Atrial Fibrillation Catheter Ablation among Cancer Patients: Utilization Trends and In-Hospital Outcomes
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Study Population and Variables
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Study Population
3.2. In-Hospital Course and Outcomes by Cancer/Non-Cancer Groups
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.P.; Choi, E.-K.; Han, K.-D.; Park, S.H.; Jung, J.-H.; Park, S.H.; Ahn, H.-J.; Lim, J.-H.; Lee, S.-R.; Oh, S. Risk of Atrial Fibrillation According to Cancer Type: A Nationwide Population-Based Study. JACC Cardio Oncol. 2021, 3, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Guha, A.; Fradley, M.G.; Dent, S.F.; Weintraub, N.L.; Lustberg, M.B.; Alonso, A.; Addison, D. Incidence, risk factors, and mortality of atrial fibrillation in breast cancer: A SEER-Medicare analysis. Eur. Heart J. 2022, 43, 300–312. [Google Scholar] [CrossRef]
- Conen, D.; Wong, J.A.; Sandhu, R.K.; Cook, N.R.; Lee, I.-M.; Buring, J.E.; Albert, C.M. Risk of Malignant Cancer Among Women with New-Onset Atrial Fibrillation. JAMA Cardiol. 2016, 1, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Lyon, A.R.; López-Fernández, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. ESC Scientific Document Group 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur. Heart J. 2022, 43, 4229–4361. [Google Scholar] [CrossRef]
- Guha, A.; Dey, A.K.; Jneid, H.; Ibarz, J.P.; Addison, D.; Fradley, M. Atrial fibrillation in the era of emerging cancer therapies. Eur. Heart J. 2019, 40, 3007–3010. [Google Scholar] [CrossRef]
- Farmakis, D.; Parissis, J.; Filippatos, G. Insights into onco-cardiology: Atrial fibrillation in cancer. J. Am. Coll. Cardiol. 2014, 63, 945–953. [Google Scholar] [CrossRef]
- Alexandre, J.; Salem, J.E.; Moslehi, J.; Sassier, M.; Ropert, C.; Cautela, J.; Thuny, F.; Ederhy, S.; Cohen, A.; Damaj, G.; et al. Identification of anticancer drugs associated with atrial fibrillation: Analysis of the WHO pharmacovigilance database. Eur. Heart J. Cardiovasc. Pharmacother. 2021, 7, 312–320. [Google Scholar] [CrossRef]
