Clinical Pharmacology in Sarcoidosis: How to Use and Monitor Sarcoidosis Medications
Abstract
1. Introduction
2. Glucocorticoids
2.1. Mechanism of Action
2.2. General Treatment Indications for Glucocorticoids in Sarcoidosis
2.3. Dosing
2.4. Side Effects and Monitoring
2.5. Drug Interactions
2.6. Special Situations
2.7. Counseling Points for a Patient Receiving Glucocorticoid(s)
- Take glucocorticoids with food to prevent gastrointestinal discomfort.
- Take glucocorticoids in the morning time to minimize insomnia.
- Educate the patient concerning potential glucocorticoid side effects including hyperglycemia, osteoporosis, adrenocortical insufficiency, weight gain, fluid retention, hypertension, mood change, myopathy, glaucoma, cataract, and infections.
- Contact the healthcare provider if an infection occurs, or if an invasive procedure is planned that may increase the risk of infection. Glucocorticoids may have to be held temporarily in this instance.
- Encourage vaccination prior to initiating glucocorticoids, as vaccination is a highly effective infection mitigation strategy.
- Patients receiving glucocorticoids or another immunosuppressive medication are eligible for RZV, Shingrix® (GlaxoSmithKline, Durham, NC, USA).
3. Methotrexate
3.1. Mechanism of Action
3.2. General Treatment Indications for Methotrexate in Sarcoidosis
3.3. Dosing
3.4. Side Effects and Monitoring
3.5. Drug Interactions
3.6. Special Situations
3.7. Counseling Points for a Patient Receiving MTX
- Take MTX “one day per week”.
- Take folic acid daily seven days per week, including the day of MTX use.
- Use split dosing for weekly MTX doses of >15 mg weekly: “half of the dose in the morning then half of the dose in the evening, 12 h apart, within one day every week”.
- MTX takes up to 3~6 months of use with good adherence to reach its steady state of clinical effectiveness. Encourage the patient to take MTX as prescribed despite the drug’s initial minimal efficacy.
- Contact the healthcare provider if unexplained cough develops.
- Potential MTX side effects include birth defects, liver toxicity, bone-marrow suppression, photosensitivity (use sunscreen, wear hat and long sleeves), hair loss, mouth ulcer etc.
- Frequent blood test monitoring (CBC, serum liver, and renal function tests) is required while receiving MTX.
- Hold two doses of MTX after receiving an annual influenza vaccination to maximize vaccine efficacy if sarcoidosis symptoms are minimum and the risk of a sarcoidosis exacerbation is low.
- Contact the healthcare provider if an infection occurs, or if an invasive procedure or surgery is planned. MTX may have to be held temporarily in this instance.
- Encourage vaccination prior to initiating MTX, as vaccination is a highly effective infection mitigation strategy.
- With drug-induced immunocompromised condition, the patient is eligible for RZV, Shingrix®.
4. Leflunomide
4.1. Mechanism of Action
4.2. General Treatment Indications for Leflunomide in Sarcoidosis
4.3. Dosing
4.4. Side Effects and Monitoring
4.5. Drug Interactions
4.6. Special Situations
4.7. Counseling Points for a Patient Receiving LEF
- Potential side effects include birth defects, liver toxicity, bone-marrow suppression, neuropathy, blood-pressure increase, and hair loss.
- It may take up to 3~6 months of use to reach its steady state of clinical effectiveness. Encourage the patient to take LEF as prescribed with good adherence despite the LEF’s initial minimal efficacy.
- Frequent blood-test monitoring is required while receiving LEF.
- Contact the healthcare provider if an infection occurs, or if a procedure or surgery is planned that may increase the risk of infection. LEF may have to be held temporarily in this instance.
- Encourage vaccination prior to initiating LEF, as vaccination is a highly effective infection mitigation strategy.
- With drug-induced immunocompromised condition, the patient is eligible for RZV, Shingrix®.
