Abstract
Background/Objectives: Despite procedural improvements, post-transcatheter aortic valve replacement (TAVR) conduction disorders remain high. Analyzing the data from a monocentric TAVR registry, this study aims to determine predictive factors for PPI (primary outcome), the indication for PPI, and long-term outcomes among these patients (secondary outcomes). Methods: Conducted at Clairval Hospital in Marseille, France, this retrospective study included all consecutive patients from June 2012 to June 2019. Clinical, electrocardiographic, echocardiographic, and procedural data were collected, with outcomes assessed annually. Logistic regression identified PPI predictors and survival analyses were performed. Results: Of the 1458 patients initially considered, 1157 patients were included. PPI was needed in 21.5% of patients, primarily for third-degree atrioventricular block (46.4%). Predictor factors for PPI included baseline right bundle branch block (ORadj 2.49, 95% CI 1.44 to 4.30; p = 0.001), longer baseline QRS duration (ORadj 1.01, 95% CI 1.00 to1.02, p = 0.002), and self-expandable valves (ORadj 1.82, 95% CI, 1.09 to 3.03; p = 0.021). Seven-year estimated mortality was higher in PPI (43.3%) vs. non-PPI patients (30.9%) (log rank p = 0.048). PPI was an independent predictive factor of death (ORadj 2.49, 95% CI 1.4 to 4.3; p = 0.002). Conclusions: This study reveals elevated rates of PPI post-TAVR associated with increased mortality. These results underscore the pressing necessity to refine our practices, delineate precise indications, and enhance the long-term prognosis for implanted patients.
1. Introduction
Aortic stenosis (AS) is the most common valvular heart disease in developed countries. In recent years, transcatheter aortic valve replacement (TAVR) has emerged as a method of choice for treating AS in patients considered unfit for cardiac surgery. The accumulation of operators’ expertise and technological enhancement has significantly decreased the procedure complications. However, cardiac rhythm disorders remain a cause of concern and are now regarded as the most prevalent complication occurring in up to 20% of patients post-TAVR [1]. In France, the FRANCE 2 national registry reported permanent pacemaker implantation (PPI) rate of 15.6% for patients who operated between 2010 and 2012 [2], while the FRANCE TAVI registry reported a rate of PPI of 17.5% between 2013 and 2015 [3]. Several studies attempted to determine factors predisposing to PPI [4]. Unfortunately, the occurrence of this complication appears to be highly variable and influenced by electrical factors (pre-existing conduction abnormalities), anatomical factors, and procedural factors (balloon valvuloplasty and implantation depth). Despite recent expert consensus papers [5,6], the medical management of post-TAVR conduction disorders continues to be debated with significant variability between centers [7]. The present study aimed to evaluate, in a large monocentric cohort of consecutive patients undergoing TAVR, predictive factors of PPI after TAVR and outcomes over a large period of inclusion and follow-up.
2. Materials and Methods
2.1. Study Population and Setting
This retrospective, single-center, observational study was conducted at the Clairval Private Hospital (Marseille, France). All consecutive patients undergoing TAVR for severe, symptomatic aortic valve stenosis from 1 June 2012 to 31 June 2019 were recruited. Exclusion criteria included the presence of a pacemaker at admission and valve-in-valve procedures. Demographic and clinical data were acquired from the patient’s medical files, as well as electrocardiographic, echocardiographic, and cardiac computed tomography data before and after the TAVR procedure. The data set was abstracted in Excel (Excel 2016, Microsoft Corporation, Redmond, WD, USA) for analysis. Outcomes and survival follow-up data were collected at one month and each year. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline was followed (https://www.strobe-statement.org (accessed on 4 January 2023)). This study is declared on the Health Data Hub registry, according to French legislation. The protocol was reviewed and approved by our Institutional Review Board (IRB number: 00010835). Informed consent was obtained from each patient, and data collection was performed anonymously.
