Prognostic Implication of Intestinal Wall Edema in Patients with Aortic Stenosis Receiving Trans-Catheter Aortic Valve Replacement
Abstract
:1. Background
2. Methods
2.1. Patient Selection
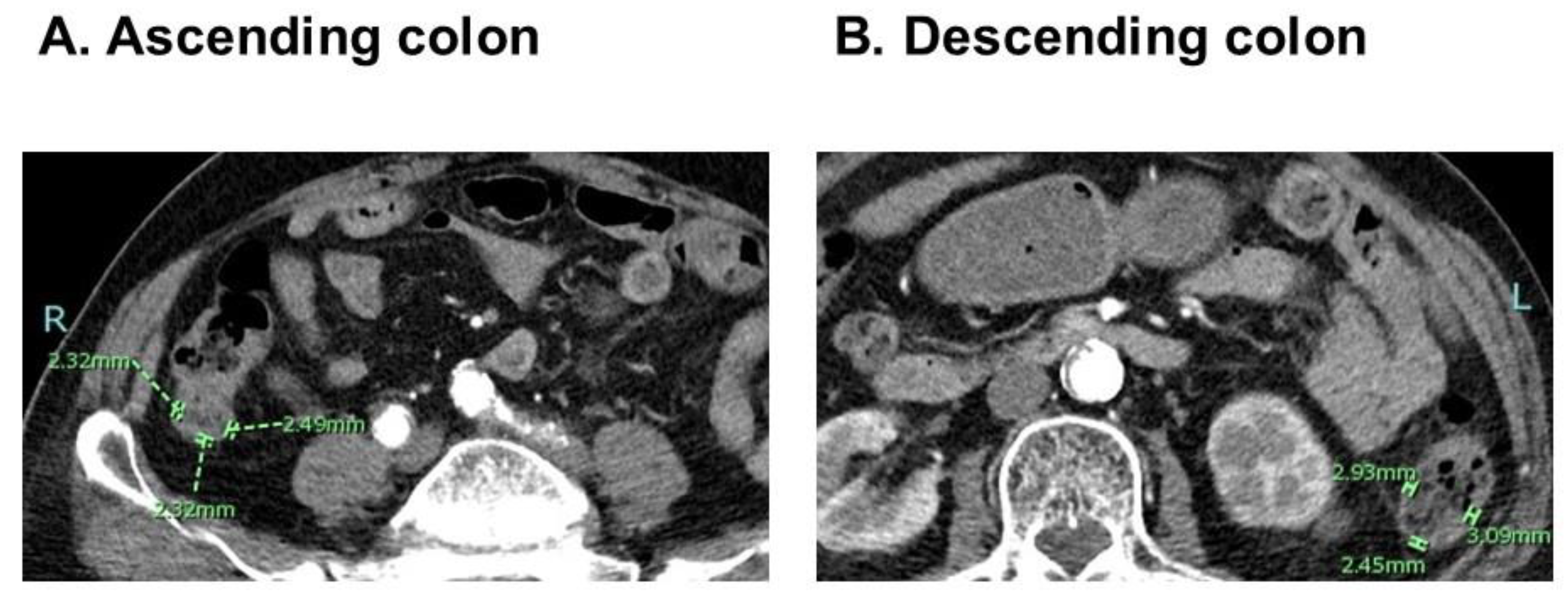
2.2. Measurement of CWT
2.3. Other Baseline Characteristics
2.4. TAVR Procedure
2.5. Post-TAVR Course and Primary Outcome
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics
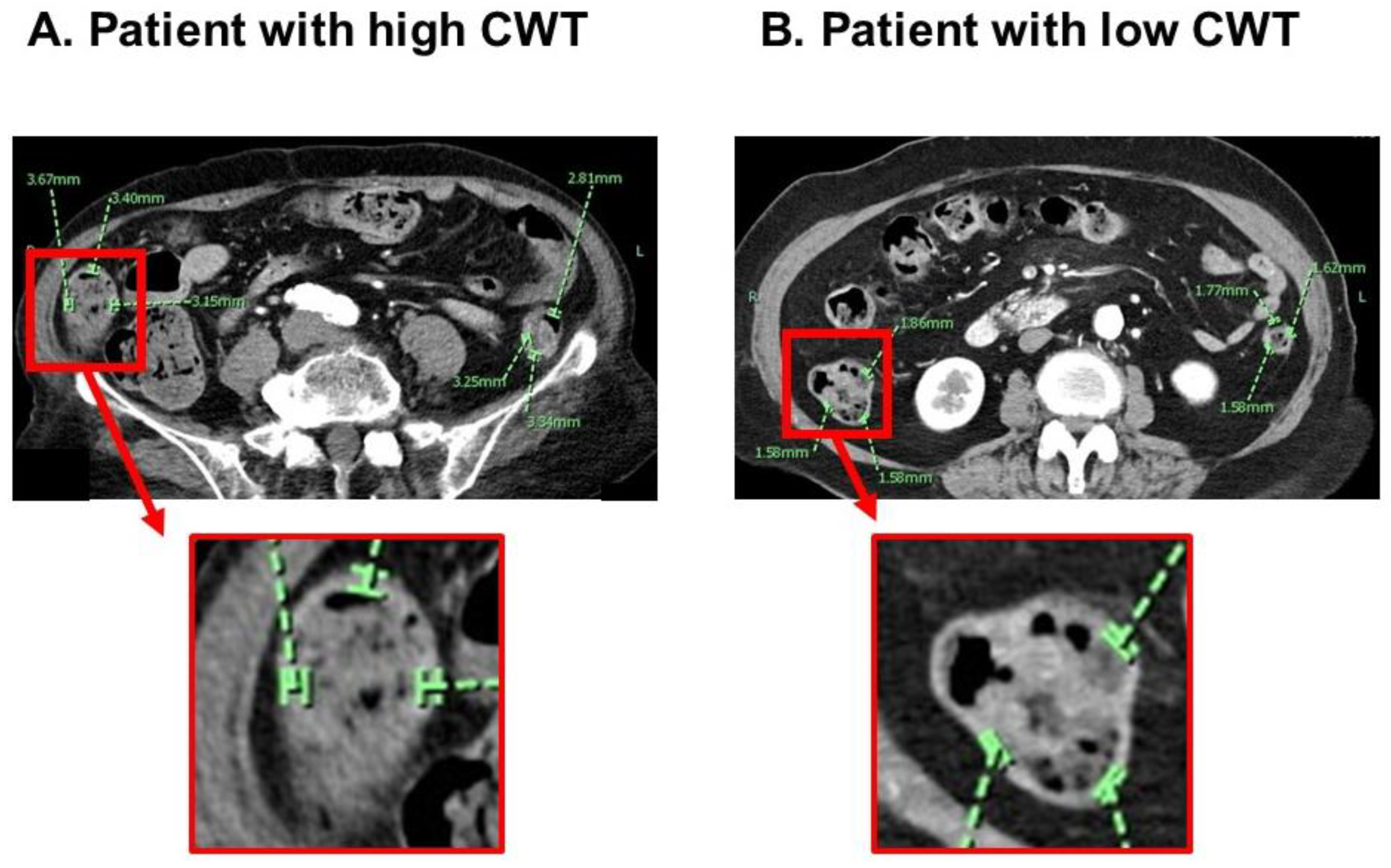
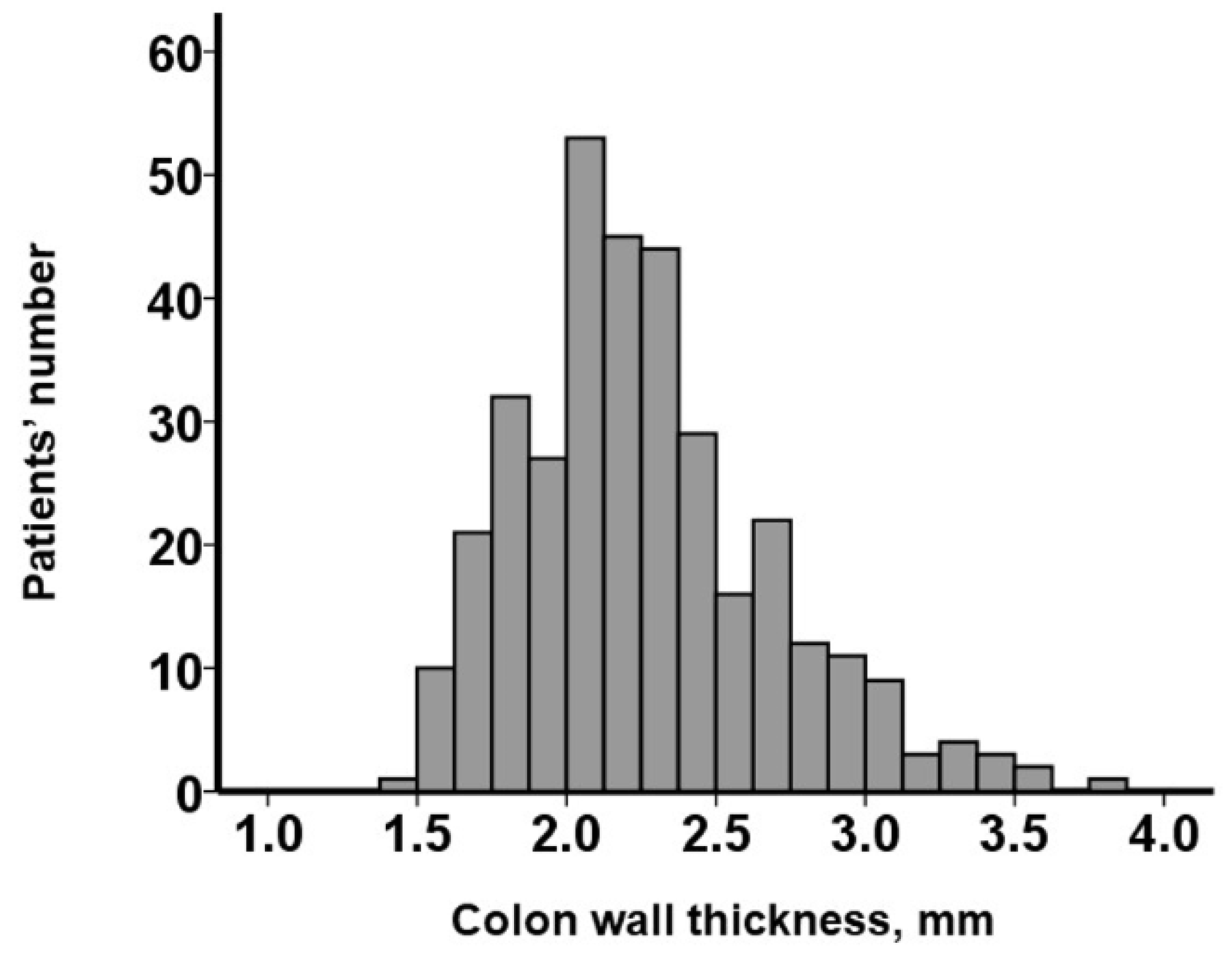
3.2. CWT Measurement
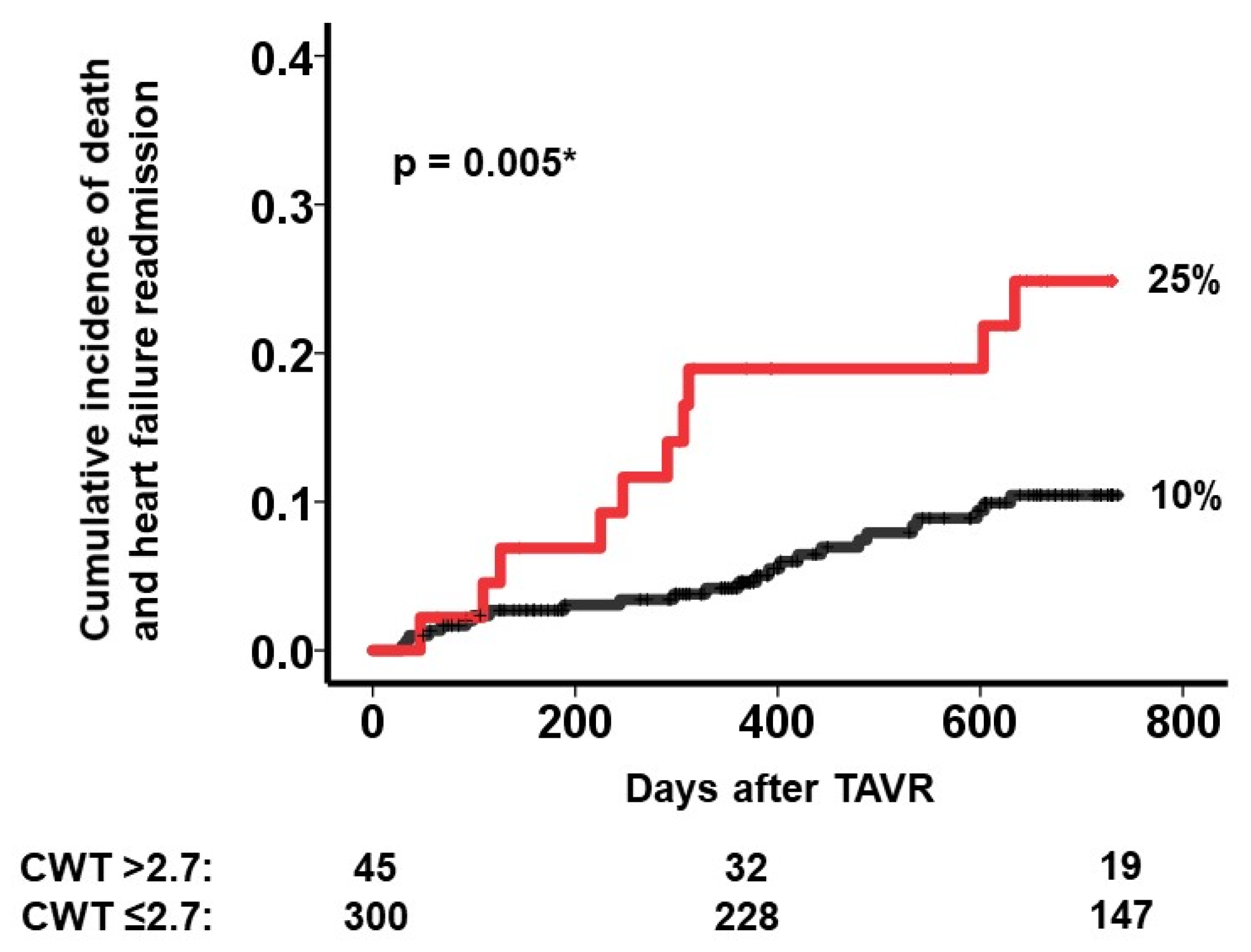
3.3. Impact of CWT on the Primary Outcome
3.4. Risk Stratification Using a Cutoff of CWT
4. Discussion
4.1. Heart Failure and Intestinal Dysfunction
4.2. Intestinal Dysfunction and Cardiac Cachexia
4.3. Clinical Implication of Our Findings
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Afilalo, J.; Lauck, S.; Kim, D.H.; Lefevre, T.; Piazza, N.; Lachapelle, K.; Martucci, G.; Lamy, A.; Labinaz, M.; Peterson, M.D.; et al. Frailty in Older Adults Undergoing Aortic Valve Replacement: The FRAILTY-AVR Study. J. Am. Coll. Cardiol. 2017, 70, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Kolte, D.; Vlahakes, G.J.; Palacios, I.F.; Sakhuja, R.; Passeri, J.J.; Inglessis, I.; Elmariah, S. Transcatheter Versus Surgical Aortic Valve Replacement in Low-Risk Patients. J. Am. Coll. Cardiol. 2019, 74, 1532–1540. [Google Scholar] [CrossRef] [PubMed]
- Osnabrugge, R.L.; Mylotte, D.; Head, S.J.; Van Mieghem, N.M.; Nkomo, V.T.; LeReun, C.M.; Bogers, A.J.; Piazza, N.; Kappetein, A.P. Aortic stenosis in the elderly: Disease prevalence and number of candidates for transcatheter aortic valve replacement: A meta-analysis and modeling study. J. Am. Coll. Cardiol. 2013, 62, 1002–1012. [Google Scholar] [CrossRef] [PubMed]
