Intravascular Lithotripsy-Assisted Transfemoral Transcatheter Aortic Valve Implantation in Patients with Severe Iliofemoral Calcifications: Expanding Transfemoral Indications
Abstract
1. Introduction
2. Methods
2.1. Patient Population and Workup
2.2. TAVI Procedures
2.3. Clinical Endpoints and Data Analysis
2.4. Statistical Analyses
3. Results
3.1. Patient Characteristics
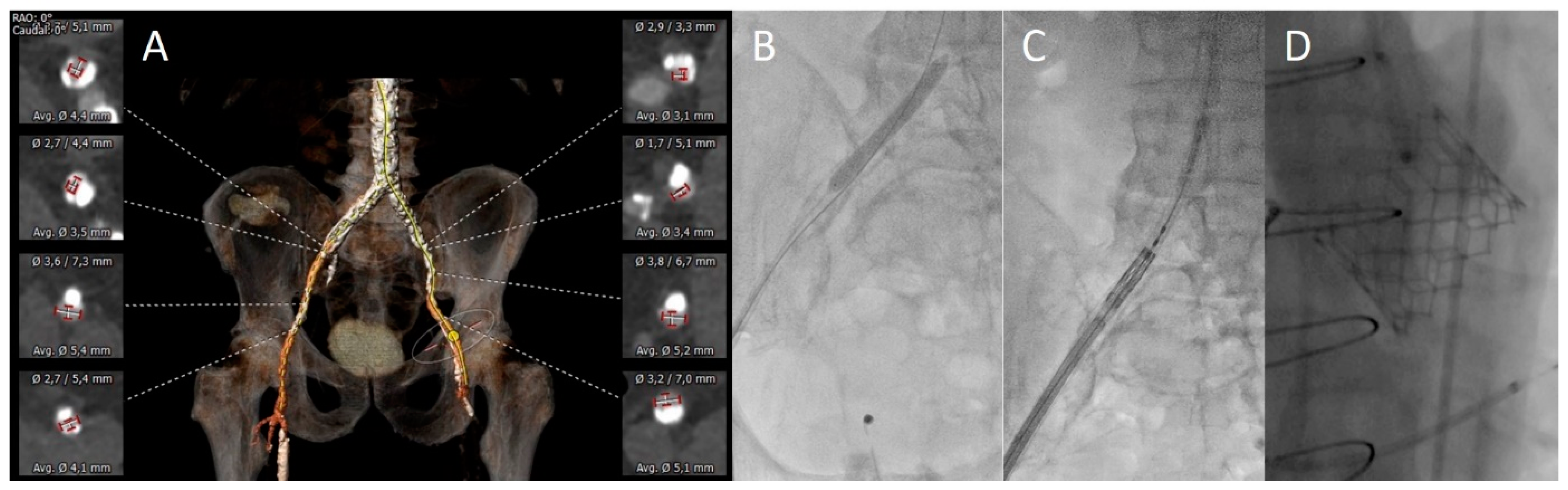
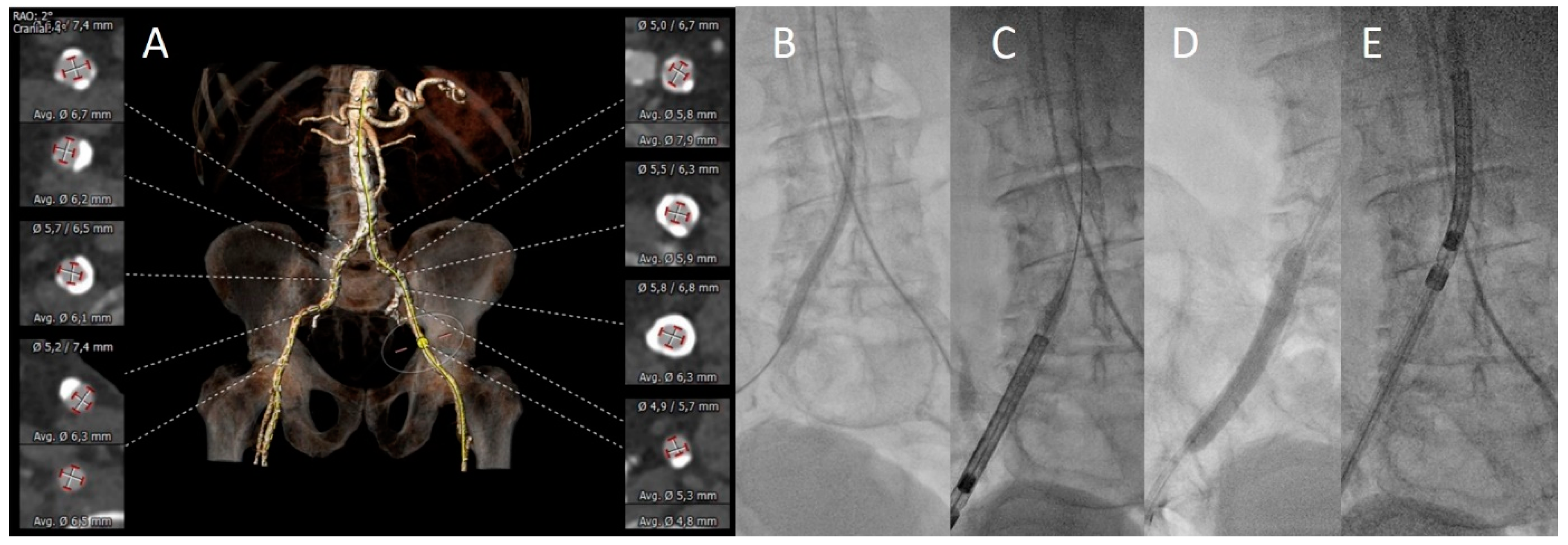
3.2. Vascular Assessment
3.3. Procedural Aspects
3.4. Outcome
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CT | Computed tomography |
| IVL | Intravascular lithotripsy |
| PTA | Percutaneous transluminal angioplasty |
| STS-PROM | Society of thoracic surgeons—predicted risk of operative mortality |
| TAVI | Transcatheter aortic valve implantation |
| TAX | Transaxillary |
| TF | Transfemoral |
References
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease: Developed by the Task Force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2021, 43, 561–632. [Google Scholar] [CrossRef]
- Dahle, T.G.; Kaneko, T.; McCabe, J.M. Outcomes Following Subclavian and Axillary Artery Access for Transcatheter Aortic Valve Replacement: Society of the Thoracic Surgeons/American College of Cardiology TVT Registry Report. JACC Cardiovasc. Interv. 2019, 12, 662–669. [Google Scholar] [CrossRef]
- Leon, M.B.; Smith, C.R.; Mack, M.J.; Makkar, R.R.; Svensson, L.G.; Kodali, S.K.; Thourani, V.H.; Tuzcu, E.M.; Miller, D.C.; Herrmann, H.C.; et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2016, 374, 1609–1620. [Google Scholar] [CrossRef] [PubMed]
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Russo, M.; Kapadia, S.R.; Malaisrie, S.C.; Cohen, D.J.; Pibarot, P.; et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1695–1705. [Google Scholar] [CrossRef]
- Popma, J.J.; Deeb, M.; Yakubov, S.J.; Mumtaz, M.; Gada, H.; O’Hair, D.; Bajwa, T.; Heiser, J.C.; Merhi, W.; Kleiman, N.S.; et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1706–1715. [Google Scholar] [CrossRef]
- Di Mario, C.; Chiriatti, N.; Stolcova, M.; Meucci, F.; Squillantini, G. Lithotripsy-assisted transfemoral aortic valve implantation. Eur. Heart J. 2018, 39, 2655. [Google Scholar] [CrossRef]
