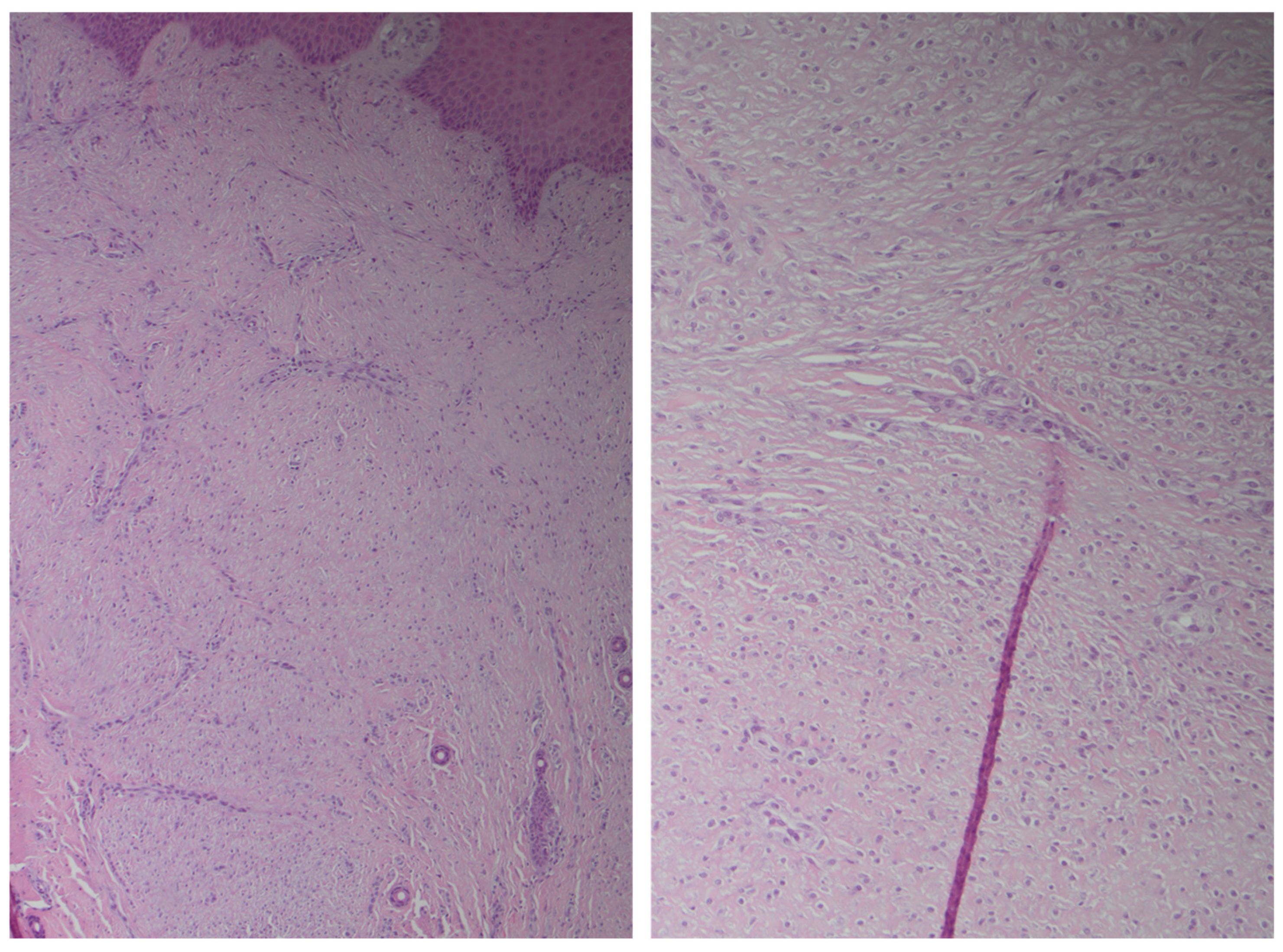
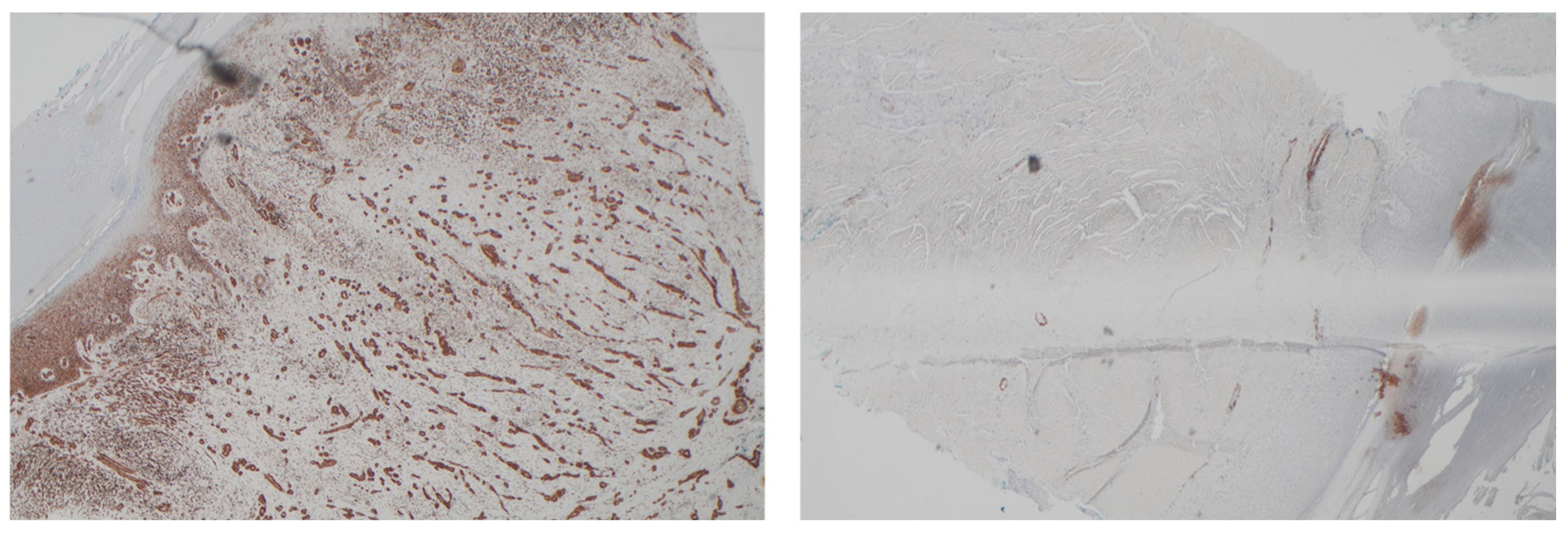
2.5. Case Description
3. Results
Questions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AN | Asymptomatic nodule |
| CPVP | Concentric perivascular proliferation |
| H&N | Head and neck |
| LE | Lower extremities |
| PN | Painful nodule |
| SMA | Smooth muscle actin |
| UE | Upper extremities |
| S | Single lesion |
| M | Multiple lesion |
| DD | Dupuytren’s Disease |
References
- Granter, S.R.; Badizadegan, K.; Fletcher, C.D. Myofibromatosis in adults, glomangiopericytoma, and myopericytoma: A spectrum of tumors showing perivascular myoid differentiation. Am. J. Surg. Pathol. 1998, 22, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, P.S.; Belfi, L.M.; Garcia, R.A.; Fufa, D.T.; Bartolotta, R.J. Myopericytoma of the hand. Skelet. Radiol. 2024, 1, 1531–1535. [Google Scholar] [CrossRef]
- McMenamin, M.; Fletcher, C. Malignant myopericytoma: Expanding the spectrum of tumours with myopericytic differentiation. Histopathology 2002, 41, 450–460. [Google Scholar] [CrossRef]
- Werker, P.M.; Pess, G.M.; van Rijssen, A.L.; Denkler, K. Correction of contracture and recurrence rates of Dupuytren contracture following invasive treatment: The importance of clear definitions. J. Hand Surg. 2012, 37, 2095–2105. [Google Scholar] [CrossRef]
- Luo, J.; Tugade, T.; Sun, E.; Pena Diaz, A.M.; O’Gorman, D.B. Sustained AWT1 expression by Dupuytren’s disease myofibroblasts promotes a proinflammatory milieu. J. Cell Commun. Signal. 2022, 16, 677–690. [Google Scholar] [CrossRef]
- Gonga-Cavé, B.C.; Pena Diaz, A.M.; O’Gorman, D.B. Biomimetic analyses of interactions between macrophages and palmar fascia myofibroblasts derived from Dupuytren’s disease reveal distinct inflammatory cytokine responses. Wound Repair. Regen. 2021, 29, 627–636. [Google Scholar] [CrossRef]
- Del Frari, B.; Zelger, B.; Piza-Katzer, H. Epithelioid sarcoma of the hand, a seldomly recognized tumor. Handchir. Mikrochir. Plast. Chir. 2004, 36, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Murad, M.H.; Sultan, S.; Haffar, S.; Bazerbachi, F. Methodological quality and synthesis of case series and case reports. BMJ Evid. Based Med. 2018, 23, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Mikami, Y.; Shiomi, T.; Manabe, T. Perivascular myoma: Case report with immunohistochemical and ultrastructural studies. Pathol. Int. 2002, 52, 69–74. [Google Scholar] [CrossRef]
- Mentzel, T.; Dei Tos, A.P.; Sapi, Z.; Kutzner, H. Myopericytoma of skin and soft tissues: Clinicopathologic and immunohistochemical study of 54 cases. Am. J. Surg. Pathol. 2006, 30, 104–113. [Google Scholar] [CrossRef]