2.2. Definitions and Outcomes
Transcatheter heart valve (THV) type was divided into the following two groups: balloon-expandable (Edwards Sapien™, Sapien XT™, and Sapien 3™ valves systems; Edwards Lifesciences, Irvine, CA, USA) and self-expandable valve [Medtronic CoreValve™, Evolut R™ systems (Medtronic, Minneapolis, MN, USA), and Accurate neo™ valve system (Boston Scientific, Marlborough, MA, USA)]. The primary outcome of this study was to assess the necessity of PPI within 30 days after TAVR, among demographic, comorbidity, electrocardiographic, clinical, anatomic, and procedural factors. Secondary outcomes included indications for PPI and survival. We also described the evolution of our practices over the study period. The oversizing degree was calculated using the following formula in patients who received self-expandable valves (nominal valve perimeter−measured perimeter)/nominal valve perimeter) × 100, and as follows for balloon-expandable valves (nominal valve area-measured area)/nominal valve area) × 100 [8]. The manufacturer provided the nominal valve perimeter and area. Chronic kidney disease was defined by a glomerular filtration rate < 60 mL/m2, and chronic respiratory disease by preoperative maximum respiratory volume per second (VEMS) < 50%. Finally, after an electrophysiological study, a threshold for the HV interval > 70 ms was used to indicate a PPI. Patients were described as having a third-degree AV block if this conductive disorder was sustained > 24 h after the procedure or if there was no underlying rhythm justifying urgent PPI after TAVR. No specific protocols were established to assess the need for pre-TAVR temporary pacemaker implantation or post-TAVR PPI, and a multidisciplinary evaluation was conducted for each patient.
2.3. Statistical Analysis
Categorial data are presented as numbers (%). Continuous variables are expressed as the means with standard deviations or medians with interquartile ranges (IQRs) with minimum and maximum values depending on the variable distribution. Patients were divided into the following two groups: with or without PPI at 30 days post-TAVR. In addition, we divided the study period into terciles (2012 to 2014, 2015 to 2017, and 2018 to 2019) to analyze variation over time. The factors associated with PPI were identified using multivariate forward stepwise logistic regression analysis, following previous univariate tests (Student’s t-test, the Mann–Whitney or Kruskal–Wallis was used to compare continuous variables, depending on their distribution, and the chi-square test or Fisher’s exact test was used to compare categorical variables). To analyze the prognostic effect on overall mortality at follow-up, univariate and multivariate stepwise logistic regression analyses were carried out. The variables tested were demographic characteristics (age, sex, BMI, NHYA classification), medical history (history of atrial fibrillation, chronic kidney disease, coronary artery disease, cerebrovascular disease, diabetes, dyslipidemia, hypertension, coronary angioplasty, coronary bypass surgery, myocardial infarction within 90 days, atrial fibrillation, chronic respiratory disease, obliterative arteriopathy in lower limbs), baseline conduction or rhythm disturbances (right bundle branch block [RBBB], left bundle branch block [LBBB]), incomplete LBBB (QRS duration between 110 and 119 ms), left anterior hemiblock (first-degree AV block, atrial fibrillation [AF] or flutter), and procedural characteristics (valve type, and oversize index). Variables were retained for the logistic regression if the p-value of the univariate test was <0.1. Further selection was based on clinical reasoning. The odds ratio was generated for all prediction values, and a significant level of p < 0.05 was used to indicate statistical significance. Kaplan–Meier analysis was performed for survival analysis to provide survival estimates, which were evaluated with a log-rank test. A 2-sided p-value of < 0.05 was considered statistically significant. All analyses were performed using the SAS release 9.6 (SAS Institute Inc., Cary, NC, USA).
