Novel Compound Heterozygous Mutation of the ABCA3 Gene in a Patient with Neonatal-Onset Interstitial Lung Disease
Abstract
1. Introduction
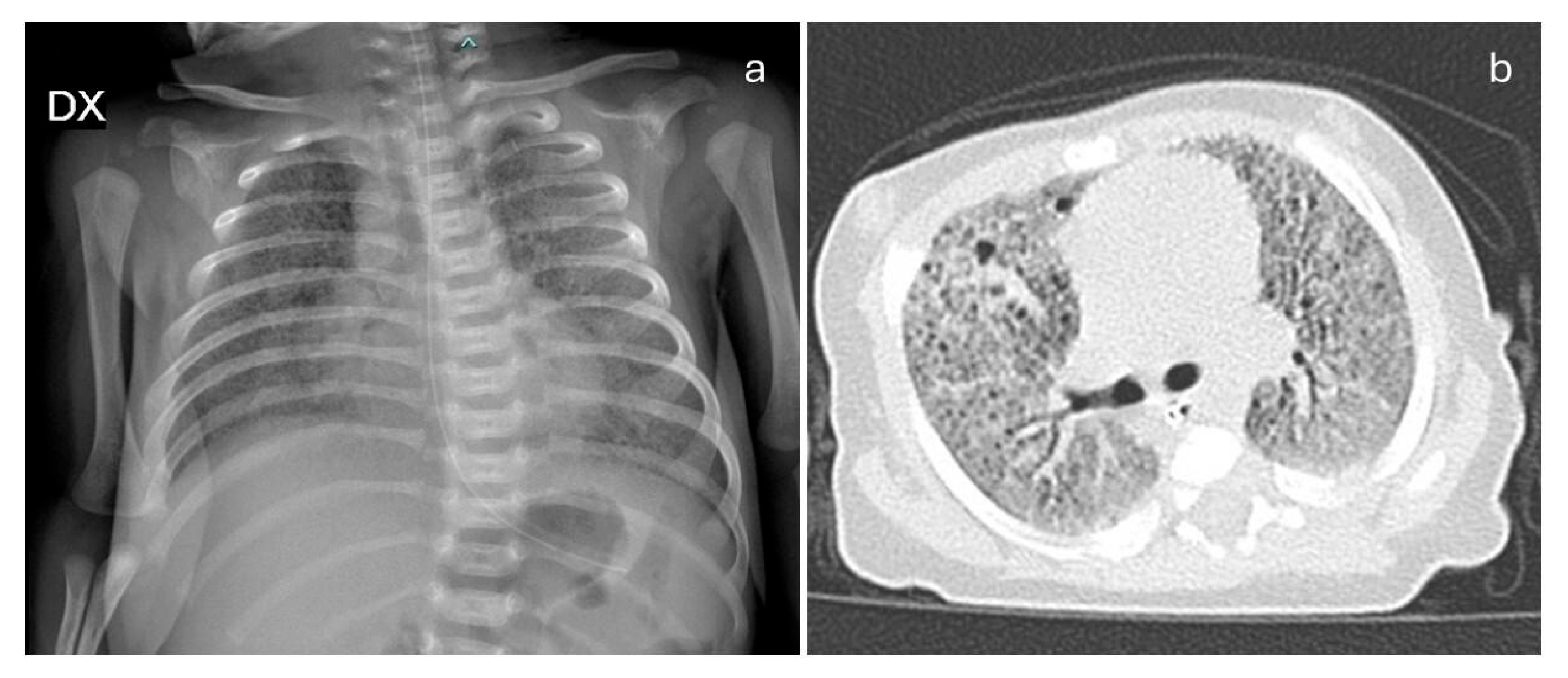
2. Case Report and Literature Review
2.1. Genetic Analysis
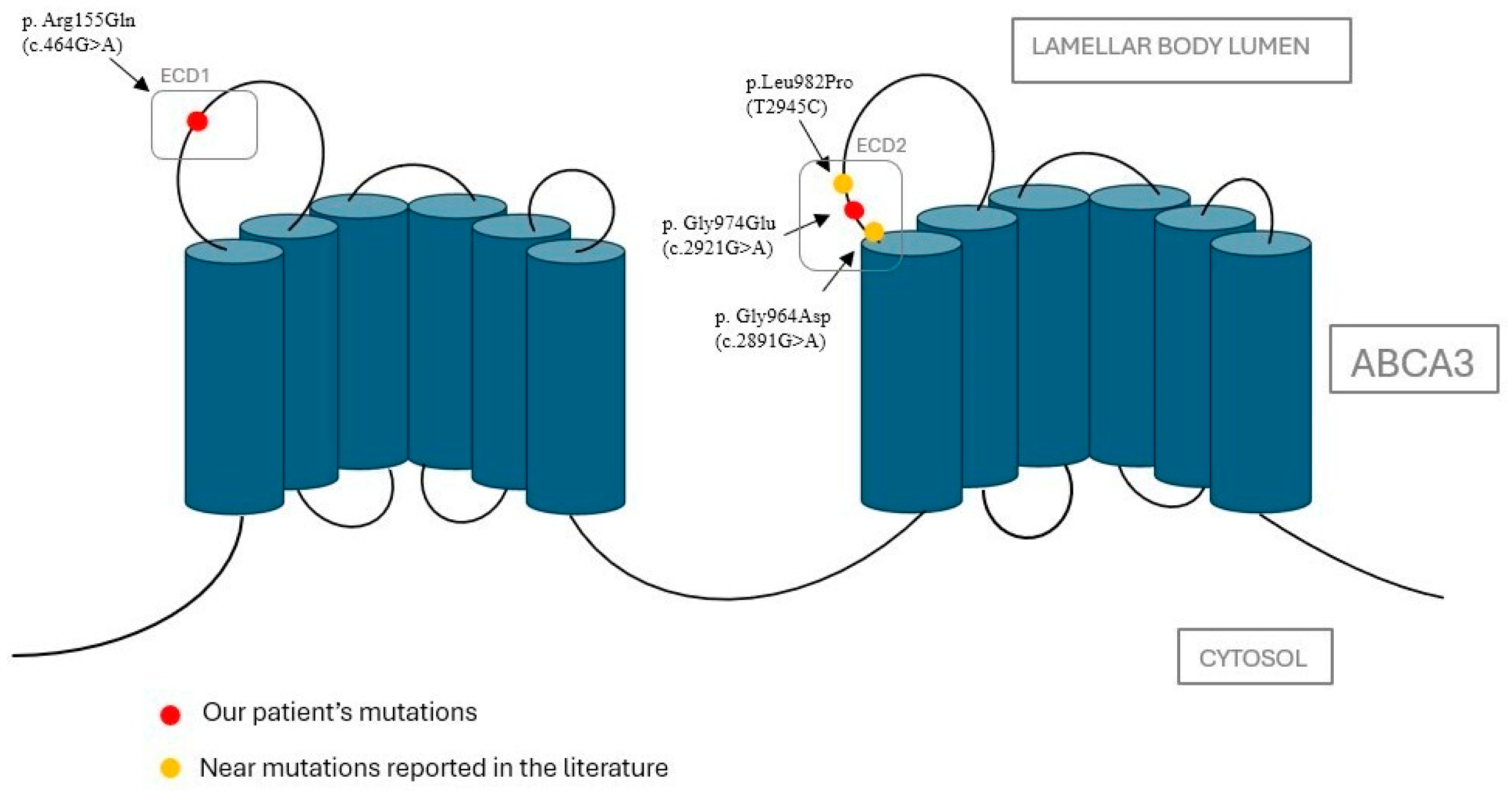
2.2. Bioinformatic Analysis on Protein Function of Identified Variants
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement:
Acknowledgments
Conflicts of Interest
References
- Cunningham, S.; Jaffe, A.; Young, L.R. Children’s interstitial and diffuse lung disease. Lancet Child. Adolesc. Health 2019, 3, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Kurland, G.; Deterding, R.R.; Hagood, J.S.; Young, L.R.; Brody, A.S.; Castile, R.G.; Dell, S.; Fan, L.L.; Hamvas, A.; Hilman, B.C.; et al. American Thoracic Society Committee on Childhood Interstitial Lung Disease (chILD) and the chILD Research Network. An official American Thoracic Society clinical practice guideline: Classification, evaluation, and management of childhood interstitial lung disease in infancy. Am. J. Respir. Crit. Care Med. 2013, 188, 376–394. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Serra, G.; Memo, L.; Cavicchioli, P.; Cutrone, M.; Giuffrè, M.; La Torre, M.L.; Schierz, I.A.M.; Corsello, G. Novel mutations of the ABCA12, KRT1 and ST14 genes in three unrelated newborns showing congenital ichthyosis. Ital. J. Pediatr. 2022, 48, 145. [Google Scholar] [CrossRef] [PubMed]