- Alexandre, J.; Moslehi, J.J.; Bersell, K.R.; Funck-Brentano, C.; Roden, D.M.; Salem, J.E. Anticancer drug-induced cardiac rhythm disorders: Current knowledge and basic underlying mechanisms. Pharmacol. Ther. 2018, 189, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Dobrev, D.; Aguilar, M.; Heijman, J.; Guichard, J.B.; Nattel, S. Postoperative atrial fibrillation: Mechanisms, manifestations and management. Nat. Rev. Cardiol. 2019, 16, 417–436. [Google Scholar] [CrossRef] [PubMed]
- Asnani, A.; Manning, A.; Mansour, M.; Ruskin, J.; Hochberg, E.P.; Ptaszek, L.M. Management of atrial fibrillation in patients taking targeted cancer therapies. Cardiooncology 2017, 3, 2. [Google Scholar] [CrossRef]
- Kanmanthareddy, A.; Vallakati, A.; Reddy Yeruva, M.; Dixit, S.; DI Biase, L.; Mansour, M.; Boolani, H.; Gunda, S.; Bunch, T.J.; Day, J.D.; et al. Pulmonary vein isolation for atrial fibrillation in the postpneumonectomy population: A feasibility, safety, and outcomes study. J. Cardiovasc. Electrophysiol. 2015, 26, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Shabtaie, S.A.; Luis, S.A.; Ward, R.C.; Karki, R.; Connolly, H.M.; Pellikka, P.A.; Kapa, S.; Asirvatham, S.J.; Packer, D.L.; DeSimone, C.V. Catheter Ablation in Patients with Neuroendocrine (Carcinoid) Tumors and Carcinoid Heart Disease: Outcomes, Peri-Procedural Complications, and Management Strategies. JACC Clin. Electrophysiol. 2021, 7, 151–160. [Google Scholar] [CrossRef]
- Giustozzi, M.; Ali, H.; Reboldi, G.; Balla, C.; Foresti, S.; de Ambroggi, G.; Lupo, P.P.; Agnelli, G.; Cappato, R. Safety of catheter ablation of atrial fibrillation in cancer survivors. J. Interv. Card. Electrophysiol. 2021, 60, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Eitel, C.; Sciacca, V.; Bartels, N.; Saraei, R.; Fink, T.; Keelani, A.; Gaßmann, A.; Kuck, K.-H.; Vogler, J.; Heeger, C.-H.; et al. Safety and Efficacy of Cryoballoon Based Pulmonary Vein Isolation in Patients with Atrial Fibrillation and a History of Cancer. J. Clin. Med. 2021, 10, 3669. [Google Scholar] [CrossRef] [PubMed]