- Brodmann, M.; Werner, M.; Holden, A.; Tepe, G.; Scheinert, D.; Schwindt, A.; Wolf, F.; Jaff, M.; Lansky, A.; Zeller, T. Primary outcomes and mechanism of action of intravascular lithotripsy in calcified, femoropopliteal lesions: Results of Disrupt PAD II. Catheter. Cardiovasc. Interv. 2019, 93, 335–342. [Google Scholar] [CrossRef]
- Tepe, G.; Brodmann, M.; Werner, M.; Bachinsky, W.; Holden, A.; Zeller, T.; Mangalmurti, S.; Nolte-Ernsting, C.; Bertolet, B.; Scheinert, D.; et al. Intravascular Lithotripsy for Peripheral Artery Calcification: 30-Day Outcomes From the Randomized Disrupt PAD III Trial. JACC. Cardiovasc. Interv. 2021, 14, 1352–1361. [Google Scholar] [CrossRef]
- Grundmann, D.; Linder, M.; Goßling, A.; Voigtländer, L.; Ludwig, S.; Waldschmidt, L.; Demal, T.; Bhadra, O.D.; Schäfer, A.; Schirmer, J.; et al. End-stage renal disease, calcification patterns and clinical outcomes after TAVI. Clin. Res. Cardiol. 2022, 111, 1313–1324. [Google Scholar] [CrossRef]
- Schäfer, U.; Deuschl, F.; Schofer, N.; Frerker, C.; Schmidt, T.; Kuck, K.H.; Kreidel, F.; Schirmer, J.; Mizote, I.; Reichenspurner, H.; et al. Safety and efficacy of the percutaneous transaxillary access for transcatheter aortic valve implantation using various transcatheter heart valves in 100 consecutive patients. Int. J. Cardiol. 2017, 232, 247–254. [Google Scholar] [CrossRef]
- Schaefer, A.; Schirmer, J.; Schofer, N.; Schneeberger, Y.; Deuschl, F.; Blankenberg, S.; Reichenspurner, H.; Conradi, L.; Schäfer, U. Transaxillary transcatheter aortic valve implantation utilizing a novel vascular closure device with resorbable collagen material: A feasibility study. Clin. Res. Cardiol. 2019, 108, 779–786. [Google Scholar] [CrossRef]
- Généreux, P.; Piazza, N.; Alu, M.C.; Nazif, T.; Hahn, R.T.; Pibarot, P.; Bax, J.J.; Leipsic, J.A.; Blanke, P.; Blackstone, E.H.; et al. Valve Academic Research Consortium 3: Updated Endpoint Definitions for Aortic Valve Clinical Research. J. Am. Coll. Cardiol. 2021, 77, 2717–2746. [Google Scholar] [CrossRef] [PubMed]