- Auffret, V.; Bakhti, A.; Leurent, G.; Bedossa, M.; Tomasi, J.; Belhaj Soulami, R.; Verhoye, J.P.; Donal, E.; Galli, E.; Loirat, A.; et al. Determinants and Impact of Heart Failure Readmission Following Transcatheter Aortic Valve Replacement. Circ. Cardiovasc. Interv. 2020, 13, e008959. [Google Scholar] [CrossRef] [PubMed]
- Genereux, P.; Pibarot, P.; Redfors, B.; Mack, M.J.; Makkar, R.R.; Jaber, W.A.; Svensson, L.G.; Kapadia, S.; Tuzcu, E.M.; Thourani, V.H.; et al. Staging classification of aortic stenosis based on the extent of cardiac damage. Eur. Heart J. 2017, 38, 3351–3358. [Google Scholar] [CrossRef] [PubMed]
- Shimura, T.; Yamamoto, M.; Kano, S.; Kagase, A.; Kodama, A.; Koyama, Y.; Tsuchikane, E.; Suzuki, T.; Otsuka, T.; Kohsaka, S.; et al. Impact of the Clinical Frailty Scale on Outcomes After Transcatheter Aortic Valve Replacement. Circulation 2017, 135, 2013–2024. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.; Wang, Z.; Levison, B.S.; Koeth, R.A.; Britt, E.B.; Fu, X.; Wu, Y.; Hazen, S.L. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N. Engl. J. Med. 2013, 368, 1575–1584. [Google Scholar] [CrossRef] [PubMed]
- Krack, A.; Sharma, R.; Figulla, H.R.; Anker, S.D. The importance of the gastrointestinal system in the pathogenesis of heart failure. Eur. Heart J. 2005, 26, 2368–2374. [Google Scholar] [CrossRef] [PubMed]