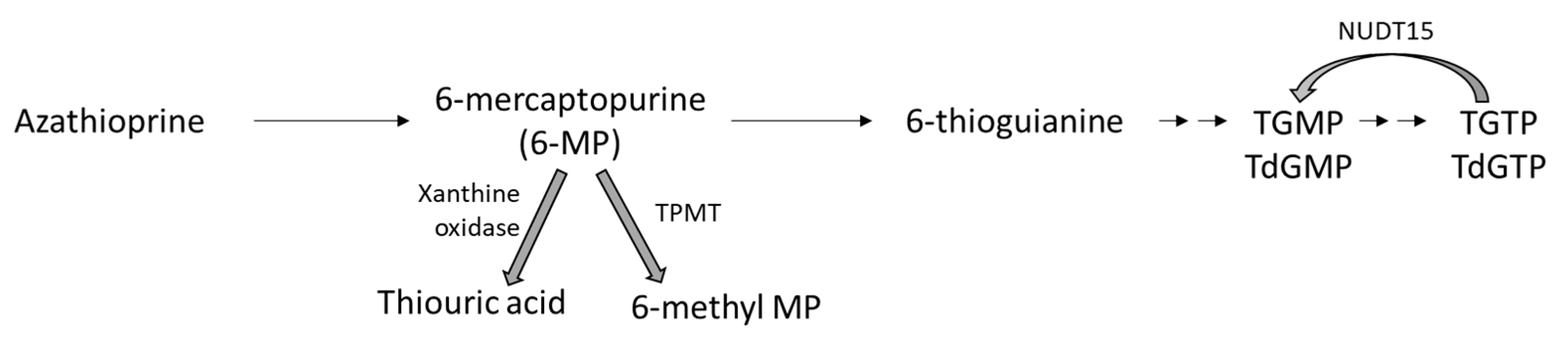
5. Azathioprine
5.1. Mechanism of Action
5.2. General Treatment Indications for Azathioprine in Sarcoidosis
5.3. Dosing
5.4. Side Effects and Monitoring
5.5. Drug Interactions
5.6. Special Situations
5.7. Counseling Points for a Patient Receiving AZA
- The potential side effects of AZA include liver toxicity and bone-marrow suppression.
- It takes up to 3~6 months of use to reach its steady state of clinical effectiveness. Encourage the patient take AZA as prescribed with good adherence despite the drug’s initial minimal efficacy.
- Frequent blood-test monitoring is required while receiving AZA.
- Contact the healthcare provider if an infection occurs, or if a procedure or surgery is planned that may increase the risk of infection. AZA may have to be held temporarily in this instance.
- Encourage vaccination prior to initiating AZA, as vaccination is a highly effective infection-mitigation strategy.
- With a drug-induced immunocompromised condition, the patient is eligible for RZV, Shingrix®.
6. Mycophenolate (Mycophenolate Mofetil, Mycophenolate Sodium)
6.1. Mechanism of Action
6.2. General Treatment Indications for Mycophenolate in Sarcoidosis
6.3. Dosing
6.4. Side Effects and Monitoring
6.5. Drug Interactions
6.6. Special Situations
6.7. Counseling Points for a Patient Receiving MMF
- Educate the patient concerning the potential side effects of MMF including gastrointestinal intolerance, liver toxicity, and bone-marrow suppression.
- Educate the patient that MMF takes up to 3~6 months of use to reach its steady state of clinical effectiveness. Encourage the patient take MMF as prescribed with good adherence despite the drug’s initial minimal efficacy.
- The 500 mg MMF tablets or capsules may be too big to swallow for some patients. Inform the patient that a smaller size (250 mg) capsule formulation is available. Also, suspension formulation can be considered.
- Frequent blood-test monitoring is required while receiving MMF.
- Contact the healthcare provider if an infection occurs, or if a procedure or surgery is planned that may increase the risk of infection. MMF may have to be held temporarily in this instance.
- Encourage vaccination prior to initiating MMF, as vaccination is a highly effective infection-mitigation strategy.
- With drug-induced immunocompromised condition, the patient is eligible for RZV, Shingrix®.
7. Hydroxychloroquine
7.1. Mechanism of Action
7.2. General Treatment Indications for Hydroxychloroquine in Sarcoidosis
7.3. Dosing
7.4. Side Effects and Monitoring
7.5. Drug Interactions
7.6. Special Situations
7.7. Counseling Points for a Patient Receiving HCQ
- Educate the patient concerning potential side effects of HCQ, especially retinal toxicity, gastrointestinal intolerance, liver toxicity, and bone-marrow suppression.
- It takes up to 3~6 months of use to reach its steady state of clinical effectiveness. Encourage the patient to take HCQ as prescribed with good adherence despite the drug’s initial minimal efficacy.
- Counsel the patient that ophthalmology evaluations as surveillance for retinopathy is required while receiving HCQ.
- Educate the patient to monitor his/her body weight. Individuals weighing <80 kg (177 pounds) should receive a weight-based daily dose (not to exceed 5 mg/kg/day). Counsel the patient to report to their healthcare provider if significant weight change occurs, as HCQ dose adjustment is needed. Individuals who weigh more than 80 kg should not exceed a daily dose of 400 mg. The maximum dose of HCQ is 400 mg daily, in divided dose, regardless of the patient’s weight.
- Educate the patient that a psoriatic rash can develop or worsen while receiving HCQ, and the patient should contact their provider if such a skin reaction occurs.