- Kurra, V.; Schoenhagen, P.; Roselli, E.E.; Kapadia, S.R.; Tuzcu, E.M.; Greenberg, R.; Akhtar, M.; Desai, M.Y.; Flamm, S.D.; Halliburton, S.S.; et al. Prevalence of significant peripheral artery disease in patients evaluated for percutaneous aortic valve insertion: Preprocedural assessment with multidetector computed tomography. J. Thorac. Cardiovasc. Surg. 2009, 137, 1258–1264. [Google Scholar] [CrossRef]
- Patel, J.S.; Krishnaswamy, A.; Svensson, L.G.; Tuzcu, E.M.; Mick, S.; Kapadia, S.R. Access Options for Transcatheter Aortic Valve Replacement in Patients with Unfavorable Aortoiliofemoral Anatomy. Curr. Cardiol. Rep. 2016, 18, 110. [Google Scholar] [CrossRef]
- Abdel-Wahab, M.; Hartung, P.; Dumpies, O.; Obradovic, D.; Wilde, J.; Majunke, N.; Boekstegers, P.; Müller, R.; Seyfarth, M.; Vorpahl, M.; et al. Comparison of a Pure Plug-Based Versus a Primary Suture-Based Vascular Closure Device Strategy for Transfemoral Transcatheter Aortic Valve Replacement: The CHOICE-CLOSURE Randomized Clinical Trial. Circulation 2022, 145, 170–183. [Google Scholar] [CrossRef]
- Barbanti, M.; van Mourik, M.S.; Spence, M.S.; Iacovelli, F.; Martinelli, G.L.; Muir, D.F.; Saia, F.; Bortone, A.S.; Densem, C.G.; van der Kley, F.; et al. Optimising patient discharge management after transfemoral transcatheter aortic valve implantation: The multicentre European FAST-TAVI trial. EuroIntervention 2019, 15, 147–154. [Google Scholar] [CrossRef]
- Covarrubias, H.A.A.; Joner, M.; Presch, A.; Pellegrini, C.; Rheude, T.; Mayr, N.P.; Kastrati, A.; Xhepa, E. Iliofemoral artery predilation prior to transfemoral transcatheter aortic valve implantation in patients with aortic valve stenosis and advanced peripheral artery disease. Catheter. Cardiovasc. Interv. 2023, 101, 628–638. [Google Scholar] [CrossRef]
- Sorini Dini, C.; Tomberli, B.; Mattesini, A.; Ristalli, F.; Valente, S.; Stolcova, M.; Meucci, F.; Baldereschi, G.; Fanelli, F.; Shlofmitz, R.; et al. Intravascular lithotripsy for calcific coronary and peripheral artery stenoses. EuroIntervention 2019, 15, 714–721. [Google Scholar] [CrossRef]