- Ganatra, S.; Abraham, S.; Kumar, A.; Parikh, R.; Patel, R.; Khadke, S.; Kumar, A.; Liu, V.; Diaz, A.N.R.; Neilan, T.G.; et al. Efficacy and safety of catheter ablation for atrial fibrillation in patients with history of cancer. Cardiooncology 2023, 9, 19. [Google Scholar] [CrossRef]
- Thotamgari, S.R.; Sheth, A.R.; Patel, H.P.; Sandhyavenu, H.; Patel, B.; Grewal, U.S.; Bhuiyan, M.A.N.; Dani, S.S.; Dominic, P. Safety of catheter ablation for atrial fibrillation in patients with cancer: A nationwide cohort study. Postgrad. Med. 2023, 135, 562–568. [Google Scholar] [CrossRef] [PubMed]
- López-Fernández, T.; Martín-García, A.; Roldán Rabadán, I.; Mitroi, C.; Mazón Ramos, P.; Díez-Villanueva, P.; Escobar Cervantes, C.; Alonso Martín, C.; Alonso Salinas, G.L.; Arenas, M.; et al. Expert reviewers Atrial fibrillation in active cancer patients: Expert position paper and recommendations. Rev. Esp. Cardiol. (Engl. Ed.) 2019, 72, 749–759. [Google Scholar] [CrossRef] [PubMed]
- January, C.T.; Wann, L.S.; Calkins, H.; Chen, L.Y.; Cigarroa, J.E.; Cleveland, J.C.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E.; Furie, K.L.; et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the american college of cardiology/american heart association task force on clinical practice guidelines and the heart rhythm society. J. Am. Coll. Cardiol. 2019, 74, 104–132. [Google Scholar] [CrossRef]
- Steiner, C.; Elixhauser, A.; Schnaier, J. The healthcare cost and utilization project: An overview. Eff. Clin. Pract. 2002, 5, 143–151. [Google Scholar]
- Deshmukh, A.; Patel, N.J.; Pant, S.; Shah, N.; Chothani, A.; Mehta, K.; Grover, P.; Singh, V.; Vallurupalli, S.; Savani, G.T.; et al. In-hospital complications associated with catheter ablation of atrial fibrillation in the United States between 2000 and 2010: Analysis of 93 801 procedures. Circulation 2013, 128, 2104–2112. [Google Scholar] [CrossRef]
- Rozen, G.; Elbaz-Greener, G.; Marai, I.; Andria, N.; Hosseini, S.M.; Biton, Y.; Heist, E.K.; Ruskin, J.N.; Gavrilov, Y.; Carasso, S.; et al. Utilization and complications of catheter ablation for atrial fibrillation in patients with hypertrophic cardiomyopathy. J. Am. Heart Assoc. 2020, 9, e015721. [Google Scholar] [CrossRef]