8. Tumor Necrosis Factor Alpha Inhibitors (TNFi)
8.1. Mechanism of Action
8.2. General Treatment Indications for Tumor Necrosis Factor Alpha Inhibitors in Sarcoidosis
8.3. Dosing
8.4. Side Effects and Monitoring
8.5. Drug Interactions
8.6. Special Situations
8.7. Counseling Points for a Patient Receiving TNFi
- Educate the patient concerning potential TNFi side effects, infections, malignancy, possible onset or worsening of congestive heart failure, or demyelinating diseases such as multiple sclerosis.
- Educate the patient that IFX or ADA may take up to three to six months to reach their steady states of clinical effectiveness. Encourage the patient take these medications as prescribed with good adherence despite the drugs initial minimal efficacy.
- ADA is a subcutaneous injection medication that can be used at home.
- IFX is administered via intravenous infusion at a clinic setting, and it typically takes several hours.
- For ADA, educate the patient on the injection technique. The first injection should be conducted in the presence of a health care professional for patient safety.
- For IFX, educate the patient that (s)he will receive pre-medications per the institution’s protocol to prevent an IFX infusion reaction.
- Inform the patient not to compensate for a missed ADA dose with an additional dose. If the patient forgets an ADA injection, the patient should perform that injection as soon as possible and consider that day as the start of a new injection cycle.
- Contact the healthcare provider if an infection occurs, or if a procedure or surgery is planned that may increase the risk of infection. The TNFi agent may have to be held temporarily in this instance.
- Three to six months may take for the medication to build up to reach its maximum effectiveness. Be patient and adhere to the medication.
- The patient should inform the healthcare provider if there is a previous history of tuberculosis, hepatitis B, or hepatitis C infection.
- Emphasize that TNFi drugs are immunosuppressants and encourage vaccine adherence to mitigate risks of vaccine-preventable diseases.
- Live vaccine is contraindicated with TNFi agents.
- With drug-induced immunocompromised conditions, the patient is eligible for RZV, Shingrix®.
9. Rituximab
9.1. Mechanism of Action
9.2. General Treatment Indications for Rituximab in Sarcoidosis
9.3. Dosing
9.4. Side Effects and Monitoring
9.5. Drug Interactions
9.6. Special Situations
9.7. Counseling Points for a Patient Receiving RTX
- Educate the patient concerning potential side effects of RTX.
- RTX is an intravenous infusion medication, which may take several hours to infuse.
- Contact the healthcare provider if an infection occurs while receiving RTX, or if a procedure or surgery is planned that may increase the risk of infection and follow their recommendation.
- Before you receive RTX, inform your provider if you have untreated hepatitis B, hepatitis C, tuberculosis, or previous infections that have been treated.
- Educate the patient that RTX is contraindicated in pregnancy. Pregnancy should be avoided while receiving RTX, and the patient will be monitored for pregnancy while receiving the drug.
- Emphasize that RTX is an immunosuppressant and encourage the patient to receive vaccines.
- Live vaccine is contraindicated with RTX.
- With a drug-induced immunocompromised condition, the patient is eligible for RZV, Shingrix®.
- Counsel the patient concerning PML symptoms such as loss of coordination, loss of language ability, memory loss, vision problems, and progressive weakness in arms and legs.
10. Repository Corticotropin Injection
10.1. Mechanism of Action
10.2. General Treatment Indications for Repository Corticotropin Injection in Sarcoidosis
10.3. Dosing
10.4. Side Effects and Monitoring
10.5. Drug Interactions
10.6. Special Situations
10.7. Counseling Points for a Patient Receiving RCI
- Educate the patient concerning potential side effects of RCI, which are practically the same as glucocorticoids plus increased pigmentation.
- RCI is a subcutaneous injection.
- RCI should be stored in a refrigerator.
- Contact the healthcare provider if an infection occurs, or if a procedure or surgery is planned that may increase the risk of infection. RCI may have to be held temporarily in this instance.
- The patient should inform the healthcare provider if there is a previous history of untreated or previously treated tuberculosis, hepatitis B, or hepatitis C infection.
- Emphasize that RTX is an immunosuppressant and encourage the patient to receive vaccines.
- Live vaccine is contraindicated in patients receiving RCI, per prescribing information.
- With drug-induced immunocompromised conditions, the patient is eligible for RZV, Shingrix®.