- Achim, A.; Alampi, C.; Krivoshei, L.; Leibundgut, G. In vitro effect of intravascular lithotripsy on the polymer of a drug-eluting stent. EuroIntervention 2022, 18, e333–e334. [Google Scholar] [CrossRef]
- Nardi, G.; De Backer, O.; Saia, F.; Søndergaard, L.; Ristalli, F.; Meucci, F.; Stolcova, M.; Mattesini, A.; Demola, P.; Wang, X.; et al. Peripheral intravascular lithotripsy for transcatheter aortic valve implantation: A multicentre observational study. EuroIntervention 2022, 17, E1397–E1406. [Google Scholar] [CrossRef]
- Sawaya, F.J.; Bajoras, V.; Vanhaverbeke, M.; Wang, C.; Bieliauskas, G.; Søndergaard, L.; De Backer, O. Intravascular Lithotripsy-Assisted Transfemoral TAVI: The Copenhagen Experience and Literature Review. Front. Cardiovasc. Med. 2021, 8, 739750. [Google Scholar] [CrossRef]
- Palmerini, T.; Saia, F.; Kim, W.-K.; Renker, M.; Iadanza, A.; Fineschi, M.; Bruno, A.G.; Ghetti, G.; Vanhaverbeke, M.; Søndergaard, L.; et al. Vascular Access in Patients With Peripheral Arterial Disease Undergoing TAVR. JACC Cardiovasc. Interv. 2023, 16, 396–411. [Google Scholar] [CrossRef]
- Chung, C.J.; Kaneko, T.; Tayal, R.; Dahle, T.G.; McCabe, J.M. Percutaneous versus surgical transaxillary access for transcatheter aortic valve replacement: A propensity-matched analysis of the US experience. EuroIntervention 2022, 17, 1514–1522. [Google Scholar] [CrossRef] [PubMed]
| All (n = 74) | IVL (n = 30) | TAX (n = 44) | p-Value | |
|---|---|---|---|---|
| Age, years | 78.2 [74.3, 82.6] | 80.0 [76.7, 84.2] | 77.7 [73.5,82.3] | 0.112 |
| Female, n | 34 (45.9%) | 16 (53.3%) | 18 (40.9%) | 0.346 |
| Body mass index, kg/m2 | 24.6 [21.7, 27.6] | 24.2 [21.6, 27.1] | 25.2 [21.8, 28.3] | 0.510 |
| STS-PROM, % | 3.6 [2.3, 6.0] | 3.9 [2.5, 5.5] | 3.5 [2.2, 6.0] | 0.613 |
| Aortic valve area, cm2 | 0.8 [0.7, 0.9] | 0.8 [0.8, 0.9] | 0.8 [0.7, 0.9] | 1.000 |
| Mean aortic valve gradient, mmHg | 30.9 [27.8, 33.9] | 30.1 [24.8, 35.5] | 31.4 [27.6, 35.2] | 0.732 |
| NYHA IV, n | 6 (8.3%) | 2 (6.9%) | 4 (9.3%) | 1.000 |
| Arterial hypertension, n | 64 (86.5%) | 25 (83.3%) | 39 (88.6%) | 0.514 |
| Severely impaired LVEF (<30%), n | 10 (13.5%) | 2 (6.7%) | 8 (18.2%) | 0.187 |