- Sandek, A.; Bauditz, J.; Swidsinski, A.; Buhner, S.; Weber-Eibel, J.; von Haehling, S.; Schroedl, W.; Karhausen, T.; Doehner, W.; Rauchhaus, M.; et al. Altered intestinal function in patients with chronic heart failure. J. Am. Coll. Cardiol. 2007, 50, 1561–1569. [Google Scholar] [CrossRef] [PubMed]
- Sandek, A.; Rauchhaus, M.; Anker, S.D.; von Haehling, S. The emerging role of the gut in chronic heart failure. Curr. Opin. Clin. Nutr. Metab. Care. 2008, 11, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Sandek, A.; Swidsinski, A.; Schroedl, W.; Watson, A.; Valentova, M.; Herrmann, R.; Scherbakov, N.; Cramer, L.; Rauchhaus, M.; Grosse-Herrenthey, A.; et al. Intestinal blood flow in patients with chronic heart failure: A link with bacterial growth, gastrointestinal symptoms, and cachexia. J. Am. Coll. Cardiol. 2014, 64, 1092–1102. [Google Scholar] [CrossRef] [PubMed]
- Peschel, T.; Schonauer, M.; Thiele, H.; Anker, S.D.; Schuler, G.; Niebauer, J. Invasive assessment of bacterial endotoxin and inflammatory cytokines in patients with acute heart failure. Eur. J. Heart Fail. 2003, 5, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Ishiyama, Y.; Hoshide, S.; Mizuno, H.; Kario, K. Constipation-induced pressor effects as triggers for cardiovascular events. J. Clin. Hypertens. 2019, 21, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Ishii, S.; Fujita, T.; Iida, Y.; Kaida, T.; Nabeta, T.; Maekawa, E.; Yanagisawa, T.; Koitabashi, T.; Takeuchi, I.; et al. Prognostic impact of intestinal wall thickening in hospitalized patients with heart failure. Int. J. Cardiol. 