- Serra, G.; Antona, V.; Cannata, C.; Giuffrè, M.; Piro, E.; Schierz, I.A.M.; Corsello, G. Distal Arthrogryposis type 5 in an Italian family due to an autosomal dominant gain-of-function mutation of the PIEZO2 gene. Ital. J. Pediatr. 2022, 48, 133. [Google Scholar] [CrossRef]
- Valutazione Antropometrica Neonatale. Riferimento Carte INeS. Available online: http://www.inescharts.com (accessed on 12 May 2025).
- Bush, A.; Cunningham, S.; de Blic, J.; Barbato, A.; Clement, A.; Epaud, R.; Hengst, M.; Kiper, N.; Nicholson, A.G.; Wetzke, M.; et al. chILD-EU Collaboration. European protocols for the diagnosis and initial treatment of interstitial lung disease in children. Thorax 2015, 70, 1078–1084. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Child Growth Standards. 2021. Available online: https://www.who.int/tools/child-growth-standards/standards (accessed on 12 May 2025).
- Hamvas, A.; Nogee, L.M.; Wegner, D.J.; Depass, K.; Christodoulou, J.; Bennetts, B.; McQuade, L.R.; Gray, P.H.; Deterding, R.R.; Carroll, T.R.; et al. Inherited surfactant deficiency caused by uniparental disomy of rare mutations in the surfactant protein-B and ATP binding cassette, subfamily a, member 3 genes. J. Pediatr. 2009, 155, 854–859.e1. [Google Scholar] [CrossRef][Green Version]