| Glomerular filtration rate, mL/min | 52.0 [38.3, 73.6] | 52.9 [40.9, 74.1] | 37.0 [21.3, 79.3] | 0.451 |
| Chronic pulmonary disease, n | 25 (33.8%) | 9 (30.0%) | 16 (36.4%) | 0.624 |
| Peripheral artery disease, n | 56 (75.7%) | 24 (80.0%) | 32 (72.7%) | 0.585 |
| Atrial fibrillation, n | 25 (33.8%) | 14 (46.7%) | 11 (25.0%) | 0.079 |
| History of stroke, n | 11 (14.9%) | 7 (23.3%) | 4 (9.1%) | 0.108 |
| Diabetes, n | 17 (23.0%) | 8 (26.7%) | 9 (20.5%) | 0.581 |
| Coronary artery disease, n | 57 (77%) | 23 (76.7%) | 34 (77.3%) | 1.000 |
| All (n = 74) | IVL (n = 30) | TAX (n = 44) | p-Value | |
|---|---|---|---|---|
| Severe tortuosity of iliofemoral vessels, n | 16 (21.6%) | 7 (23.3%) | 9 (20.5%) | 0.781 |
| Severe calcification at target lesion, n | 57 (77.0%) | 27 (90.0%) | 30 (68.2%) | 0.047 |
| Reference vessel diameter, mm | 7.8 [6.8, 8.9] | 7.8 [6.9, 8.6] | 7.8 [6.7, 9.4] | 0.969 |
| Target lesion: common or external iliac artery, n | 69 (93.2%) | 29 (96.7%) | 40 (90.1%) | 0.642 |
| Target lesion diameter, mm | 4.3 [3.6, 4.8] | 4.4 [3.5, 4.8] | 4.2 [3.6, 4.7] | 0.567 |
| Diameter stenosis, % | 56.2 [43.6, 62.9] | 58.2 [46.6, 61.4] | 54.5 [42.9, 63.2] | 0.560 |
| Target lesion length, mm | 54.5 [39.8, 73.1] | 63.0 [48.6, 80.3] | 48.5 [33.1, 68.8] | 0.043 |
| Maximal arc calcification, ° | 360.0 [262.5, 360.0] | 360.0 [297.5, 360.0] | 360.0 [180.0, 360.0] | 0.033 |
| Circular calcification (360°), n | 42 (56.7%) | 19 (63.3%) | 23 (52.3%) | 0.474 |
| Horseshoe-like calcification (270°), n | 14 (18.9%) | 11 (36.7%) | 3 (6.8%) | 0.002 |
| All (n = 74) | IVL (n = 30) | TAX (n = 44) | p-Value | |
|---|---|---|---|---|
| Local anesthesia, n | 51 (68.9%) | 26 (86.7%) | 25 (56.8%) | 0.010 |
| Sheath size ≥16 Fr, n | 34 (50.0%) | 5 (19.2%) | 29 (69.0%) | <0.001 |
| Cerebral protection, n | 6 (8.1%) | 0 | 6 (13.6%) | 0.075 |
| Balloon-expandable THV, n | 25 (33.8%) | 13 (43.3%) | 12 (27.2%) | 0.211 |
| Planned valve-in-valve, n | 4 (6.3%) | 1 (5.3%) | 3 (6.8%) | 1.000 |
| Successful delivery of THV, n | 74 (100%) | 30 (100%) | 44 (100%) | 1.000 |
| IVL balloon size 7 × 60 mm/8 × 60 mm, n | 29 (96.7%)/1 (3.3%) | - | ||
| Number of pulses, n | 257.3 [232.0, 300.0] | - | ||