3. Results
3.1. Study Population and Procedural Characteristics
Among 1458 consecutive patients who underwent TAVR during the study period, 205 patients were excluded because of prior pacemakers, 89 because they had undergone a valve-in-valve procedure, and 7 patients did not consent to the inclusion. Ultimately, 1157 patients were included in the analysis. The median follow-up time was 44.9 months (95% CI: 43–47.2). The flowchart of the study population is described in Figure 1. The baseline and procedural characteristics of the population are summarized in Table 1. The mean age was 82.01 ± 6.9 years and 592 patients (52%) were women. The mean logistic EuroSCORE was 16.71 ± 11.06. The mean aortic gradient was 50.5 ± 14 mmHg. The transfemoral approach provided the most frequent access route for TAVR, performed in 74.6% of cases. The peri and post-procedural characteristics are described in Table 2. One hundred and thirty-one patients (11.3%) had self-expandable THV whereas 1025 (88.6%) had balloon-expandable THV. Post-procedural paravalvular aortic regurgitation was mild in 346 patients (30.6%), moderate in 48 patients (4.2%), and severe in 4 patients (0.4%). Twenty patients (1.7%) had a stroke post-procedure, 6 patients (0.6%) had a myocardial infarction, and pericardial tamponade occurred in 18 patients (1.6%). No patient had valve migration. The mean hospital stay after TAVR was 10 ± 6 days.
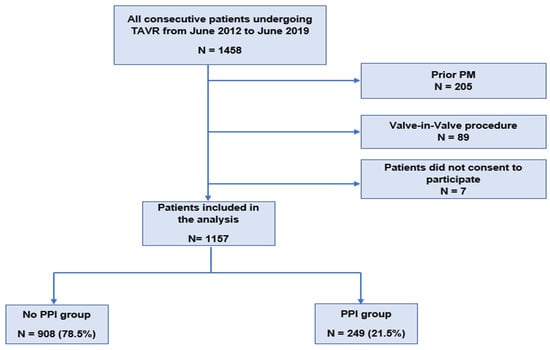
Figure 1.
Flowchart of the study population. PM: pacemaker; PPI: permanent pacemaker implantation; and TAVR: transcatheter aortic valve replacement.

Table 1.
Baseline and preprocedural characteristics of the study population (n = 1157): patients with or without 30-day permanent pacemaker implantation (PPI) after TAVR.

Table 2.
Periprocedural and postprocedural characteristics.
3.2. PPI and Predictive Factor
PPI was required in 249 patients (21.5%). The median time between TAVR and PPI was 4 days (IQR: 3–5). The main indications for PPI were: third-degree atrioventricular (AV) block (46.4%), followed by LBBB with pathological HV (24.6%), and LBBB without electrophysiological exploration (21.4%). The mean oversize index was estimated at 13.8 ± 12.2% in the PPI group and 12.6 ± 10.3% in the non-PPI group (p = 0.99). The variables significantly associated with PPI in univariate and multivariate analysis are presented in Table 3. Baseline RBBB (ORadj 2.49, 95% CI 1.44 to 4.30; p = 0.001), longer baseline QRS duration (ORadj 1.01, 95% CI 1.00 to1.02, p = 0.002), and self-expandable valves (ORadj 1.82, 95% CI, 1.09 to 3.03; p = 0.021) were found to be independent predictive factors of PPI.

Table 3.
Independent factors for 30-day permanent pacemaker implantation after transcatheter aortic valve replacement.
3.3. Change in Practices over Time
An overview of the evolution of practices during the study period is provided in Table 4. The PPI incidence rate was 29.5% from 2012 to 2014 and gradually decreased over time as follows: 24.2% between 2015 and 2017 and 13.7% between 2018 and 2019 (p < 0.0001). The mean oversize index evolved significantly between 2012 and 2019. It was estimated to be 15.21 ± 16.7% from 2012 to 2014 and gradually decreased over time to 13.68 ± 8.3% (from 2015 to 2017) and 10.36 ± 9.8% (from 2018 to 2019), respectively (p < 0.0001). The transfemoral approach was the most frequent access route, whatever the period, and peaked in the 2018–2019 period (86.3%). Balloon-expandable valves implantation peaked implanted in the 2015–2017 period, whereas self-expandable valves implantation peaked over the 2012–2014 period (28.6%) (p < 0.001).