- Ciantelli, M.; Ghirri, P.; Presi, S.; Sigali, E.; Vuerich, M.; Somaschini, M.; Ferrari, M.; Boldrini, A.; Carrera, P. Fatal respiratory failure in a full-term newborn with two ABCA3 gene mutations: A case report. J. Perinatol. 2011, 31, 70–72. [Google Scholar] [CrossRef][Green Version]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Laboratory Quality Assurance Committee. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef]
- Rehder, C.; Bean, L.J.H.; Bick, D.; Chao, E.; Chung, W.; Das, S.; O’Daniel, J.; Rehm, H.; Shashi, V.; Vincent, L.M.; et al. Next-generation sequencing for constitutional variants in the clinical laboratory, 2021 revision: A technical standard of the American College of Medical Genetics and Genomics (ACMG). Genet. Med. 2021, 23, 1399–1415. [Google Scholar] [CrossRef]
- Matthijs, G.; Souche, E.; Alders, M.; Corveleyn, A.; Eck, S.; Feenstra, I.; Race, V.; Sistermans, E.; Sturm, M.; Weiss, M.; et al. EuroGentest; European Society of Human Genetics. Guidelines for diagnostic next-generation sequencing. Eur. J. Hum. Genet. 2016, 24, 2–5. [Google Scholar] [CrossRef]
- ISO 9001:2015; Standard Title: Quality Management Systems. International Organization for Standardization (ISO): Geneva, Switzerland, 2015.