| Elective/bail-out IVL, n | 19 (63.3%)/11 (36.7%) | - | ||
| Vascular closure | <0.001 | |||
| Suture-based closure system | 41 (56.9%) | 9 (31%) | 32 (74.4%) | <0.001 |
| Plug-based closure system | 30 (41.7%) | 19 (65.5%) | 11 (25.6%) | 0.001 |
| Planned surgical vascular access | 1 (1.4%) | 1 (3.4%) | 0 | 0.403 |
| Procedure time, min | 105 [85.0, 130.0] | 93.0 [80.0, 124.1] | 110.0 [92.1, 130.0] | 0.090 |
| Fluoroscopy time, min | 34.0 [24.4, 39.2] | 29.5 [20.9, 36.1] | 36.1 [28.4, 45.0] | 0.008 |
| Contrast agent, mL | 192.5 [157.7, 260.0] | 179.0 [135.7, 197.3] | 220.0 [171.7, 303.3] | 0.004 |
| All (n = 74) | IVL (n = 30) | TAX (n = 44) | p-Value | |
|---|---|---|---|---|
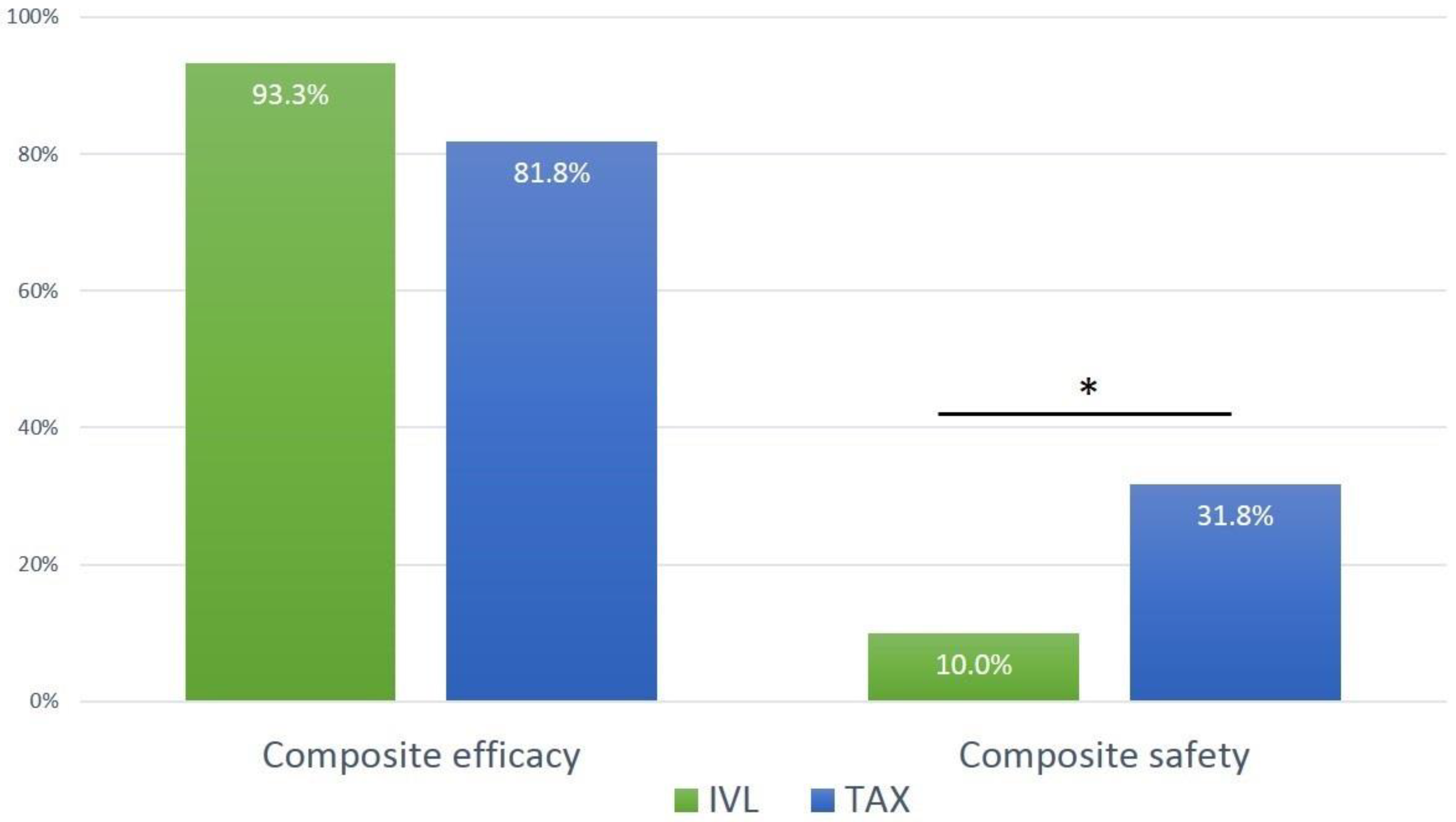
| Composite efficacy endpoint, n (at exit from procedure room) | 64 (86.5%) | 28 (93.3%) | 36 (81.8%) | 0.187 |
| Freedom from mortality, n | 74 (100%) | 30 (100%) | 44 (100%) | 1.000 |
| Successful access, delivery of the device, and retrieval of the delivery system, n | 74 (100%) | 30 (100%) | 44 (100%) | 1.000 |
| Correct positioning of a single THV, n | 74 (100%) | 30 (100%) | 44 (100%) | 1.000 |
| Freedom from surgery or intervention related to the device or to a major complication, n | 64 (86.5%) | 28 (93.3%) | 36 (81.8%) | 0.187 |
| Composite safety endpoint, n (at 30 days) | 17 (23.0%) | 3 (10.0%) | 14 (31.8%) | 0.047 |
| All-cause mortality, n | 5 (6.8%) | 1 (3.3%) | 4 (9.1%) | 0.642 |
| Disabling or non-disabling stroke, n | 5 (6.8%) | 1 (3.3%) | 4 (9.1%) | 0.642 |
| Major vascular access-site complication, n | 10 (13.5%) | 1 (3.3%) | 9 (20.5%) | 0.042 |
| Access-related bleeding ≥ type 2 <24 h, n | 8(10.8%) | 1 (3.3%) | 7 (15.9%) | 0.132 |
| Minor vascular access-site complication, n | 20 (27.0%) | 8 (26.7%) | 12 (27.3%) | 1.000 |
| Unplanned treatment due to vascular complication at primary vascular access location | ||||
| Covered stent implantation | 11 (14.9%) | 0 (0%) | 11 (25.0%) | 0.002 |
| Endovascular balloon inflatation | 5 (6.8%) | 2 (6.6%) | 3 (6.8%) | 1.000 |
| Thrombin injection | 1 (1.4%) | 1 (3.3%) | 0 | 0.405 |
| Vascular surgery | 4 (5.4%) | 1 (3.3%) | 3 (6.8%) | 0.642 |