2017, 230, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.K. Normal colon wall thickness on CT. Radiology 1982, 145, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Haber, H.P.; Benda, N.; Fitzke, G.; Lang, A.; Langenberg, M.; Riethmuller, J.; Stern, M. Colonic wall thickness measured by ultrasound: Striking differences in patients with cystic fibrosis versus healthy controls. Gut 1997, 40, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Carabello, B.A.; Paulus, W.J. Aortic stenosis. Lancet 2009, 373, 956–966. [Google Scholar] [CrossRef] [PubMed]
- Vaduganathan, M.; Greene, S.J.; Butler, J.; Sabbah, H.N.; Shantsila, E.; Lip, G.Y.; Gheorghiade, M. The immunological axis in heart failure: Importance of the leukocyte differential. Heart Fail. Rev. 2013, 18, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Verbrugge, F.H.; Dupont, M.; Steels, P.; Grieten, L.; Malbrain, M.; Tang, W.H.; Mullens, W. Abdominal contributions to cardiorenal dysfunction in congestive heart failure. J. Am. Coll. Cardiol. 2013, 62, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Kida, K.; Miyajima, I.; Suzuki, N.; Greenberg, B.H.; Akashi, Y.J. Nutritional management of heart failure. J. Cardiol. 2023, 81, 283–291. [Google Scholar] [CrossRef] [PubMed]
| Total (N = 345) | CWT > 2.7 mm (N = 45) | CWT ≤ 2.7 mm (N = 300) | p-c | |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 85 (82, 88) | 87 (82, 90) | 85 (82, 88) | 0.23 |
| Male sex | 99 (29%) | 14 (31%) | 85 (28%) | 0.41 |
| Body surface area, m2 | 1.38 (1.30, 1.52) | 1.35 (1.27, 1.44) | 1.39 (1.31, 1.52) | 0.79 |
| Systolic blood pressure, mm Hg | 116 (106, 127) | 118 (106, 124) | 115 (106, 127) | 0.94 |
| Pulse rate, bpm | 71 (63, 78) | 74 (71, 78) | 70 (63, 78) | 0.52 |