- Dray, M.S.; McCarthy, S.W.; Palmer, A.A.; Bonar, S.F.; Stalley, P.D.; Marjoniemi, V.; Millar, E.; Scolyer, A.R. Myopericytoma: A unifying term for a spectrum of tumours that show overlapping features with myofibroma. A review of 14 cases. J. Clin. Pathol. 2006, 59, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Sadahira, C.; Yoneda, K.; Moriue, T.; Takai, I.; Kushida, Y.; Haba, R.; Kubota, Y. Periungual myopericytoma. J. Am. Acad. Dermatol. 2006, 54, 1107–1108. [Google Scholar] [CrossRef]
- Harreld, K.L.; Li, H.; Li, Z. Orthopaedic Radiology Pathology Conference: Painful palmar mass in a 36-year-old woman. Clin. Orthop. Relat. Res. 2010, 468, 917–920. [Google Scholar] [CrossRef] [PubMed]
- Wagner, E.R.; Shin, A.Y. Myopericytoma of the hypothenar eminence: Case report. Hand 2015, 10, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Kara, A.; Keskinbora, M.; Kayaalp, M.E.; Şeker, A.; Erdil, M.; Bülbül, M. An atypical presentation of myopericytoma in palmar arch and review of the literature. Case Rep. Orthop. 2014, 2014, 759329. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Shi, X.; Li, J.; Qian, L. Imaging features of myopericytoma of the breast: A case report and review of the literature. Radiol. Case Rep. 2021, 16, 98–102. [Google Scholar] [CrossRef]
- Seen, K.F. A case review of myopericytoma: Radiological, macroscopic and histological correlation. Pathology 2014, 46, S69. [Google Scholar] [CrossRef]
- Jairajpuri, Z.S.; Hasan, M.J.; Madan, G.B.; Jetley, S. Myopericytoma: A unifying term or a unique entity? Malays. J. Pathol. 2016, 38, 159. [Google Scholar]
- Morzycki, A.; Joukhadar, N.; Murphy, A.; Williams, J. Digital myopericytoma: A case report and systematic literature review. J. Hand Microsurg. 2017, 9, 32–36. [Google Scholar] [CrossRef]
- Gulhane, S.; Sinha, R.; Gangane, N. Myopericytoma of hand: A case report with review of literature. Galore Int. J. Heal. Sci. Res. 2017, 2, 1–5. [Google Scholar]
- Van Camp, L.; Goubau, J.; Van den Berghe, I.; Mermuys, K. Myopericytoma of the base of the finger: Radiological and pathological description of a rare benign entity. J. Hand Surg. 2019, 44, e1–e69. [Google Scholar] [CrossRef] [PubMed]
- Crombé, A.; Bouhamama, A.; Loarer, F.L.; Kind, M. Radiopathological correlations of myopericytomas of the hand: Emphasis on the MRI perivascular pushing growth pattern. BJR Case Rep. 2020, 6, 20190074. [Google Scholar] [CrossRef]