Table 4.
Evolution of practices over time.
3.4. Survival Analysis
Overall, the all-cause estimated mortality rate was 58% (95% CI: 53–64) at 7 years. During follow-up, mortality was higher in patients with PPI (43.3%) compared to those without (30.9%) (log-rank test, p = 0.048). Kaplan–Meier survival analysis comparing mortality according to the presence of PPI is presented in Figure 2. The univariate and multivariate analyses of factors associated with mortality are described in Table 5. After adjustment, PPI (ORadj 2.49, 95% CI 1.4 to 4.3; p = 0.002), chronic kidney disease (ORadj 1.53, 95% CI 1.11 to 2.12; p = 0.01), chronic respiratory disease (ORadj 1.45, 95% CI 1.04 to 2.03; p = 0.03), obliterative arteriopathy in the lower limbs (ORadj 1.38, 95% CI 1.02 to 1.87; p = 0.04), and AF (ORadj 1.95, 95% CI 1.4 to 2.71; p < 0.0001) were the only remaining factors associated with death. Survival according to PPI indication was not different between third-degree AV block, LBBB with pathological HV, and LBBB (Supplementary Figure S1).
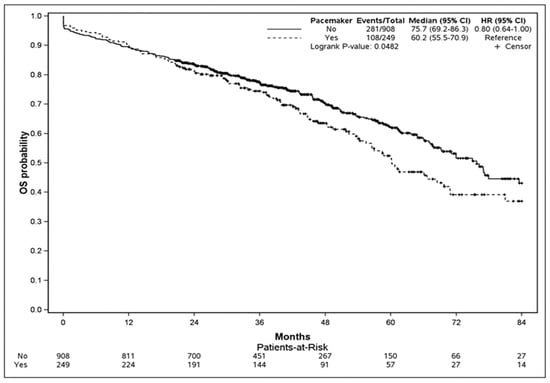
Figure 2.
Kaplan-Meier curves comparing survival stratified by PPI and the non-PPI group. The test comparing the two groups was based on the log-rank test.

Table 5.
Independent risk factors of overall mortality at logistic regression analysis.
4. Discussion
The key findings of this study, which involved a large single-center cohort of patients undergoing TAVR with an extended follow-up of up to 7 years, can be summarized as follows: (i) the rate of PPI remained notably high at 21.5%, and its incidence decreased over time. (ii) Three independent predictive factors for PPI were identified as follows: a longer baseline QRS duration, the presence of RBBB at the baseline, and the use of self-expandable valves. (iii) Patients in the PPI group exhibited higher long-term mortality compared to their counterparts. (iv) PPI emerged as an independent predictive factor for long-term mortality, along with other factors, such as chronic kidney disease, chronic respiratory disease, obliterative arteriopathy, and AF.
In this study, the rate of PPI was elevated at 21.5%, with a higher prevalence observed in patients receiving self-expandable valves (35.6%). While this rate exceeded that of contemporary cohorts [9,10,11], the range across the literature was highly variable, ranging from 3.4% to 25.9%, according to the 2021 guidelines on cardiac pacing [12]. Several factors contributed to this variability. Firstly, the included patients might carry a higher risk of conduction disorders based on their profiles, as evidenced by PPI rates of 8.1% in the intermediate-risk cohort and 6.5% in the low-risk cohort of the PARTNER trials [13,14]. Secondly, this rate appeared to be dependent on the type of valve used, with self-expandable valves consistently associated with higher rates compared to balloon-expandable valves. Thirdly, variations in indications and protocols among different centers can significantly influence the PPI rate. For instance, in our cohort, less than half of the patients had a third-degree block warranting PPI, and the majority of implanted patients had LBBB. However, indications for pacemaker implantation in LBBB post-TAVR are a subject of debate, and current guidelines exhibit a liberal stance, suggesting electrophysiology studies or ambulatory ECG monitoring with the same level of evidence (Class IIa). These findings hold clinical significance for our daily practice and underscore the imperative need for standardized protocols and further evidence to determine the optimal approach for this substantial patient population.