| Comorbidity | ||||
| Hypertension | 252 (73%) | 35 (78%) | 217 (72%) | 0.31 |
| Dyslipidemia | 165 (48%) | 19 (42%) | 146 (49%) | 0.25 |
| Diabetes mellitus | 61 (18%) | 8 (18%) | 53 (18%) | 0.60 |
| Atrial fibrillation | 56 (16%) | 4 (9%) | 52 (17%) | 0.11 |
| History of stroke | 45 (13%) | 3 (7%) | 42 (14%) | 0.12 |
| Coronary heart disease | 88 (26%) | 14 (31%) | 74 (25%) | 0.23 |
| Laboratory data | ||||
| Hemoglobin, g/dL | 11.4 (10.0, 12.4) | 10.3 (8.9, 11.7) | 11.4 (10.1, 12.5) | 0.012 * |
| Serum albumin, g/dL | 3.8 (3.5, 4.0) | 3.6 (3.3, 4.0) | 3.8 (3.5, 4.1) | 0.068 |
| Serum cholinesterase, U/L | 234 (195, 278) | 205 (169, 281) | 238 (198, 277) | 0.063 |
| Serum sodium, mEq/L | 141 (139, 142) | 140 (137, 142) | 141 (139, 142) | 0.074 |
| Serum potassium, mEq/L | 4.4 (4.1, 4.6) | 4.4 (4.1, 4.7) | 4.3 (4.0, 4.6) | 0.94 |
| Serum total bilirubin, mg/dL | 0.5 (0.4, 0.7) | 0.5 (0.4, 0.6) | 0.5 (0.4, 0.7) | 0.76 |
| eGFR, mL/min/1.73 m2 | 48 (37, 61) | 41 (31, 58) | 49 (38, 64) | 0.24 |
| Plasma B-type natriuretic peptide, pg/mL | 216 (113, 516) | 268 (151, 563) | 200 (109, 449) | 0.30 |
| Total cholesterol, mg/dL | 165 (147, 194) | 156 (142, 192) | 167 (148, 194) | 0.060 |
| C-reactive protein, mg/dL | 0.11 (0.04, 0.36) | 0.14 (0.04, 0.83) | 0.11 (0.04, 0.33) | 0.087 |
| Echocardiography | ||||
| Left ventricular end-diastolic diameter, mm | 46 (42, 51) | 46 (40, 51) | 46 (42, 51) | 0.58 |
| Left ventricular ejection fraction, % | 64 (54, 70) | 67 (55, 72) | 63 (54, 70) | 0.62 |
| Left atrial diameter, mm | 43 (39, 49) | 45 (41, 50) | 43 (38, 49) | 0.28 |