- Deyo, R.A.; Cherkin, D.C.; Ciol, M.A. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J. Clin. Epidemiol. 1992, 45, 613–619. [Google Scholar] [CrossRef]
- Glasheen, W.P.; Cordier, T.; Gumpina, R.; Haugh, G.; Davis, J.; Renda, A. Charlson Comorbidity Index: ICD-9 Update and ICD-10 Translation. Am. Health Drug Benefits 2019, 12, 188–197. [Google Scholar] [PubMed]
- Chu, Y.-T.; Ng, Y.-Y.; Wu, S.-C. Comparison of different comorbidity measures for use with administrative data in predicting short- and long-term mortality. BMC Health Serv. Res. 2010, 10, 140. [Google Scholar] [CrossRef] [PubMed]
- Radovanovic, D.; Seifert, B.; Urban, P.; Eberli, F.R.; Rickli, H.; Bertel, O.; Puhan, M.A.; Erne, P.; AMIS Plus Investigators. Validity of Charlson Comorbidity Index in patients hospitalised with acute coronary syndrome. Insights from the nationwide AMIS Plus registry 2002–2012. Heart 2014, 100, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.M.; Moazzami, K.; Rozen, G.; Vaid, J.; Saleh, A.; Heist, K.E.; Vangel, M.; Ruskin, J.N. Utilization and in-hospital complications of cardiac resynchronization therapy: Trends in the United States from 2003 to 2013. Eur. Heart J. 2017, 38, 2122–2128. [Google Scholar] [CrossRef] [PubMed]
- Navi, B.B.; Reiner, A.S.; Kamel, H.; Iadecola, C.; Okin, P.M.; Tagawa, S.T.; Panageas, K.S.; DeAngelis, L.M. Arterial thromboembolic events preceding the diagnosis of cancer in older persons. Blood 2019, 133, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Pastori, D.; Marang, A.; Bisson, A.; Herbert, J.; Lip, G.Y.H.; Fauchier, L. Performance of the HAS-BLED, ORBIT, and ATRIA Bleeding Risk Scores on a Cohort of 399 344 Hospitalized Patients with Atrial Fibrillation and Cancer: Data from the French National Hospital Discharge Database. J. Am. Heart Assoc. 2022, 11, e026388. [Google Scholar] [CrossRef] [PubMed]
- Kupo, P.; Riesz, T.J.; Saghy, L.; Vamos, M.; Bencsik, G.; Makai, A.; Kohari, M.; Benak, A.; Miklos, M.; Pap, R. Ultrasound guidance for femoral venous access in patients undergoing pulmonary vein isolation: A quasi-randomized study. J. Cardiovasc. Electrophysiol. 2023, 34, 1177–1182. [Google Scholar] [CrossRef] [PubMed]