We found a robust association between PPI and the presence of RBBB (OR 2.51, 95% CI 1.45–4.36). The link between conduction disorders and the need for PPI following TAVR is a well-established association, with pre-existing RBBB consistently identified as the most significant and powerful contributing factor [15,16]. The proximity of the conduction pathways, especially the left bundle to the aortic valve, makes them susceptible to injury during aortic prosthesis deployment through various mechanisms—with hemorrhagic, compressive, and ischemic effects on the conductive tissue. These mechanisms result in high-grade conduction disorders and complete atrioventricular block. Additionally, other conduction disorders and the use of self-expandable valves are also found to be associated with PPI, aligning with the existing literature available [17,18]. One aspect not explored in our study, yet notably associated with PPI, is the depth of valve implantation. This parameter is gaining recognition, and operators are employing various techniques, such as deploying the valve in the cusp overlap view, to position the valve at the highest reachable level [19,20]. However, concerns about future coronary re-access in cases of the high implantation of THV are now emerging [19]. Thus, few modifiable risks are currently identified, with the majority being established as non-modifiable risk factors (preoperative conductive disorders). While these data allow for the prediction of risk, they may not necessarily lead to its prevention. The solution might lie not only in improving practices but also in enhancing valves to minimize trauma post-deployment. Nevertheless, achieving very low rates of pacemaker implantation in this type of procedure remains challenging. In our study, although we observed a higher proportion of women requiring pacemaker implantation after TAVI, this factor was not predictive in multivariate analysis. This observation contrasts with the literature, where women are often described as less frequently requiring pacemaker implantation [21].
To the best of our knowledge, this study represents one of the largest single-center TAVR registries in France, offering an extensive long-term follow-up. This prolonged observation period enabled the identification of robust predictors of mortality. Among these factors, PPI emerged as the most significant, exhibiting a two-fold increase in mortality. This phenomenon may be attributed to various factors. Firstly, patients with indications for PPI may be more likely to have comorbidities, and PPI could serve as a marker of frailty. Secondly, right ventricular stimulation has been linked to heart failure hospitalization and mortality, even in individuals without pre-existing health issues [20]. This association is tied to electromechanical dyssynchrony. These findings emphasize the non-trivial impact of PPI on patients and underscore the caution required in its indication. Furthermore, when the indication is confirmed, physiological pacing options, such as bundle pacing or left bundle branch pacing, should be considered. Additionally, given the low rates of long-term pacing dependency, especially in patients with LBBB, algorithms promoting spontaneous atrioventricular conduction should be employed. Moreover, the increasing utilization of leadless pacemakers as a safe alternative for high-risk patients has generated significant interest. Some studies have highlighted safety and efficacity, supporting their utilization [22]. However, recent findings underscore potential concerns, with evidence suggesting higher inhospitality rates compared to traditional transvenous devices [23]. Given these conflicting findings, larger randomized trials are essential to elucidate the true benefits and risks associated with the use of leadless pacemakers in this setting.
Study Limitations
Our analysis has several limitations. The primary constraint arises from the retrospective and single-center nature of this study, introducing biases inherent to the methodology and associated with data collection in a registry. The presence of unmeasured confounders cannot be ruled out. Furthermore, certain variables known to be predictive factors of PPI, such as the implantation depth and modality of valve deployment, were not recorded. Only life status was reported during follow-up, and information on re-hospitalization for heart failure or the cause of death was not available. Lastly, we did not investigate long-term PPI dependency and had no access to specific data concerning the programming and pacing parameters of the pacing devices. It is important to note that the absence of data on the burden of pacing limits our ability to ascertain the true population of patients who genuinely require a pacemaker. The inability to identify cases where the device was truly necessary underscores the importance of a more precise evaluation of indications. It is plausible that a subset of patients may have been exposed to increased mortality without a confirmed need for a pacemaker, highlighting the necessity for enhanced caution in defining indications.