- Vaser, R.; Adusumalli, S.; Leng, S.N.; Sikic, M.; Ng, P.C. SIFT missense predictions for genomes. Nat. Protoc. 2016, 11, 1–9. [Google Scholar] [CrossRef]
- Adzhubei, I.; Jordan, D.M.; Sunyaev, S.R. Predicting functional effect of human missense mutations using PolyPhen-2. Curr. Protoc. Hum. Genet. 2013, 7, Unit7.20. [Google Scholar] [CrossRef]
- Steinhaus, R.; Proft, S.; Schuelke, M.; Cooper, D.N.; Schwarz, J.M.; Seelow, D. MutationTaster2021. Nucleic. Acids Res. 2021, 49, W446–W451. [Google Scholar] [CrossRef] [PubMed]
- Panther Classification System. PANTHER™ Website News. Available online: https://www.pantherdb.org (accessed on 17 September 2023).
- Beers, M.F.; Mulugeta, S. The biology of the ABCA3 lipid transporter in lung health and disease. Cell Tissue Res. 2017, 367, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Chai, A.B.; Ammit, A.J.; Gelissen, I.C. Examining the role of ABC lipid transporters in pulmonary lipid homeostasis and inflammation. Respir. Res. 2017, 18, 41. [Google Scholar] [CrossRef] [PubMed]
- Tirelli, C.; Pesenti, C.; Miozzo, M.; Mondoni, M.; Fontana, L.; Centanni, S. The Genetic and Epigenetic Footprint in Idiopathic Pulmonary Fibrosis and Familial Pulmonary Fibrosis: A State-of-the-Art Review. Diagnostics 2022, 12, 3107. [Google Scholar] [CrossRef]
- Kuning, A.M.; Parker, T.A.; Nogee, L.M.; Abman, S.H.; Kinsella, J.P. ABCA3 deficiency presenting as persistent pulmonary hypertension of the newborn. J. Pediatr. 2007, 151, 322–324. [Google Scholar] [CrossRef]
- Anandarajan, M.; Paulraj, S.; Tubman, R. ABCA3 Deficiency: An unusual cause of respiratory distress in the newborn. Ulster Med. J. 2009, 78, 51–52. [Google Scholar]
- Hallik, M.; Annilo, T.; Ilmoja, M.L. Different course of lung disease in two siblings with novel ABCA3 mutations. Eur. J. Pediatr. 2014, 173, 1553–1556. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, J.P.; Pinheiro, L.; Costa, M.; Silva, A.; Gonçalves, A.; Pereira, A. Novel ABCA3 mutations as a cause of respiratory distress in a term newborn. Gene 2014, 534, 417–420. [Google Scholar] [CrossRef]
- Malý, J.; Navrátilová, M.; Hornychová, H.; Looman, A.C. Respiratory failure in a term newborn due to compound heterozygous ABCA3 mutation: The case report of another lethal variant. J. Perinatol. 2014, 34, 951–953. [Google Scholar] [CrossRef] [PubMed]
- Moore, G.P.; Lines, M.A.; Geraghty, M.T.; de Nanassy, J.; Kovesi, T. Novel mutation in ABCA3 resulting in fatal congenital surfactant deficiency in two siblings. Am. J. Respir. Crit. Care Med. 2014, 189, 750–752. [Google Scholar] [CrossRef] [PubMed]
- Jackson, T.; Wegner, D.J.; White, F.V.; Hamvas, A.; Cole, F.S.; Wambach, J.A. Respiratory failure in a term infant with cis and trans mutations in ABCA3. J. Perinatol. 2015, 35, 231–232. [Google Scholar] [CrossRef]
- Piersigilli, F.; Peca, D.; Campi, F.; Corsello, M.; Landolfo, F.; Boldrini, R.; Danhaive, O.; Dotta, A. New ATP-binding cassette A3 mutation causing surfactant metabolism dysfunction pulmonary type 3. Pediatr. Int. 2015, 57, 970–974. [Google Scholar] [CrossRef]
- Pachajoa, H.; Ruiz-Botero, F.; Meza-Escobar, L.E.; Villota-Delgado, V.A.; Ballesteros, A.; Padilla, I.; Duarte, D. Fatal respiratory disease due to a homozygous intronic ABCA3 mutation: A case report. J. Med. Case Rep. 2016, 10, 266. [Google Scholar] [CrossRef]