- Kupó, P.; Pap, R.; Sághy, L.; Tényi, D.; Bálint, A.; Debreceni, D.; Basu-Ray, I.; Komócsi, A. Ultrasound guidance for femoral venous access in electrophysiology procedures-systematic review and meta-analysis. J. Interv. Card. Electrophysiol. 2020, 59, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Potts, J.; Mohamed, M.O.; Lopez Mattei, J.C.; Iliescu, C.A.; Konopleva, M.; Rashid, M.; Bagur, R.; Mamas, M.A. Percutaneous coronary intervention and in-hospital outcomes in patients with leukemia: A nationwide analysis. Catheter. Cardiovasc. Interv. 2020, 96, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Calkins, H.; Hindricks, G.; Cappato, R.; Kim, Y.-H.; Saad, E.B.; Aguinaga, L.; Akar, J.G.; Badhwar, V.; Brugada, J.; Camm, J.; et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: Executive summary. Heart Rhythm. 2017, 14, e445–e494. [Google Scholar] [CrossRef] [PubMed]
- Chehab, M.A.; Thakor, A.S.; Tulin-Silver, S.; Connolly, B.L.; Cahill, A.M.; Ward, T.J.; Padia, S.A.; Kohi, M.P.; Midia, M.; Chaudry, G.; et al. Adult and Pediatric Antibiotic Prophylaxis during Vascular and IR Procedures: A Society of Interventional Radiology Practice Parameter Update Endorsed by the Cardiovascular and Interventional Radiological Society of Europe and the Canadian Association for Interventional Radiology. J. Vasc. Interv. Radiol. 2018, 29, 1483–1501.e2. [Google Scholar] [CrossRef] [PubMed]
- Kuck, K.H.; Brugada, J.; Fürnkranz, A.; Metzner, A.; Ouyang, F.; Chun, K.R.; Elvan, A.; Arentz, T.; Bestehorn, K.; Pocock, S.J.; et al. Cryoballoon or Radiofrequency Ablation for Paroxysmal Atrial Fibrillation. N. Engl. J. Med. 2016, 374, 2235–2245. [Google Scholar] [CrossRef]
- Reddy, V.Y.; Gerstenfeld, E.P.; Natale, A.; Whang, W.; Cuoco, F.A.; Patel, C.; Mountantonakis, S.E.; Gibson, D.N.; Harding, J.D.; Ellis, C.R.; et al. Pulsed Field or Conventional Thermal Ablation for Paroxysmal Atrial Fibrillation. N. Engl. J. Med. 2023, 389, 1660–1671. [Google Scholar] [CrossRef]