5. Conclusions
This monocentric study highlights elevated rates of PPI post-TAVR, reaching 21.5%, associated with increased mortality in affected patients. These findings underscore the urgent need to reassess our practices, refine indications, and optimize the future outlook for implanted patients, particularly in reducing high PPI rates and improving long-term outcomes.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm13113050/s1, Figure S1: Kaplan-Meier curves comparing survival stratified by third-degree atrioventricular block and no third-degree atrioventricular block in the PPI group. The test comparing the two groups was based on the log-rank test.
Author Contributions
Conceptualization, M.-P.G.; methodology, M.-P.G.; software, V.P.; validation, M.-P.G., V.P., E.C., A.B., J.V., E.L.-V., P.R. and F.C.; formal analysis, M.-P.G. and V.P.; investigation, M.-P.G.; resources, E.S.; data curation, M.-P.G.; writing—original draft preparation, M.-P.G. and V.P.; writing—review and editing, V.P.; visualization, V.P.; supervision, M.-P.G. and V.P.; project administration, E.S.; funding acquisition, M.-P.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Le GCS pour l’Enseignement et la Recherche Ramsay Santé, grant numbers 12-039, 2019.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of “Le GCS Ramsay Santé pour l’Enseignement et la Recherche” on 12 April 2023 (IRB number: 00010835).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors would like to thank Nesrine BENHIZIA-BENAOUICHA and Benoit BERGE from Euraxi Pharma (Joué-les-Tours, France) for their contribution to the drafting of this manuscript and providing editorial support, as well as for conducting the statistical analysis. Furthermore, the authors would like to thank the research assistants and nurses from the department of Clairval Private Hospital for their outstanding work and diligent patient follow-up.
Conflicts of Interest
V.P. has received institutional research grants from Medtronic, Boston Scientific, and Microport. Other authors declare no conflict of interest.
References
- Urena, M.; Webb, J.G.; Cheema, A.; Serra, V.; Toggweiler, S.; Barbanti, M.; Cheung, A.; Ye, J.; Dumont, E.; DeLarochellière, R.; et al. Impact of new-onset persistent left bundle branch block on late clinical outcomes in patients undergoing transcatheter aortic valve implantation with a balloon-expandable valve. JACC Cardiovasc. Interv. 2014, 7, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Gilard, M.; Eltchaninoff, H.; Iung, B.; Donzeau-Gouge, P.; Chevreul, K.; Fajadet, J.; Leprince, P.; Leguerrier, A.; Lievre, M.; Prat, A.; et al. Registry of Transcatheter Aortic-Valve Implantation in High-Risk Patients. N. Engl. J. Med. 2012, 366, 1705–1715. [Google Scholar] [CrossRef] [PubMed]
- Auffret, V.; Lefevre, T.; Van Belle, E.; Eltchaninoff, H.; Iung, B.; Koning, R.; Motreff, P.; Leprince, P.; Verhoye, J.P.; Manigold, T.; et al. Temporal Trends in Transcatheter Aortic Valve Replacement in France: FRANCE 2 to FRANCE TAVI. J. Am. Coll. Cardiol. 2017, 70, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Ullah, W.; Zahid, S.; Zaidi, S.R.; Sarvepalli, D.; Haq, S.; Roomi, S.; Mukhtar, M.; Khan, M.A.; Gowda, S.N.; Ruggiero, N.; et al. Predictors of Permanent Pacemaker Implantation in Patients Undergoing Transcatheter Aortic Valve Replacement—A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2021, 10, e020906. [Google Scholar] [CrossRef] [PubMed]