- Tan, J.K.; Murray, C.; Schultz, A. ABCA3 lung disease in an ex 27 week preterm infant responsive to systemic glucocorticosteroids. Pediatr. Pulmonol. 2016, 51, E1–E3. [Google Scholar] [CrossRef]
- Ota, C.; Kimura, M.; Kure, S. ABCA3 mutations led to pulmonary fibrosis and emphysema with pulmonary hypertension in an 8-year-old girl. Pediatr. Pulmonol. 2016, 51, E21–E23. [Google Scholar] [CrossRef] [PubMed]
- Uchida, D.A.; Wert, S.E.; Nogee, L.M.; Carroll, T.R.; Chatfield, B.A. Pulmonary nodules in a newborn with ATP-binding cassette transporter A3 (ABCA3) mutations. Pediatrics 2011, 127, e1347–e1351. [Google Scholar] [CrossRef]
- Akil, N.; Fischer, A.J. Surfactant deficiency syndrome in an infant with a C-terminal frame shift in ABCA3: A case report. Pediatr. Pulmonol. 2018, 53, E12–E14. [Google Scholar] [CrossRef]
- Mitsiakos, G.; Tsakalidis, C.; Karagianni, P.; Gialamprinou, D.; Chatziioannidis, I.; Papoulidis, I.; Tsanakas, I.; Soubasi, V. A New ABCA3 Gene Mutation c.3445G > A (p.Asp1149Asn) as a Causative Agent of Newborn Lethal Respiratory Distress Syndrome. Medicina 2019, 55, 389. [Google Scholar] [CrossRef]
- Oltvai, Z.N.; Smith, E.A.; Wiens, K.; Nogee, L.M.; Luquette, M.; Nelson, A.C.; Wikenheiser-Brokamp, K.A. Neonatal respiratory failure due to novel compound heterozygous mutations in the ABCA3 lipid transporter. Cold Spring Harb. Mol. Case Stud. 2020, 6, a005074. [Google Scholar] [CrossRef]
- Gupta, N.P.; Batra, A.; Puri, R.; Meena, V. Novel homozygous missense mutation in ABCA3 protein leading to severe respiratory distress in term infant. BMJ Case Rep. 2020, 13, e235520. [Google Scholar] [CrossRef]
- Shaaban, W.; Hammoud, M.; Abdulraheem, A.; Elsayed, Y.Y.; Alkazemi, N. Hydroxychloroquine, a successful treatment for lung disease in ABCA3 deficiency gene mutation: A case report. J. Med. Case Rep. 2021, 15, 54. [Google Scholar] [CrossRef]
- Bozkurt, H.B.; Şahin, Y. Whole exome sequencing identifies a novel variant in ABCA3 in an individual with fatal congenital surfactant protein deficiency. Turk. J. Pediatr. 2021, 63, 703–707. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, Z.; Lin, Y.; Wang, R.; Xu, J.; He, Y.; Zhang, F.; Wu, L.; Chen, D. A novel synonymous ABCA3 variant identified in a Chinese family with lethal neonatal respiratory failure. BMC Med. Genom. 2021, 14, 256. [Google Scholar] [CrossRef] [PubMed]
- Si, X.; Steffes, L.C.; Schymick, J.C.; Hazard, F.K.; Tracy, M.C.; Cornfield, D.N. Three Infants with Pathogenic Variants in the ABCA3 Gene: Presentation, Treatment, and Clinical Course. J. Pediatr. 2021, 231, 278–283.e2. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Xie, Z.; Zhang, V.W.; Chen, C.; Fan, H.; Zhang, D.; Jiang, W.; Wang, C.; Wu, P. Case Report: Report of Two Cases of Interstitial Lung Disease Caused by Novel Compound Heterozygous Variants in the ABCA3 Gene. Front. Genet. 2022, 13, 875015. [Google Scholar] [CrossRef]
- Shulenin, S.; Nogee, L.M.; Annilo, T.; Wert, S.E.; Whitsett, J.A.; Dean, M. ABCA3 gene mutations in newborns with fatal surfactant deficiency. N. Engl. J. Med. 2004, 350, 1296–1303. [Google Scholar] [CrossRef]
- Schindlbeck, U.; Wittmann, T.; Höppner, S.; Kinting, S.; Liebisch, G.; Hegermann, J.; Griese, M. ABCA3 missense mutations causing surfactant dysfunction disorders have distinct cellular phenotypes. Hum. Mutat. 2018, 39, 841–850. [Google Scholar] [CrossRef]