- Cutler, M.J.; Sattayaprasert, P.; Pivato, E.; Jabri, A.; AlMahameed, S.T.; Ziv, O. Low voltage-guided ablation of posterior wall improves 5-year arrhythmia-free survival in persistent atrial fibrillation. J. Cardiovasc. Electrophysiol. 2022, 33, 2475–2484. [Google Scholar] [CrossRef]
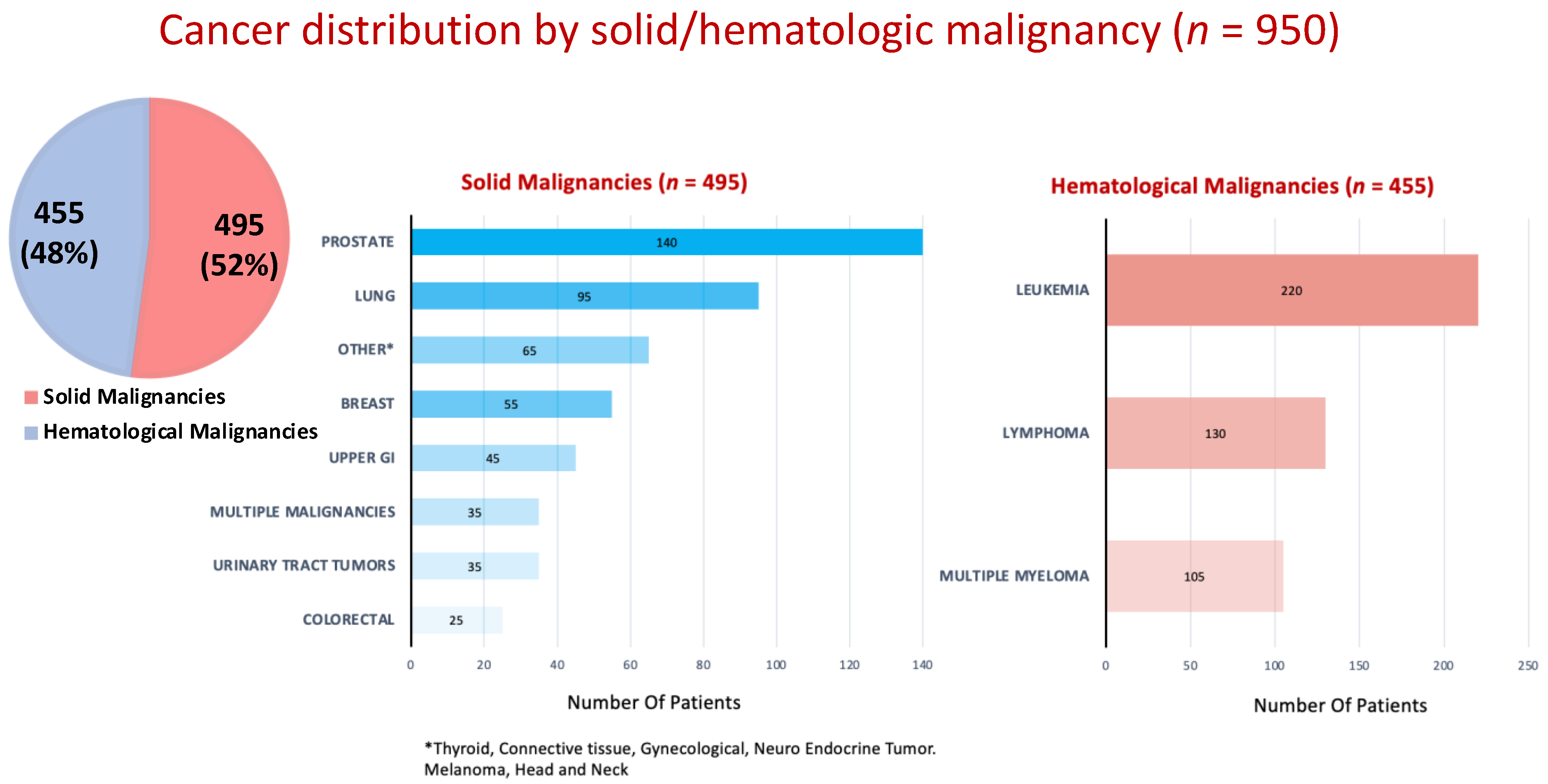
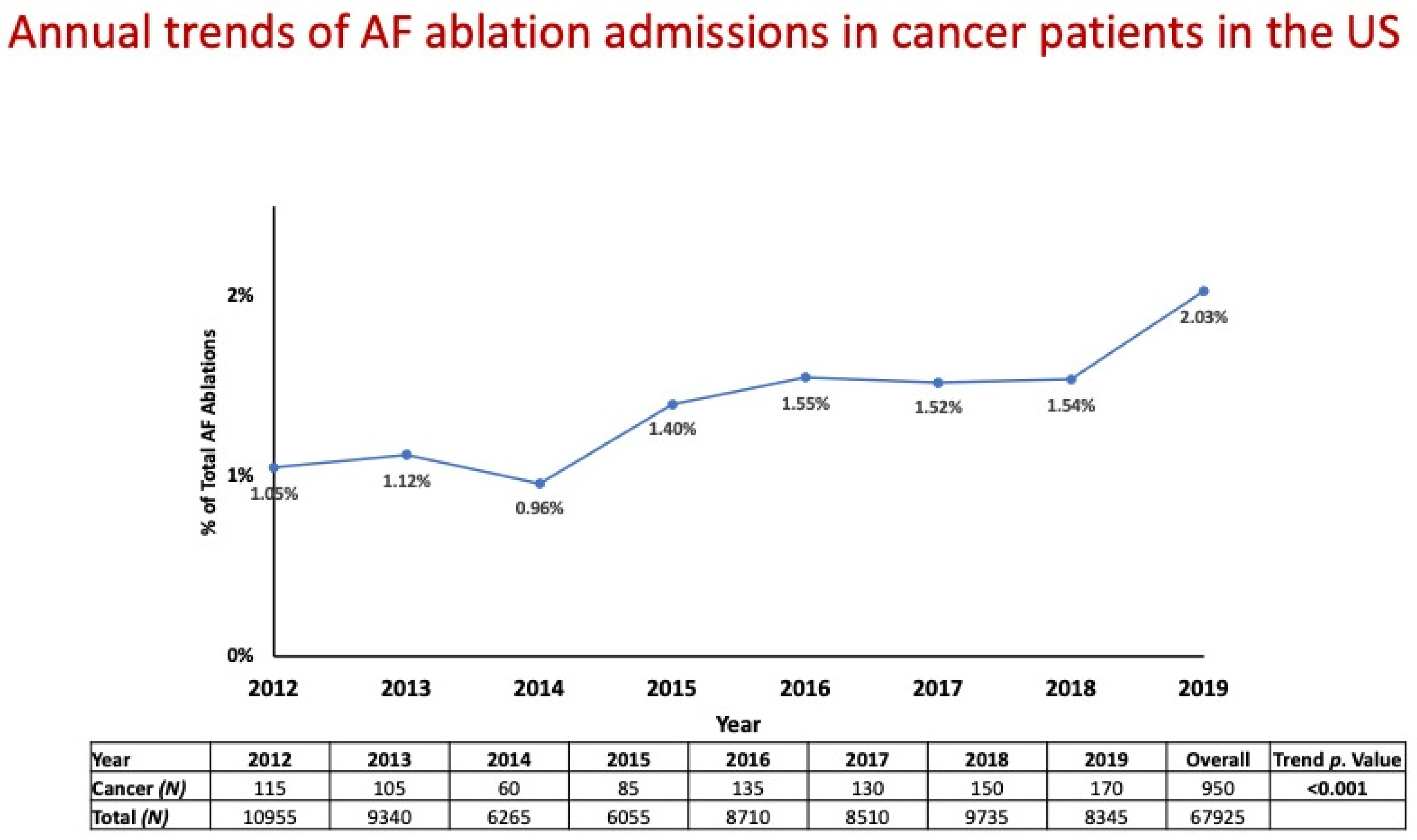
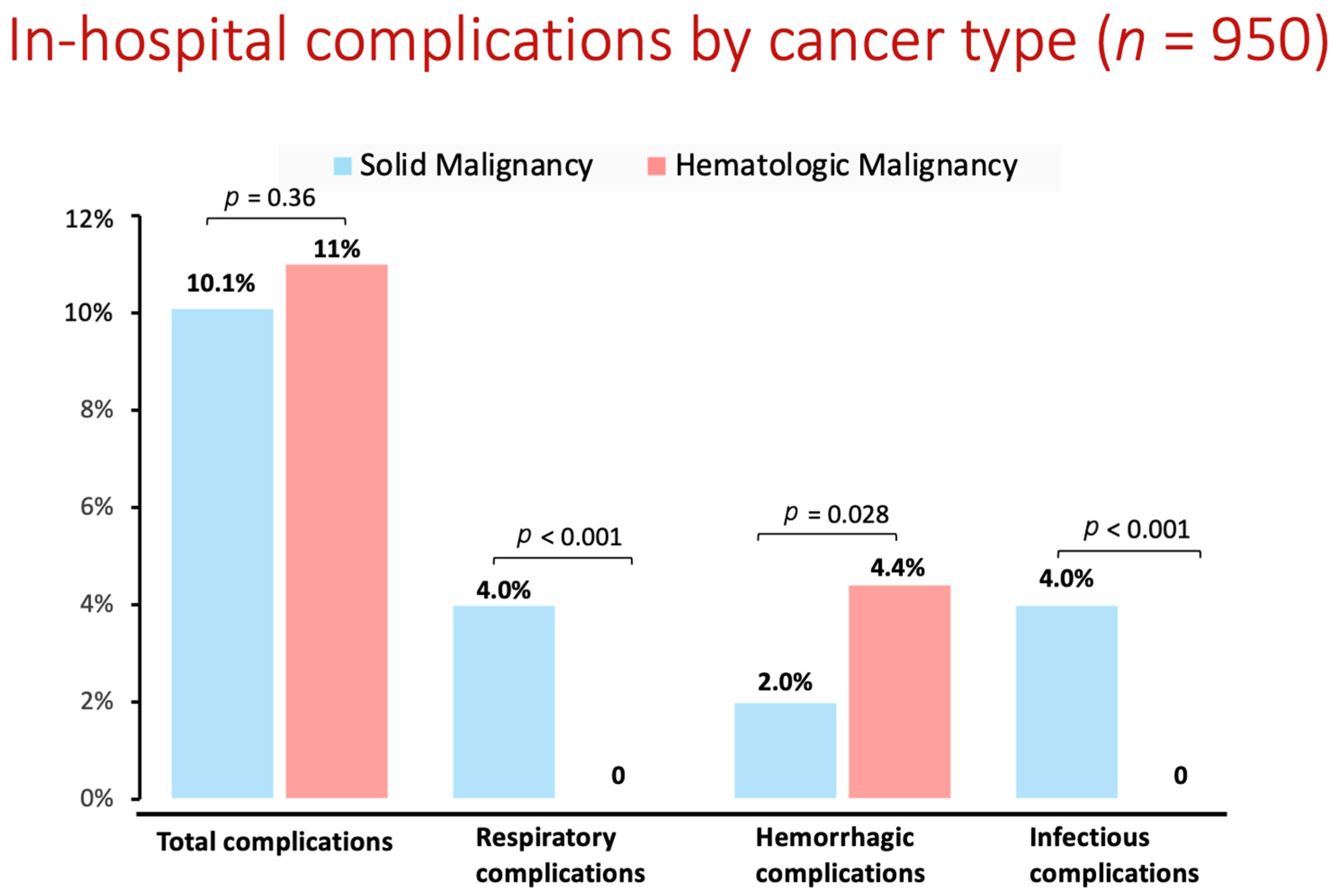
| Variable | Non-Cancer Patients | Cancer Patients | p Value |
|---|---|---|---|
| (n = 66,965) | (n = 950) | ||
| Age (years) | 64 ± 11 | 69 ± 9 | <0.001 |
| Female | 26,140 (39%) | 310 (33%) | <0.001 |
| White ethnicity | 57,250 (86%) | 840 (88%) | 0.006 |
| Diabetes mellitus | 13,945 (21%) | 225 (24%) | 0.017 |
| Hypertension | 38,265 (57%) | 500 (53%) | 0.003 |
| Heart failure | 16,585 (25%) | 370 (39%) | <0.001 |
| Valvular heart disease | 8385 (13%) | 140 (15%) | 0.023 |
| Chronic kidney disease | 6655 (10%) | 170 (18%) | <0.001 |
| Chronic Pulmonary Disease | 9875 (15%) | 195 (21%) | <0.001 |
| Obesity (BMI > 30) | 15,430 (23%) | 215 (23%) | 0.398 |
| Obstructive sleep apnea | 14,985 (22%) | 190 (20%) | 0.044 |
| Prior stroke | 1155 (1.7%) | 15 (1.6%) | 0.414 |
| Prior myocardial infarction | 3760 (6%) | 60 (6%) | 0.003 |
| Hypertrophic cardiomyopathy | 610 (0.9%) | None | NA |
| Peripheral artery disease | 2670 (4%) | 55 (6%) | 0.003 |
| Anemia | 4000 (6%) | 195 (21%) | <0.001 |
| Charlson comorbidity index ≥ 2 | 15,700 (24%) | 900 (95%) | <0.001 |
| CHA2DS2-VASc score | 2.32 ± 1.45 | 2.72 ± 1.36 | <0.001 |
| 0–1 | 21,455 (32%) | 185 (19%) | <0.001 |
| ≥2 | 45,510 (68%) | 765 (81%) | |
| ATRIA bleeding score | 1.21 ± 1.37 | 1.91 ± 1.86 | <0.001 |
| 0–1 | 51,130 (76%) | 520 (55%) | <0.001 |
| ≥2 | 15,835 (24%) | 430 (45%) | |
| Income percentile | |||
| 0–25 | 13,700 (21%) | 150 (16%) | 0.001 |
| 26–50 | 15,540 (23%) | 255 (27%) | |
| 51–75 | 17,610 (26%) | 240 (25%) | |
| 76–100 | 20,115 (30%) | 305 (32%) | |
| Teaching hospital | 55,000 (82%) | 770 (81%) | 0.206 |
| Hospital region | |||
| South/west | 37,900 (57%) | 515 (54%) | 0.075 |
| Midwest/northeast | 29,065 (43%) | 435 (46%) |