- Lilly, S.M.; Deshmukh, A.J.; Epstein, A.E.; Ricciardi, M.J.; Shreenivas, S.; Velagapudi, P.; Wyman, J.F. 2020 ACC Expert Consensus Decision Pathway on Management of Conduction Disturbances in Patients Undergoing Transcatheter Aortic Valve Replacement: A Report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll. Cardiol. 2020, 76, 2391–2411. [Google Scholar] [CrossRef] [PubMed]
- Rodés-Cabau, J.; Ellenbogen, K.A.; Krahn, A.D.; Latib, A.; Mack, M.; Mittal, S.; Muntané-Carol, G.; Nazif, T.M.; Sondergaard, L.; Urena, M.; et al. Management of Conduction Disturbances Associated with Transcatheter Aortic Valve Replacement: JACC Scientific Expert Panel. J. Am. Coll. Cardiol. 2019, 74, 1086–1106. [Google Scholar] [CrossRef] [PubMed]
- Subramani, S.; Arora, L.; Krishnan, S.; Hanada, S.; Sharma, A.; Ramakrishna, H. Analysis of Conduction Abnormalities and Permanent Pacemaker Implantation After Transcatheter Aortic Valve Replacement. J. Cardiothorac. Vasc. Anesth. 2020, 34, 1082–1093. [Google Scholar] [CrossRef] [PubMed]
- Ki, Y.-J.; Kang, J.; Lee, H.S.; Chang, M.; Han, J.-K.; Yang, H.-M.; Park, K.W.; Kang, H.-J.; Koo, B.-K.; Kim, H.-S. Optimal Oversizing Index Depending on Valve Type and Leakage-Proof Function for Preventing Paravalvular Leakage after Transcatheter Aortic Valve Implantation. J. Clin. Med. 2020, 9, 3936. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.J.; Yan, C.J.; Lin, D.Q.; Cheng, Y.B.; Yu, S.J.; Li, J.; Zhang, X.P.; Cheng, W. Prognostic impact of new permanent pacemaker implantation following transcatheter aortic valve replacement. Catheter. Cardiovasc. Interv. 2023, 102, 743–750. [Google Scholar] [CrossRef]
- Panagides, V.; Cheema, A.N.; Urena, M.; Nombela-Franco, L.; Veiga-Fernandez, G.; Vilalta, V.; Regueiro, A.; Del Val, D.; Asmarats, L.; del Trigo, M.; et al. Optimal Degree of Balloon-Expandable Transcatheter Valve Oversizing in Patients With Borderline Aortic Annulus Measurements: Insights From a Multicenter Real-World Experience. Circ. Cardiovasc. Interv. 2023, 16, e012554. [Google Scholar] [CrossRef]
- Carroll, J.D.; Mack, M.J.; Vemulapalli, S.; Herrmann, H.C.; Gleason, T.G.; Hanzel, G.; Deeb, G.M.; Thourani, V.H.; Cohen, D.J.; Desai, N.; et al. STS-ACC TVT Registry of Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2020, 76, 2492–2516. [Google Scholar] [CrossRef]
- Glikson, M.; Nielsen, J.C.; Kronborg, M.B.; Michowitz, Y.; Auricchio, A.; Barbash, I.M.; Barrabés, J.A.; Boriani, G.; Braunschweig, F.; Brignole, M.; et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur. Heart J. 2021, 42, 3427–3520. [Google Scholar] [CrossRef] [PubMed]
- Leon, M.B.; Smith, C.R.; Mack, M.J.; Makkar, R.R.; Svensson, L.G.; Kodali, S.K.; Thourani, V.H.; Tuzcu, E.M.; Miller, D.C.; Herrmann, H.C.; et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2016, 374, 1609–1620. [Google Scholar] [CrossRef] [PubMed]