- Campo, I.; Zorzetto, M.; Mariani, F.; Kadija, Z.; Morbini, P.; Dore, R.; Kaltenborn, E.; Frixel, S.; Zarbock, R.; Liebisch, G.; et al. A large kindred of pulmonary fibrosis associated with a novel ABCA3 gene variant. Respir. Res. 2014, 15, 43. [Google Scholar] [CrossRef]
- Rindler, T.N.; Stockman, C.A.; Filuta, A.L.; Brown, K.M.; Snowball, J.M.; Zhou, W.; Veldhuizen, R.; Zink, E.M.; Dautel, S.E.; Clair, G.; et al. Alveolar injury and regeneration following deletion of ABCA3. JCI Insight 2017, 2, e97381. [Google Scholar] [CrossRef] [PubMed]
- Peers de Nieuwburgh, M.; Wambach, J.A.; Griese, M.; Danhaive, O. Towards personalized therapies for genetic disorders of surfactant dysfunction. Semin. Fetal Neonatal Med. 2023, 28, 101500. [Google Scholar] [CrossRef] [PubMed]
- Serra, G.; Giambrone, C.; Antona, V.; Cardella, F.; Carta, M.; Cimador, M.; Corsello, G.; Giuffrè, M.; Insinga, V.; Maggio, M.C.; et al. Congenital hypopituitarism and multiple midline defect sin a new born with non-familial Cat Eye syndrome. Ital. J. Pediatr. 2022, 48, 170. [Google Scholar] [CrossRef] [PubMed]
- Presti, S.; Parisi, G.F.; Papale, M.; Gitto, E.; Manti, S.; Leonardi, S. Interstitial Lung Disease in Children: “Specific Conditions of Undefined Etiology” Becoming Clearer. Children 2022, 9, 1744. [Google Scholar] [CrossRef]
- Drobňaková, S.; Vargová, V.; Barkai, L. The Clinical Approach to Interstitial Lung Disease in Childhood: A Narrative Review Article. Children 2024, 11, 904. [Google Scholar] [CrossRef]
| Authors, Year, Country | Type of Mutation in the ABCA3 Gene | Sex | GA | Onset | Clinical Features | Surfactant | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|
| Kuning et al. [21] 2007, Colorado (USA) | p.Leu326Arg, in homozygosity | M | Term | Early onset | Refractory pulmonary hypertension, later parenchymal lung disease | No (due to clinical instability) | Nitric oxide, dopamine (for pulmonary hypertension) | Death due to withdrawal of intensive care |
| Anandarajan et al. [22] 2009, United Kingdom | p.Gly378Arg, p.Gly1002Arg | F | Term | Within few hours of birth | RDS | Yes | Antibiotics (not specified) | Death at 25 days of life due to withdrawal of intensive care |
| Ciantelli et al. [9] 2011, Italy | p.Pro186Thr, p.Met878Trp fs36X | M | Term | Within few hours of birth | RDS | Yes (9 doses) | Dexamethasone | Death at 72 days of life |
| Hallik et al. [23] 2013, Estonia | p.Asp507AlafsTer508, p.Asp696Asn | F | Term | Within few hours of birth | RDS | Yes (1 dose) | Methylprednisolone, azithromycin, hydroxychloroquine | Discharged at age 12 months with oxygen therapy and gastrostomy. In the following months, mild neuro-motor delay. |
| M | Not specified | Asymptomatic for 4 years | ||||||
| Goncalves et al. [24] 2014, Portugal | p.Leu798Pro, p.Arg1612Pro | F | Term | Within few hours of birth | RDS | Yes (4 doses) | Methylprednisolone, hydroxychloroquine | Death at 101 days due to withdrawal of intensive care |
| Maly et al. [25] 2014, Czech Republic | p. Met1227Arg, p. Ins1510fsTer1519 | M | Term | Within few hours of birth | RDS | Yes | Dexamethasone and macrolides | Death at 33 days of life due to withdrawal of intensive care |