| Moderate or greater MR | 34 (10%) | 8 (18%) | 26 (9%) | 0.056 |
| Moderate or greater TR | 18 (5%) | 3 (7%) | 15 (5%) | 0.64 |
| E/e’ ratio | 16.5 (12.6, 22.7) | 17.3 (14.0, 23.3) | 16.5 (12.3, 22.6) | 0.25 |
| Aortic valve peak velocity, m/s | 4.4 (4.0, 4.9) | 4.5 (4.0, 4.9) | 4.4 (4.0, 4.8) | 0.46 |
| Mean aortic valve velocity, m/s | 46 (38, 57) | 47 (38, 57) | 46 (38, 56) | 0.55 |
| Aortic valve area, cm2 | 0.54 (0.44, 0.67) | 0.54 (0.47, 0.71) | 0.54 (0.44, 0.66) | 0.58 |
| Hemodynamics | ||||
| CVP, mm Hg | 5 (3, 7) | 5 (3, 8) | 5 (3, 7) | 0.72 |
| Mean pulmonary artery pressure, mm Hg | 19 (16, 23) | 19 (16, 25) | 19 (15, 23) | 0.92 |
| PAWP, mm Hg | 11 (8, 15) | 11 (7, 16) | 11 (8, 15) | 0.81 |
| Cardiac index, L/min/m2 | 2.7 (2.3, 3.0) | 2.6 (2.3, 2.9) | 2.7 (2.3, 3.0) | 0.95 |
| CVP/PCAWP ratio | 0.45 (0.33, 0.57) | 0.44 (0.30, 0.57) | 0.45 (0.33, 0.57) | 0.98 |
| PAPi | 3.5 (2.4, 6.0) | 3.3 (2.4, 4.8) | 3.6 (2.6, 6.0) | 0.087 |
| RVSWI, g/m | 7.3 (5.3, 9.9) | 7.0 (5.4, 9.6) | 7.4 (5.1, 9.8) | 0.32 |
| Scores | ||||
| STS score | 5.2 (3.9, 7.3) | 5.6 (4.5, 9.3) | 5.1 (3.8, 7.2) | 0.091 |
| EURO II score | 3.4 (2.4, 4.6) | 3.6 (2.9, 4.6) | 3.4 (2.4, 4.5) | 0.24 |
| GNRI | 97 (91, 104) | 96 (86, 105) | 98 (91, 105) | 0.45 |
| Medication | ||||
| Beta-blocker | 112 (32%) | 15 (33%) | 97 (32%) | 0.51 |
| Renin-angiotensin system inhibitor | 213 (62%) | 26 (58%) | 187 (62%) | 0.33 |
| Mineralocorticoid receptor antagonist | 98 (28%) | 15 (33%) | 83 (28%) | 0.27 |
| Loop diuretics | 189 (55%) | 25 (56%) | 164 (55%) | 0.52 |
| Hazard Ratio (95% Confidence Interval) | p-Value | |
|---|---|---|