- Kumar, M.K.; Panchal, S.; Dabral, L.; Prabhakar, A. Myopericytoma involving proximal phalanx of index finger: Masquerading as tenosynovial giant cell tumor—A case report. J. Orthop. Case Rep. 2021, 11, 88. [Google Scholar] [CrossRef]
- Alhujayri, A.K.; Alsugair, S.I.; Al Mishal, O. Fast growing myopericytoma of the hand: Case report and literature review. Int. J. Surg. Case Rep. 2021, 85, 106220. [Google Scholar] [CrossRef]
- Ahatov, R.R.; Hoyer, P.; Weisert, E.; Kelly, B. Exophytic nodule on the finger. Cureus 2021, 13, e19137. [Google Scholar] [CrossRef] [PubMed]
- Menozzi, M.; Acciaro, A.L.; Vanni, S.; Adani, R. Myoperycitoma at the finger: Case-report and literary review. Riv. Ital. Di Chir. Della Mano 2022, 59, 41–44. [Google Scholar]
- Awosusi, B.L.; Attia, O.M.; Nwadiokwu, J. Myopericytoma of the right middle finger: A case report of an uncommon perivascular neoplasm. Cureus 2024, 16, e61938. [Google Scholar] [CrossRef]
- Michot, A.; Chaput, B.; Alet, J.-M.; Pelissier, P. Arterial myopericytoma resulting in Guyon’s canal syndrome. Hand Surg. Rehabil. 2016, 35, 231–233. [Google Scholar] [CrossRef]
- Seth, I.; Marcaccini, G.; Lim, K.; Castrechini, M.; Cuomo, R.; Ng, S.K.-H.; Ross, R.J.; Rozen, W.M. Management of Dupuytren’s Disease: A Multi-Centric Comparative Analysis Between Experienced Hand Surgeons Versus Artificial Intelligence. Diagnostics 2025, 15, 587. [Google Scholar] [CrossRef]


| Case Report | Cases, n | Gender | Age, Years | Site | Clinical Presentation | Lesions | Size, mm | Histopathologic Features | Positive Immunohistochemistry | Local Recurrence (Follow-Up Period) |
|---|---|---|---|---|---|---|---|---|---|---|
| Mikami et al. [9] | 1 | Female | 61 | UE (hand) | AN | S | 20 | CPVP of ovoid spindle cells, vascular channels | SMA | Unknown |
| Mentzel et al. [10] | 16 | Mixed | 13–87 | UE | Unknown | S, multiple | Various | CPVP of spindle and plump myoid cells | SMA, h-caldesmon, desmin | Malignant in 1 (1 year), benign in others |
| Dray et al. [11] | 5 | Mixed | 13–71 | UE (hand) | AN | S, multiple | 6–90 | Bland round/oval cells, vascular channels | SMA | No recurrence (0.5–4 years) |
| Sadahira et al. [12] | 1 | Male | 70 | Volar aspect of left third, fourth and fifth digits | Non-painful | M, 3 | 5 | Proliferation of spindle to oval shaped cells showing a concentric growth pattern | Positive for smooth muscle actin and vimentin | NS |
| Harreld et al. [13] | 1 | Female | 36 | Left palm mid diaphyseal region between index and long metacarpals | Sharp pain over left palm, no visible mass | S | 5 | Spindle cells with multiple small, scattered vascular spaces | Positive for smooth muscle actin | None (2 years) |