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Russo, M.; Kapadia, S.R.; Malaisrie, S.C.; Cohen, D.J.; Pibarot, P.; et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1695–1705. [Google Scholar] [CrossRef] [PubMed]
- Mangieri, A.; Lanzillo, G.; Bertoldi, L.; Jabbour, R.J.; Regazzoli, D.; Ancona, M.B.; Tanaka, A.; Mitomo, S.; Garducci, S.; Montalto, C.; et al. Predictors of Advanced Conduction Disturbances Requiring a Late (≥48 H) Permanent Pacemaker Following Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2018, 11, 1519–1526. [Google Scholar] [CrossRef] [PubMed]
- Mauri, V.; Reimann, A.; Stern, D.; Scherner, M.; Kuhn, E.; Rudolph, V.; Rosenkranz, S.; Eghbalzadeh, K.; Friedrichs, K.; Wahlers, T.; et al. Predictors of Permanent Pacemaker Implantation After Transcatheter Aortic Valve Replacement with the SAPIEN 3. JACC Cardiovasc. Interv. 2016, 9, 2200–2209. [Google Scholar] [CrossRef] [PubMed]
- Giustino, G.; Van der Boon, R.; de Nicolas, J.M.-M.; Dumonteil, N.; Chieffo, A.; de Jaegere, P.; Tchetche, D.; Marcheix, B.; Millischer, D.; Cassagneau, R.; et al. Impact of permanent pacemaker on mortality after transcatheter aortic valve implantation: The PRAGMATIC (Pooled Rotterdam-Milan-Toulouse in Collaboration) Pacemaker substudy. EuroIntervention 2016, 12, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Auffret, V.; Puri, R.; Urena, M.; Chamandi, C.; Rodriguez-Gabella, T.; Philippon, F.; Rodés-Cabau, J. Conduction Disturbances After Transcatheter Aortic Valve Replacement: Current Status and Future Perspectives. Circulation 2017, 136, 1049–1069. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, T.; Yamanaka, F.; Shishido, K.; Moriyama, N.; Komatsu, I.; Yokoyama, H.; Miyashita, H.; Sato, D.; Sugiyama, Y.; Hayashi, T.; et al. Impact of High Implantation of Transcatheter Aortic Valve on Subsequent Conduction Disturbances and Coronary Access. JACC Cardiovasc. Interv. 2023, 16, 1192–1204. [Google Scholar] [CrossRef]
- Defaye, P.; Biffi, M.; El-Chami, M.; Boveda, S.; Glikson, M.; Piccini, J.; Vitolo, M. Cardiac pacing and lead devices management: 25 years of research at EP Europace journal. EP Eur. 2023, 25, euad202. [Google Scholar] [CrossRef]
- Ravaux, J.M.; Di Mauro, M.; Vernooy, K.; Hof, A.W.V.; Veenstra, L.; Kats, S.; Maessen, J.G.; Lorusso, R. Do Women Require Less Permanent Pacemaker After Transcatheter Aortic Valve Implantation? A Meta-Analysis and Meta-Regression. J. Am. Heart Assoc. 2021, 10, e019429. [Google Scholar] [CrossRef] [PubMed]
- MitMitacchione, G.; Schiavone, M.; Gasperetti, A.; Arabia, G.; Breitenstein, A.; Cerini, M.; Palmisano, P.; Montemerlo, E.; Ziacchi, M.; Gulletta, S.; et al. Outcomes of leadless pacemaker implantation following transvenous lead extraction in high-volume referral centers: Real-world data from a large international registry. Heart Rhythm. 2023, 20, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Alhuarrat, M.A.D.; Khawrawala, A.; Renjithlal, S.; Eid, M.M.; Varrias, D.; Mohammed, M.; Grushko, M.; Di Biase, L. Comparison of in-hospital outcomes and complications of leadless pacemaker and traditional transvenous pacemaker implantation. Europace 2023, 25, euad269. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).