| IVL-specific outcome | ||||
| Perforation, n | 0 | - | ||
| Major dissection, n | 0 | - | ||
| Minor dissection (conservative treatment), n | 2 (6.7%) | - | ||
| Stent implantation at IVL location | 1 (3.3%) | - | ||
| Myocardial infarction | 0 | 0 | 0 | |
| Acute kidney injury (stage 3) | 3 (4.7%) | 1 (5.0%) | 2 (4.5%) | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Linder, M.; Grundmann, D.; Kellner, C.; Demal, T.; Waldschmidt, L.; Bhadra, O.; Ludwig, S.; Voigtländer, L.; von der Heide, I.; Nebel, N.; et al. Intravascular Lithotripsy-Assisted Transfemoral Transcatheter Aortic Valve Implantation in Patients with Severe Iliofemoral Calcifications: Expanding Transfemoral Indications. J. Clin. Med. 2024, 13, 1480. https://doi.org/10.3390/jcm13051480
Linder M, Grundmann D, Kellner C, Demal T, Waldschmidt L, Bhadra O, Ludwig S, Voigtländer L, von der Heide I, Nebel N, et al. Intravascular Lithotripsy-Assisted Transfemoral Transcatheter Aortic Valve Implantation in Patients with Severe Iliofemoral Calcifications: Expanding Transfemoral Indications. Journal of Clinical Medicine. 2024; 13(5):1480. https://doi.org/10.3390/jcm13051480
Chicago/Turabian StyleLinder, Matthias, David Grundmann, Caroline Kellner, Till Demal, Lara Waldschmidt, Oliver Bhadra, Sebastian Ludwig, Lisa Voigtländer, Ina von der Heide, Nicole Nebel, and et al. 2024. "Intravascular Lithotripsy-Assisted Transfemoral Transcatheter Aortic Valve Implantation in Patients with Severe Iliofemoral Calcifications: Expanding Transfemoral Indications" Journal of Clinical Medicine 13, no. 5: 1480. https://doi.org/10.3390/jcm13051480
APA StyleLinder, M., Grundmann, D., Kellner, C., Demal, T., Waldschmidt, L., Bhadra, O., Ludwig, S., Voigtländer, L., von der Heide, I., Nebel, N., Hannen, L., Schirmer, J., Reichenspurner, H., Blankenberg, S., Conradi, L., Schofer, N., Schäfer, A., & Seiffert, M. (2024). Intravascular Lithotripsy-Assisted Transfemoral Transcatheter Aortic Valve Implantation in Patients with Severe Iliofemoral Calcifications: Expanding Transfemoral Indications. Journal of Clinical Medicine, 13(5), 1480. https://doi.org/10.3390/jcm13051480