| Variable | Non-Cancer Patients | Cancer Patients | p Value |
|---|---|---|---|
| (n = 66,965) | (n = 950) | ||
| Total complications | 5300 (7.9%) | 100 (10.5%) | 0.002 |
| Cardiac complications | |||
| Cardiogenic shock | 80 (0.1%) | None | NA |
| Cardiac arrest | 100 (0.1%) | None | NA |
| Acute heart failure | 445 (0.7%) | ≤10 | NA |
| Hemopericardium/cardiac tamponade | 1085 (1.6%) | ≤10 | NA |
| Vascular complications | |||
| Hemorrhage/hematoma/blood transfusion | 1230 (1.8%) | 30 (3.2%) | 0.002 |
| Vascular injury | 1065 (1.6%) | 15 (1.6%) | 0.557 |
| Respiratory complications | |||
| Post-procedural respiratory failure/invasive ventilation > 24 h | 1125 (1.6%) | 20 (2.1%) | 0.188 |
| Diaphragmatic disorders | 190 (0.2%) | None | NA |
| Neurologic complications (stroke) | 95 (0.1%) | ≤10 | NA |
| Infectious complications (bacteremia, sepsis) | 530 (0.8%) | 20 (2.1%) | <0.001 |
| Length of stay | 2.75 ± 3.05 | 4.91 ± 5.8 | <0.001 |
| In-hospital mortality | 165 (0.2%) | ≤10 | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Margolis, G.; Goldhaber, O.; Kazatsker, M.; Kobo, O.; Roguin, A.; Leshem, E. Atrial Fibrillation Catheter Ablation among Cancer Patients: Utilization Trends and In-Hospital Outcomes. J. Clin. Med. 2024, 13, 1318. https://doi.org/10.3390/jcm13051318
Margolis G, Goldhaber O, Kazatsker M, Kobo O, Roguin A, Leshem E. Atrial Fibrillation Catheter Ablation among Cancer Patients: Utilization Trends and In-Hospital Outcomes. Journal of Clinical Medicine. 2024; 13(5):1318. https://doi.org/10.3390/jcm13051318
Chicago/Turabian StyleMargolis, Gilad, Ofir Goldhaber, Mark Kazatsker, Ofer Kobo, Ariel Roguin, and Eran Leshem. 2024. "Atrial Fibrillation Catheter Ablation among Cancer Patients: Utilization Trends and In-Hospital Outcomes" Journal of Clinical Medicine 13, no. 5: 1318. https://doi.org/10.3390/jcm13051318
APA StyleMargolis, G., Goldhaber, O., Kazatsker, M., Kobo, O., Roguin, A., & Leshem, E. (2024). Atrial Fibrillation Catheter Ablation among Cancer Patients: Utilization Trends and In-Hospital Outcomes. Journal of Clinical Medicine, 13(5), 1318. https://doi.org/10.3390/jcm13051318