11. Summary
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Baughman, R.P.; Valeyre, D.; Korsten, P.; Mathioudakis, A.G.; Wuyts, W.A.; Wells, A.; Rottoli, P.; Nunes, H.; Lower, E.E.; Judson, M.A.; et al. ERS clinical practice guidelines on treatment of sarcoidosis. Eur. Respir. J. 2021, 58, 2004079. [Google Scholar] [CrossRef]
- Hupfeld, C.J.; Iñiguez-Lluhí, J.A. Adrenocorticotropic Hormone, Adrenal Steroids, and the Adrenal Cortex. In Goodman & Gilman’s: The Pharmacological Basis of Therapeutics, 14th ed.; Brunton, L.L., Knollmann, B.C., Eds.; McGraw-Hill Education: New York, NY, USA, 2023. [Google Scholar]
- Judson, M.A. Corticosteroids in Sarcoidosis. Rheum. Dis. Clin. North. Am. 2016, 42, 119–135. [Google Scholar] [CrossRef]
- Chrousos, G.P. Adrenocorticosteroids & Adrenocortical Antagonists. In Katzung’s Basic & Clinical Pharmacology, 16th ed.; Vanderah, T.W., Ed.; McGraw-Hill: New York, NY, USA, 2024. [Google Scholar]
- Liu, D.; Ahmet, A.; Ward, L.; Krishnamoorthy, P.; Mandelcorn, E.D.; Leigh, R.; Brown, J.P.; Cohen, A.; Kim, H. A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy. Allergy Asthma Clin. Immunol. 2013, 9, 30. [Google Scholar] [CrossRef]
- Goldman, C.; Judson, M.A. Corticosteroid refractory sarcoidosis. Respir. Med. 2020, 171, 106081. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, M.B.; Russell, L.; Danila, M.I.; Fink, H.A.; Guyatt, G.; Cannon, M.; Caplan, L.; Gore, S.; Grossman, J.; Hansen, K.E.; et al. 2022 American College of Rheumatology Guideline for the Prevention and Treatment of Glucocorticoid-Induced Osteoporosis. Arthritis Rheumatol. 2023, 75, 2088–2102. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 139, e1082–e1143. [Google Scholar] [CrossRef] [PubMed]
- Moghadam-Kia, S.; Werth, V.P. Prevention and treatment of systemic glucocorticoid side effects. Int. J. Dermatol. 2010, 49, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Ahmet, A.; Kim, H.; Spier, S. Adrenal suppression: A practical guide to the screening and management of this under-recognized complication of inhaled corticosteroid therapy. Allergy Asthma Clin. Immunol. 2011, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Virani, S.S.; Newby, L.K.; Arnold, S.V.; Bittner, V.; Brewer, L.C.; Demeter, S.H.; Dixon, D.L.; Fearon, W.F.; Hess, B.; Johnson, H.M.; et al. 2023 AHA/ACC/ACCP/ASPC/NLA/PCNA Guideline for the Management of Patients with Chronic Coronary Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2023, 148, e9–e119. [Google Scholar] [CrossRef] [PubMed]
- de La Red, G.; Mejia, J.C.; Cervera, R.; Llado, A.; Mensa, J.; Font, J. Bilateral Achilles tendinitis with spontaneous rupture induced by levofloxacin in a patient with systemic sclerosis. Clin. Rheumatol. 2003, 22, 367–368. [Google Scholar] [CrossRef]
- Naggar, V.F.; Khalil, S.A.; Gouda, M.W. Effect of concomitant administration of magnesium trisilicate on GI absorption of dexamethasone in humans. J. Pharm. Sci. 1978, 67, 1029–1030. [Google Scholar] [CrossRef]
- Uribe, M.; Casian, C.; Rojas, S.; Sierra, J.G.; Go, V.L. Decreased bioavailability of prednisone due to antacids in patients with chronic active liver disease and in healthy volunteers. Gastroenterology 1981, 80, 661–665. [Google Scholar] [CrossRef]
- Albin, H.; Vincon, G.; Demotes-Mainard, F.; Begaud, B.; Bedjaoui, A. Effect of aluminium phosphate on the bioavailability of cimetidine and prednisolone. Eur. J. Clin. Pharmacol. 1984, 26, 271–273. [Google Scholar] [CrossRef]
- Tanner, A.R.; Caffin, J.A.; Halliday, J.W.; Powell, L.W. Concurrent administration of antacids and prednisone: Effect on serum levels of prednisolone. Br. J. Clin. Pharmacol. 1979, 7, 397–400. [Google Scholar] [CrossRef]
- Hazlewood, K.A.; Fugate, S.E.; Harrison, D.L. Effect of oral corticosteroids on chronic warfarin therapy. Ann. Pharmacother. 2006, 40, 2101–2106. [Google Scholar] [CrossRef]
- Bartoszek, M.; Brenner, A.M.; Szefler, S.J. Prednisolone and methylprednisolone kinetics in children receiving anticonvulsant therapy. Clin. Pharmacol. Ther. 1987, 42, 424–432. [Google Scholar] [CrossRef]
- McAllister, W.A.; Thompson, P.J.; