Characteristics and Risk Factors of Delayed Perforation in Endoscopic Submucosal Dissection for Early Gastric Cancer
Abstract
1. Introduction
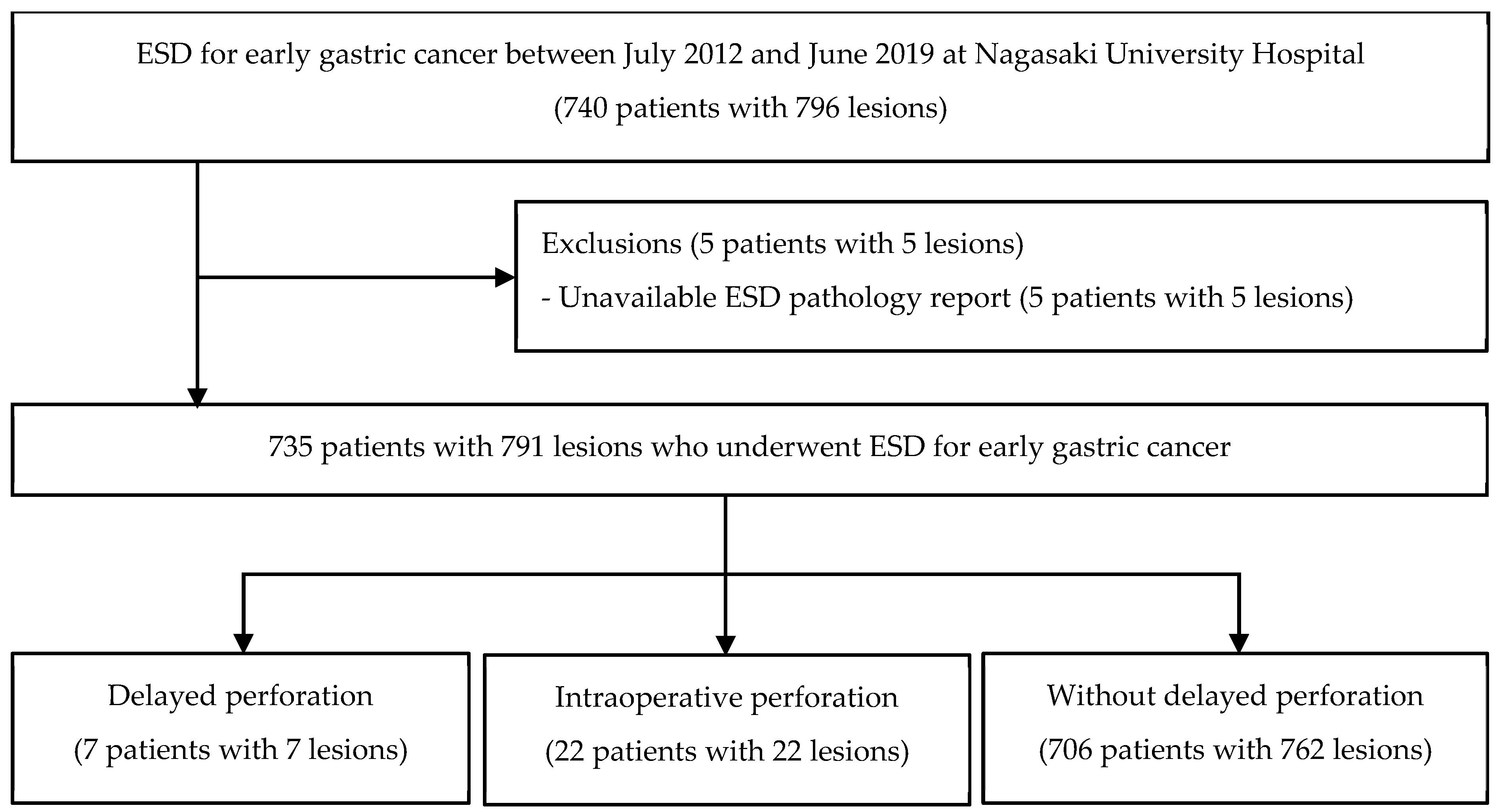
2. Materials and Methods
2.1. Patients
2.2. ESD Procedures
2.3. Definition of Delayed Perforation
2.4. Evaluation of Clinical Pathological Features, Risk Factors for Delayed Perforation, and Optimal Management
2.5. Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shiotsuki, K.; Takizawa, K.; Ono, H. Indications of endoscopic submucosal dissection for undifferentiated early gastric cancer: Current status and future perspectives for further expansion. Digestion 2022, 103, 76–82. [Google Scholar] [CrossRef]
- Suzuki, H.; Takizawa, K.; Hirasawa, T.; Takeuchi, Y.; Ishido, K.; Hoteya, S.; Yano, T.; Tanaka, S.; Endo, M.; Nakagawa, M.; et al. Short-term outcomes of multicenter prospective cohort study of gastric endoscopic resection: “Real-world evidence” in Japan. Dig. Endosc. 2019, 31, 30–39. [Google Scholar] [CrossRef]
- Iiizuka, H.; Kakizaki, S.; Sohara, N.; Onozato, Y.; Ishihara, H.; Okamura, S.; Itoh, H.; Mori, M. Stricture after endoscopic submucosal dissection for early gastric cancers and adenomas. Dig. Endosc. 2010, 22, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Kakushima, N.; Tanaka, M.; Sawai, H.; Imai, K.; Kawata, N.; Hagiwara, T.; Takao, T.; Hotta, K.; Yamaguchi, Y.; Takizawa, K.; et al. Gastric obstruction after endoscopic submucosal dissection. United Eur. Gastroenterol. J. 2013, 1, 184–190. [Google Scholar] [CrossRef]
- Ohta, T.; Ishihara, R.; Uedo, N.; Takeuchi, Y.; Nagai, K.; Matsui, F.; Kawada, N.; Yamashina, T.; Kanzaki, H.; Hanafusa, M.; et al. Factors predicting perforation during endoscopic submucosal dissection for gastric cancer. Gastrointest. Endosc. 2012, 75, 1159–1165. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Kikuchi, D.; Nagami, Y.; Nonaka, K.; Tsuji, Y.; Fujimoto, A.; Sanomura, Y.; Tanaka, K.; Abe, S.; Zhang, S.; et al. Management of adverse events related to endoscopic resection of upper gastrointestinal neoplasms: Review of the literature and recommendations from experts. Dig. Endosc. 2019, 31 (Suppl. S1), 4–20. [Google Scholar] [CrossRef]
- Imagawa, A.; Okada, H.; Kawahara, Y.; Takenaka, R.; Kato, J.; Kawamoto, H.; Fujiki, S.; Takata, R.; Yoshino, T.; Shiratori, Y. Endoscopic submucosal dissection for early gastric cancer: Results and degrees of technical difficulty as well as success. Endoscopy 2006, 38, 987–990. [Google Scholar] [CrossRef]
- Minami, S.; Gotoda, T.; Ono, H.; Oda, I.; Hamanaka, H. Complete Endoscopic Closure of Gastric Perforation Induced by Endoscopic Resection of Early Gastric Cancer Using endoclips Can Prevent Surgery (with Video). Gastrointest. Endosc. 2006, 63, 596–601. [Google Scholar] [CrossRef]
- Hanaoka, N.; Uedo, N.; Ishihara, R.; Higashino, K.; Takeuchi, Y.; Inoue, T.; Chatani, R.; Hanafusa, M.; Tsujii, Y.; Kanzaki, H.; et al. Clinical features and outcomes of delayed perforation after endoscopic submucosal dissection for early gastric cancer. Endoscopy 2010, 42, 1112–1115. [Google Scholar] [CrossRef]
- Homma, S.; Tokodai, K.; Watanabe, M.; Takaya, K.; Hashizume, E. Delayed Perforation Occurring on the 24th Day after Endoscopic Submucosal Dissection for Early Gastric Cancer. Clin. J. Gastroenterol. 2017, 10, 124–127. [Google Scholar] [CrossRef]
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer 2017, 20, 1–19. [Google Scholar] [CrossRef]
- Tanaka, M.; Ono, H.; Hasuike, N.; Takizawa, K. Endoscopic submucosal dissection of early gastric cancer. Digestion 2008, 77 (Suppl. S1), 23–28. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Takizawa, K.; Ono, H.; Igarashi, K.; Sugimoto, S.; Kawata, N.; Tanaka, M.; Kakushima, N.; Ito, S.; Imai, K.; et al. Efficacy of Endoscopic submucosal Dissection with Dental Floss Clip Traction for Gastric Epithelial Neoplasia: A Pilot Study (with Video). Surg. Endosc. 2016, 30, 3100–3106. [Google Scholar] [CrossRef] [PubMed]
- Ikezawa, K.; Michida, T.; Iwahashi, K.; Maeda, K.; Naito, M.; Ito, T.; Katayama, K. Delayed perforation occurring after endoscopic submucosal dissection for early gastric cancer. Gastric. Cancer 2012, 15, 111–114. [Google Scholar] [CrossRef]
- Kato, M.; Nishida, T.; Tsutsui, S.; Komori, M.; Michida, T.; Yamamoto, K.; Kawai, N.; Kitamura, S.; Zushi, S.; Nishihara, A.; et al. Endoscopic Submucosal Dissection as a Treatment for Gastric Noninvasive Neoplasia: A Multicenter Study by Osaka University ESD Study Group. J. Gastroenterol. 2011, 46, 325–331. [Google Scholar] [CrossRef]
- Suzuki, H.; Oda, I.; Sekiguchi, M.; Abe, S.; Nonaka, S.; Yoshinaga, S.; Nakajima, T.; Saito, Y. Management and associated factors of delayed perforation after gastric endoscopic submucosal dissection. World J. Gastroenterol. 2015, 21, 12635–12643. [Google Scholar] [CrossRef]
- Nagae, S.; Kimoto, Y.; Sawada, R.; Furuta, K.; Ito, Y.; Takeuchi, N.; Takayanagi, S.; Kano, Y.; Ishii, R.; Sakuno, T.; et al. Perigastric abscess caused by delayed perforation after gastric endoscopic submucosal dissection: Successful conservative treatment without perforation closure: A case report. J. Med. Case Rep. 2023, 17, 92. [Google Scholar] [CrossRef] [PubMed]
- Yano, T.; Tanabe, S.; Ishido, K.; Azuma, M.; Wada, T.; Suzuki, M.; Kawanishi, N.; Yamane, S.; Sasaki, T.; Katada, C.; et al. Delayed perforation after endoscopic submucosal dissection for early gastric cancer: Clinical features and treatment. World J. Gastrointest. Endosc. 2016, 8, 368–373. [Google Scholar] [CrossRef]
- Kim, T.S.; Min, B.H.; Min, Y.W.; Lee, H.; Rhee, P.L.; Kim, J.J.; Lee, J.H. Delayed perforation occurring after gastric endoscopic submucosal dissection: Clinical features and management strategy. Gut Liver 2024, 18, 40–49. [Google Scholar] [CrossRef]
- Ono, H.; Takizawa, K.; Kakushima, N.; Tanaka, M.; Kawata, N. Application of Polyglycolic Acid Sheets for Delayed Perforation after Endoscopic Submucosal Dissection of Early Gastric Cancer. Endoscopy 2015, 47 (Suppl. S1), E18–E19. [Google Scholar] [CrossRef]
- Nonaka, S.; Oda, I.; Sato, C.; Abe, S.; Suzuki, H.; Yoshinaga, S.; Hokamura, N.; Igaki, H.; Tachimori, Y.; Taniguchi, H.; et al. Endoscopic submucosal dissection for gastric tube cancer after esophagectomy. Gastrointest. Endosc. 2014, 79, 260–270. [Google Scholar] [CrossRef]
- Yabuuchi, Y.; Kakushima, N.; Takizawa, K.; Tanaka, M.; Kawata, N.; Yoshida, M.; Kishida, Y.; Ito, S.; Imai, K.; Ishiwatari, H.; et al. Short- and long-term outcomes of endoscopic submucosal dissection for early gastric cancer in the remnant stomach after gastrectomy. J. Gastroenterol. 2019, 54, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Hatta, W.; Koike, T.; Abe, H.; Ogata, Y.; Saito, M.; Jin, X.; Kanno, T.; Uno, K.; Asano, N.; Imatani, A.; et al. Recent approach for preventing complications in upper gastrointestinal endoscopic submucosal dissection. DEN Open 2022, 2, e60. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, S.; Uedo, N.; Oda, I.; Kaneko, K.; Yamamoto, Y.; Yamashina, T.; Suzuki, H.; Kodashima, S.; Yano, T.; Yamamichi, N.; et al. Scheduled Second-Look Endoscopy Is Not Recommended after Endoscopic Submucosal Dissection for Gastric Neoplasms (the SAFE Trial): A Multicentre Prospective Randomised Controlled Non-Inferiority Trial. Gut 2015, 64, 397–405. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Delayed Perforation n = 7 |
|---|---|
| Age (years), Median (IQR) | 74 (66~79) |
| <75 | 4 (57.1) |
| ≧75 | 3 (42.9) |
| Sex | |
| Male | 6 (85.7) |
| Female | 1 (14.3) |
| Hypertension | |
| No | 3 (42.9) |
| Yes | 4 (57.1) |
| Diabetes mellitus | |
| No | 7 (100) |
| Yes | 0 (0) |
| Tumor location | |
| Upper third | 1 (14.3) |
| Middle third | 0 (0) |
| Lower third | 6 (85.7) |
| Postoperative stomach | |
| Normal stomach | 5 (71.4) |
| Postoperative stomach | 2 (28.6) |
| En bloc resection | |
| En bloc resection | 7 (100) |
| Partial resection | 0 (0) |
| En bloc complete resection | |
| En bloc complete resection | 5 (71.4) |
| Non-en bloc complete resection | 2 (28.6) |
| Curative resection | |
| Curative resection | 5 (71.4) |
| Non-curative resection | 2 (28.6) |
| Resection size (mm), median (IQR) | 42 (38~55) |
| <45 | 4 (57.1) |
| ≧45 | 3 (42.9) |
| Tumor size (mm), median (IQR) | 17 (10~22) |
| <20 | 5 (71.4) |
| ≧20 | 2 (28.6) |
| Tumor shape (endoscopy) | |
| 0-I | 1 (14.3) |
| 0-IIa | 3 (42.8) |
| 0-IIb | 1 (14.3) |
| 0-IIc | 2 (28.6) |
| Combined | 0 (0) |
| Tumor depth | |
| M | 7 (100) |
| SM1 | 0 (0) |
| SM2 | 0 (0) |
| Ulceration | |
| Absent | 3 (42.9) |
| Present | 4 (57.1) |
| Second-look endoscopy performed | 3 (42.9) |
| Symptoms of delayed perforation | |
| Abdominal pain | 6 (85.7) |
| Fever | 1 (14.3) |
| Symptom onset time (h), median (IQR) | 14.4 (5.3–17.8) |
| Time to diagnosis (h), median (IQR) | 23 (11–183.4) |
| CT findings | |
| Free air | 6 (85.7) |
| Fluid collection | 1 (14.3) |
| Other complications | |
| None | 6 (85.7) |
| Delayed bleeding | 1 (14.3) |
| Treatment | |
| Conservative treatment | 2 (28.6) |
| Surgery | 5 (71.4) |
| Characteristic | Delayed Perforation n = 7 | Without Delayed Perforation n = 706 | p Value |
|---|---|---|---|
| Age (years), median (IQR) | 74 (66–79) | 74 (67–80) | 0.764 |
| <75 | 4 (57.1) | 370 (52.4) | |
| ≧75 | 3 (42.9) | 336 (47.6) | |
| Sex | 1.000 | ||
| Male | 6 (85.7) | 541 (76.6) | |
| Female | 1 (14.3) | 165 (23.4) | |
| Hypertension | 1.000 | ||
| No | 3 (42.9) | 296 (41.9) | |
| Yes | 4 (57.1) | 410 (58.1) | |
| Diabetes mellitus | 0.362 | ||
| No | 7 (100) | 541 (76.6) | |
| Yes | 0 (0) | 165 (23.4) | |
| Tumor location * | 0.842 | ||
| Upper third | 1 (14.3) | 128 (16.8) | |
| Middle third | 0 (0) | 105 (13.8) | |
| Lower third | 6 (85.7) | 529 (69.4) | |
| Postoperative stomach * | 0.016 | ||
| Normal stomach | 5 (71.4) | 741 (97.2) | |
| Postoperative stomach | 2 (28.6) | 21 (2.8) | |
| En bloc resection * | 1.000 | ||
| En bloc resection | 7 (100) | 760 (99.7) | |
| Partial resection | 0 (0) | 2 (0.03) | |
| En bloc complete resection * | 0.043 | ||
| En bloc complete resection | 5 (71.4) | 726 (95.3) | |
| Non-en bloc complete resection | 2 (28.6) | 36 (4.7) | |
| Curative resection * | 0.224 | ||
| Curative resection | 5 (71.4) | 665 (87.3) | |
| Non-curative resection | 2 (28.6) | 97 (12.7) | |
| Resection size (mm), median (IQR) * | 42 (38–55) | 44 (35–55) | 0.661 |
| <45 | 4 (57.1) | 380 (51.1) | |
| ≧45 | 3 (42.9) | 372 (48.9) | |
| Tumor size (mm), median (IQR) * | 17 (10–22) | 15 (10–22) | 0.711 |
| <20 | 5 (71.4) | 500 (65.6) | |
| ≧20 | 2 (28.6) | 262 (34.4) | |
| Tumor shape (endoscopy) * | 0.092 | ||
| 0-I | 1 (14.3) | 19 (2.5) | |
| 0-IIa | 3 (42.9) | 233 (30.6) | |
| 0-IIb | 1 (14.3) | 31 (4.1) | |
| 0-IIc | 2 (28.5) | 433 (56.8) | |
| Combined | 0 (0) | 46 (6.0) | |
| Tumor depth * | 1.000 | ||
| M | 7 (100) | 673 (88.3) | |
| SM1 | 0 (0) | 53 (7.0) | |
| SM2 | 0 (0) | 36 (4.7) | |
| Ulceration * | 0.026 | ||
| Absent | 3 (42.9) | 622 (81.6) | |
| Present | 4 (57.1) | 140 (18.4) |
| Characteristics | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| Odds Ratio | 95% CI | p Value | Odds Ratio | 95% CI | p Value | |
| Age (years), median (IQR) | ||||||
| <75 | Reference | |||||
| ≧75 | 0.83 | 0.18–3.72 | 0.803 | |||
| Sex | ||||||
| Male | Reference | |||||
| Female | 0.55 | 0.07–4.57 | 0.577 | |||
| Postoperative stomach | ||||||
| Normal stomach | Reference | Reference | ||||
| Postoperative stomach | 14.11 | 2.59–76.97 | 0.002 | 23.1 | 3.59–148.64 | 0.001 |
| Ulceration | ||||||
| Absent | Reference | Reference | ||||
| Present | 5.92 | 1.31–26.8 | 0.021 | 8.37 | 1.64–42.7 | 0.016 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akashi, T.; Yamaguchi, N.; Shiota, J.; Tabuchi, M.; Kitayama, M.; Hashiguchi, K.; Matsushima, K.; Akazawa, Y.; Nakao, K. Characteristics and Risk Factors of Delayed Perforation in Endoscopic Submucosal Dissection for Early Gastric Cancer. J. Clin. Med. 2024, 13, 1317. https://doi.org/10.3390/jcm13051317
Akashi T, Yamaguchi N, Shiota J, Tabuchi M, Kitayama M, Hashiguchi K, Matsushima K, Akazawa Y, Nakao K. Characteristics and Risk Factors of Delayed Perforation in Endoscopic Submucosal Dissection for Early Gastric Cancer. Journal of Clinical Medicine. 2024; 13(5):1317. https://doi.org/10.3390/jcm13051317
Chicago/Turabian StyleAkashi, Taro, Naoyuki Yamaguchi, Junya Shiota, Maiko Tabuchi, Moto Kitayama, Keiichi Hashiguchi, Kayoko Matsushima, Yuko Akazawa, and Kazuhiko Nakao. 2024. "Characteristics and Risk Factors of Delayed Perforation in Endoscopic Submucosal Dissection for Early Gastric Cancer" Journal of Clinical Medicine 13, no. 5: 1317. https://doi.org/10.3390/jcm13051317
APA StyleAkashi, T., Yamaguchi, N., Shiota, J., Tabuchi, M., Kitayama, M., Hashiguchi, K., Matsushima, K., Akazawa, Y., & Nakao, K. (2024). Characteristics and Risk Factors of Delayed Perforation in Endoscopic Submucosal Dissection for Early Gastric Cancer. Journal of Clinical Medicine, 13(5), 1317. https://doi.org/10.3390/jcm13051317