| Wagner and Shin [14] | 1 | Male | 58 | UE (hand) | Hypothenar mass, wrist pain | S | 18 | Spindle cells, perivascular growth | SMA, desmin | None (2 years) |
| Kara et al. [15] | 1 | Male | 18 | UE (hand) | Painful mass, ulnar nerve compression | S | 24 | Perivascular spindle-shaped cells | SMA, caldesmon | None (14 months) |
| Yang et al. [16] | 1 | Female | 44 | Ulnar aspect of the right distal forearm | Painless, slow-growing mass present for 5 years | S | 40 × 20 | Elongated blood vessels surrounded by spindle-shaped cells with bland morphology | Vimentin, smooth muscle actin, muscle-specific actin, h-caldesmon | None at 5 months |
| KF Seen [17] | 1 | Male | 76 | Right hand | Painless solitary nodule | S | 45 | Myoid appearing plump spindled cells with abundant eosinophilic cytoplasm | Positive for smooth muscle actin and calponin. Negative for S100 and CD34. | None |
| Jairajpuri et al. [18] | 1 | Male | 52 | UE (left thumb) | Painless nodule, 9 months duration | S | 15 × 12 | Proliferating spindle-to-ovoid bland cells with eosinophilic cytoplasm | SMA positive, negative for desmin and CD34 | Unknown |
| Morzycki et al. [19] | 1 | Male | 33 | UE (left index finger) | Painless swelling, erythema | S | Not specified (extensive destruction of the distal phalanx) | Oval-to-spindle–shaped cells, eosinophilic cytoplasm, indistinct cellular border | SMA, vimentin | None (81 days) |
| Gulhane et al. [20] | 1 | Female | 66 | Hand (thumb) | Swelling, pain | S | 24 | CPVP of spindle cells, abundant myxoid stroma | SMA, vimentin | Unknown |
| Van Camp et al. [21] | 1 | Female | 46 | Base between the fourth and fifth fingers of the right hand | Non-painful, progressively enlarging mass over two years. Interfered with daily activities. | S | 23 × 15 × 31 | Well-defined tumour with spindle-shaped smooth muscle cells arranged around blood vessels. Diagnosis confirmed as a benign smooth-muscle neoplasm | Positive for alpha-smooth muscle actin (ASMA) and desmin. Negative for CD34, CD68, CD31, and Ki-67. | None (2 years) |
| Crombé et al. [22] | 3 | Mixed | Case 1: 72 Case 2: 30 Case 3: 51 | Case 1: Ulnar palmar side of the hand Case 2: Palmar and ulnar side of the left wrist Case 3: Fifth finger of the right hand. | Case 1: Slowly growing lump over 5 years, progressively painful Case 2: Discrete lump with paraesthesia in the ulnar nerve territory Case 3: Slowly progressive lump, painless | S | Case 1: 54 Case 2: 45 Case 3: 9 | Concentric perivascular growth pattern with fusiform cells, no atypia, low Ki-67 proliferative activity, strong staining for alpha-smooth muscle actin, desmin, and h-caldesmon. | Positive for ASMA, h-caldesmon, and desmin. CD34 was variably positive. | Case 1: No recurrence after 3 years Case 2: No recurrence after 9 years Case 3: No recurrence after 7 years |