| Moore et al. [26] 2014, Canada | p.Ser1116Phe, in homozygosity | M | Term | At three hours of life | RDS | Yes (a few doses) | Antibiotics, nitric oxide | Death at 48 days of life |
| F | Term | At sixteen hours of life | RDS | Yes | Death at 53 days of life | |||
| Jackson et al. [27] 2015, USA | p.Arg280Cys, p.Val1399Met, p.Gln1589Ter | F | Term | Within few hours of birth | RDS | Yes | Nitric oxide | Bilateral lung transplant at 9 months. At age 3 years, without oxygen support, nutritional supplementation tube, mild motor and speech delay. |
| Piersigilli et al. [28] 2015, Italy | p.Arg194Gly, p.Val1615GlyfsTer15 | F | Late preterm | Within few hours of birth | RDS | Yes (3 doses) | Betamethasone, hydroxychloroquine | Death at age 5 months |
| M | Early onset | RDS | Yes (three doses) | Betamethasone, hydroxychloroquine | Death at age 3 months | |||
| Pachajoa et al. [29] 2016, Colombia | IVS 25-98T in homozygosity | M | Term | Early onset | RDS | Yes | Antibiotics (vancomycin, meropenem) | Death at 60 days |
| Tan et al. [30] 2016, Australia | p.Ala307Val, in homozygosity | F | Preterm | Early onset | RDS | Yes (2 doses) | Hydroxychloroquine, azithromycin, methylprednisolone | Oxygen therapy through nasal cannula at age 1 year |
| Ota et al. [31] 2016, Japan | p.Leu34Pro, p.1203_1204del | F | Term | 8 years | Pulmonary fibrosis with emphysema, pulmonary hypertension | Not specified | Calcium antagonist, warfarin, home oxygen therapy, beraprost sodium | Improvement of pulmonary arterial pressure. No worsening of fibrosis. |
| Uchida et al. [32] 2017, USA | p.(delPhe1203)4, c.1375ins15 | F | Term | Early onset | RDS, perihilar and peripheral rounded masses of the right lung | Not specified | Systemic corticosteroids (not specified) | Death at 113 days |
| Akil et al. [33] 2018, USA | p.Glu292Val, p.Met1647fs | M | Term | Early onset | Respiratory illness, cyanosis, impaired growth. | Not specified | Prednisolone, methylprednisolone, azithromycin, hydroxychloroquine. Gastrostomy | Oxygen therapy with nasal cannula at age 1 year |
| Mitsiakos et al. [34] 2019, Greece | p.Asp1149Asn, in homozygosity | M | Term | Within few hours of birth | RDS | Yes (10 doses) | Methylprednisolone, prednisolone, azithromycin. At age 78 days hydroxychloroquine, prednisone, azithromycin | Death at 9 months |
| Oltvai et al. [35] 2020, USA | p.Ala1629GlyfsX15, p.Gly961Gly | M | Term | Within few hours of birth | RDS | Yes (a few doses) | Dexamethasone and macrolides | Successful bilateral lung transplantation at 15 weeks of age |
| Gupta et al. [36] 2020, India | p.Leu437Pro, in homozygosity | F | Late preterm | Within few hours of birth | RDS | Yes (2 doses) | Dexamethasone | Death at 100 days |
| Shaaban et al. [37] 2021, Kuwait | c.875A<T in homozygosity | M | Term | Within few hours of birth | RDS | Yes | Hydroxychloroquine, methylprednisolone, azithromycin | Discharged at 160 days with hydroxychloroquine maintenance treatment |
| Bozkurt et al. [38] 2021, Turkey | p.Leu1226Pro, in homozygosity | M | Term | Early onset | RDS, pulmonary hypertension | Yes (3 doses) | Antibiotics, nitric oxide, sildenafil | Death at 24 days |
| Zhang et al. [39] 2021, China | c.4-7del, p.Lys291Lys | Two girls | Term | Within few hours of birth | RDS | Yes | Antibiotics, nitric oxide, and methylprednisolone | Death at 23 days |