| Colon wall thickness, cm | 2.02 (1.01–4.07) | 0.049 * |
| Hemoglobin, g/dL | 0.81 (0.65–1.02) | 0.075 |
| Age, years | 1.03 (0.96–1.10) | 0.47 |
| Logarithm of plasma BNP, pg/mL | 1.48 (0.69–3.17) | 0.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akao, K.; Imamura, T.; Tanaka, S.; Onoda, H.; Ushijima, R.; Sobajima, M.; Fukuda, N.; Ueno, H.; Kinugawa, K. Prognostic Implication of Intestinal Wall Edema in Patients with Aortic Stenosis Receiving Trans-Catheter Aortic Valve Replacement. J. Clin. Med. 2023, 12, 7658. https://doi.org/10.3390/jcm12247658
Akao K, Imamura T, Tanaka S, Onoda H, Ushijima R, Sobajima M, Fukuda N, Ueno H, Kinugawa K. Prognostic Implication of Intestinal Wall Edema in Patients with Aortic Stenosis Receiving Trans-Catheter Aortic Valve Replacement. Journal of Clinical Medicine. 2023; 12(24):7658. https://doi.org/10.3390/jcm12247658
Chicago/Turabian StyleAkao, Kousuke, Teruhiko Imamura, Shuhei Tanaka, Hiroshi Onoda, Ryuichi Ushijima, Mitsuo Sobajima, Nobuyuki Fukuda, Hiroshi Ueno, and Koichiro Kinugawa. 2023. "Prognostic Implication of Intestinal Wall Edema in Patients with Aortic Stenosis Receiving Trans-Catheter Aortic Valve Replacement" Journal of Clinical Medicine 12, no. 24: 7658. https://doi.org/10.3390/jcm12247658
APA StyleAkao, K., Imamura, T., Tanaka, S., Onoda, H., Ushijima, R., Sobajima, M., Fukuda, N., Ueno, H., & Kinugawa, K. (2023). Prognostic Implication of Intestinal Wall Edema in Patients with Aortic Stenosis Receiving Trans-Catheter Aortic Valve Replacement. Journal of Clinical Medicine, 12(24), 7658. https://doi.org/10.3390/jcm12247658