| Kumar et al. [23] | 1 | Male | 83 | UE (right index finger) | Insidious, painless mass for 40 years, became painful after recent trauma. Firm, non-compressible, mobile, with overlying hyperpigmentation. | S | Not specified | Encapsulated soft tissue lesion with abundant vascular structures, proliferating spindle cells (myopericytes) with concentric perivascular growth. No nuclear atypia or abnormal mitotic activity. | Immunohistochemistry was not performed due to financial constraints | None (3 years) |
| Alhujayri et al. [24] | 1 | Male | 45 | Hand (1st webspace) | Fast-growing mass | S | 30 | Spindle cells, concentric vascular growth | SMA, caldesmon | None (6 months) |
| Ahatov et al. [25] | 1 | Female | 45 | Volar aspect of the left index finger middle phalanx | Painless slow growing mass | S | 5 | Concentric perivascular spindle cells | Positive for smooth muscle actin | None in 6 months |
| Menozzi et al. [26] | 1 | Male | 54 | Volar aspect of right thumb distal phalanx | Painful, solid, slow growing mass | S | NS | Ovoid spindle cells with elongated nuclei and eosinophilic cytoplasm | positive for smooth muscle actin stain and focal positivity for desmin | None (14 months) |
| Sullivan et al. [2] | 1 | Male | 60 | UE (hand) | Painful mass, gradual growth over 20 years | S | 18 × 12 × 21 | Non-encapsulated lesion with multiple blood vessels and concentric perivascular proliferation of spindle-shaped myoid cells. No nuclear atypia. | Positive for smooth muscle actin (SMA) and h-caldesmon. Negative for desmin and CD34 | None |
| Awosusi et al. [27] | 1 | Male | 29 | Volar aspect of the right middle finger. | Painless swelling initially suspected to be an epidermal inclusion cyst. | S | 15 × 15 | Circumscribed, nodular soft tissue mass confined to the dermis; diagnosed as myopericytoma upon histopathologic evaluation | Positive for smooth muscle actin (SMA), h-caldesmon, and vimentin. Negative for CD34, CD68, S100, and desmin | None (1 year) |
| Selection | Ascertainment | Causality | Reporting | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Case Report | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | Overall Risk of Bias |
| Mikami et al. [9] | N | Y | U | Y | N/A | N/A | Y | Y | Low risk |