| Si et al. [40] 2021, California (USA) | p.Glu292Val, p.Arg1081Trp | M | Pre term | Early onset | RDS | Yes | Azithromycin, hydroxychloroquine, methylprednisolone. Gastrostomy fundoplicatio according to Nissen | At age 2 years without oxygen support, severe motor, language and cognitive delay, impaired growth. Steroid therapy discontinued, bilevel alternating with low-flow nasal cannula respiratory support |
| p.Tyr758Ter, p.Lys915Asn | M | Term | Within few hours of birth | RDS | Yes | Methylprednisolone, hydroxychloroquine, azithromycin. Gastrostomy fundoplicatio according to Nissen | At age 14 months, language, cognitive, and motor delay, impaired growth. Steroid discontinued at 1 year. HFNC respiratory support. | |
| C.1285+1G>A, p.Asp200Gly | M | Term | Within few hours of birth | RDS | Yes | Methylprednisolone, azithromycin and hydroxychloroquine. Gastrostomy fundoplicatio according to Nissen | Removal of gastrostomy tube at age 18 months, impaired growth, mild language delay (at age 13 months). Steroid discontinued. Low-flow nasal cannula (LFNC). | |
| Chen et al. [41] 2022, China | c.4035+5G>A, p.Met223Thr, c.1285+4A>C | M | Term | After 24 h of birth | Dyspnea and cough | Not specified | Azithromycin, dexamethasone and hydroxychloroquine | Death at 94 days |
| p.Phe235Ser, p.Thr1346Asn fsTer15 | M | Term | At 9 months | Chronic cough | Not specified | Budesonide, ipratropium bromide and acetylcysteine | At age 41 months, stable clinical condition without respiratory support | |
| Our case (2025) | p.Arg155Gln, p.Gly974Glu | F | Late preterm | Early onset | RDS | Yes (4 doses) | Azithromycin, hydroxychloroquine, diuretics (furosemide, spironolactone), sildenafil. Gastrostomy | Good general condition with oxygen therapy through HFNC. Impaired growth, mild motor delay. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serra, G.; Notarbartolo, V.; Antona, V.; Cacace, C.; Di Pace, M.R.; Morreale, D.M.; Pensabene, M.; Piro, E.; Schierz, I.A.M.; Sergio, M.; et al. Novel Compound Heterozygous Mutation of the ABCA3 Gene in a Patient with Neonatal-Onset Interstitial Lung Disease. J. Clin. Med. 2025, 14, 3704. https://doi.org/10.3390/jcm14113704
Serra G, Notarbartolo V, Antona V, Cacace C, Di Pace MR, Morreale DM, Pensabene M, Piro E, Schierz IAM, Sergio M, et al. Novel Compound Heterozygous Mutation of the ABCA3 Gene in a Patient with Neonatal-Onset Interstitial Lung Disease. Journal of Clinical Medicine. 2025; 14(11):3704. https://doi.org/10.3390/jcm14113704
Chicago/Turabian StyleSerra, Gregorio, Veronica Notarbartolo, Vincenzo Antona, Caterina Cacace, Maria Rita Di Pace, Daniela Mariarosa Morreale, Marco Pensabene, Ettore Piro, Ingrid Anne Mandy Schierz, Maria Sergio, and et al. 2025. "Novel Compound Heterozygous Mutation of the ABCA3 Gene in a Patient with Neonatal-Onset Interstitial Lung Disease" Journal of Clinical Medicine 14, no. 11: 3704. https://doi.org/10.3390/jcm14113704
APA StyleSerra, G., Notarbartolo, V., Antona, V., Cacace, C., Di Pace, M. R., Morreale, D. M., Pensabene, M., Piro, E., Schierz, I. A. M., Sergio, M., Valenti, G., Giuffrè, M., & Corsello, G. (2025). Novel Compound Heterozygous Mutation of the ABCA3 Gene in a Patient with Neonatal-Onset Interstitial Lung Disease. Journal of Clinical Medicine, 14(11), 3704. https://doi.org/10.3390/jcm14113704