| Mentzel et al. [10] | N | U | Y | Y | N/A | N/A | N | Y | Moderate risk |
| Dray et al. [11] | U | Y | U | Y | N/A | N/A | U | Y | Low risk |
| Sadahira et al. [12] | N | U | Y | Y | U | N/A | N | Y | Moderate risk |
| Harreld et al. [13] | U | Y | Y | Y | N/A | N/A | Y | Y | Low risk |
| Wagner and Shin [14] | U | Y | Y | Y | N/A | N/A | U | Y | Moderate risk |
| Kara et al. [15] | U | Y | Y | Y | N/A | N/A | Y | Y | Low risk |
| Yang et al. [16] | U | Y | Y | Y | N/A | N/A | Y | Y | Low risk |
| KF Seen. [17] | N | U | Y | Y | U | N/A | N | Y | Moderate risk |
| Jairajpuri et al. [18] | U | Y | Y | Y | N/A | N/A | U | Y | Low risk |
| Morzycki et al. [19] | U | Y | Y | Y | N/A | N/A | U | Y | Low risk |
| Gulhane et al. [20] | U | Y | Y | Y | N/A | N/A | U | Y | Low risk |
| Van Camp et al. [21] | N | U | Y | Y | U | N/A | N | Y | Moderate risk |
| Crombé et al. [22] | U | Y | Y | Y | N/A | N/A | Y | Y | Low risk |
| Kumar et al. [23] | U | Y | Y | Y | N/A | N/A | Y | Y | Low risk |
| Alhujayri et al. [24] | U | Y | Y | Y | N/A | N/A | U | Y | Low risk |
| Ahatov et al. [25] | U | Y | Y | Y | N/A | N/A | U | Y | Low risk |
| Menozzi et al. [26] | U | Y | Y | Y | N/A | N/A | Y | Y | Low risk |
| Sullivan et al. [2] | U | Y | Y | Y | N/A | N/A | U | Y | Low risk |
| Awosusi et al. [27] | U | Y | Y | Y | N/A | N/A | Y | Y | Low risk |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcaccini, G.; Seth, I.; Novo, J.; Bautista, M.; Ruhunage, L.; Bhat, S.; Cuomo, R.; Rozen, W.M. Myopericytoma Masquerading as Dupuytren’s Disease: A Case Report and Systematic Literature Review. J. Clin. Med. 2025, 14, 3703. https://doi.org/10.3390/jcm14113703
Marcaccini G, Seth I, Novo J, Bautista M, Ruhunage L, Bhat S, Cuomo R, Rozen WM. Myopericytoma Masquerading as Dupuytren’s Disease: A Case Report and Systematic Literature Review. Journal of Clinical Medicine. 2025; 14(11):3703. https://doi.org/10.3390/jcm14113703
Chicago/Turabian StyleMarcaccini, Gianluca, Ishith Seth, Jennifer Novo, Marcus Bautista, Lakal Ruhunage, Saiuj Bhat, Roberto Cuomo, and Warren M. Rozen. 2025. "Myopericytoma Masquerading as Dupuytren’s Disease: A Case Report and Systematic Literature Review" Journal of Clinical Medicine 14, no. 11: 3703. https://doi.org/10.3390/jcm14113703
APA StyleMarcaccini, G., Seth, I., Novo, J., Bautista, M., Ruhunage, L., Bhat, S., Cuomo, R., & Rozen, W. M. (2025). Myopericytoma Masquerading as Dupuytren’s Disease: A Case Report and Systematic Literature Review. Journal of Clinical Medicine, 14(11), 3703. https://doi.org/10.3390/jcm14113703








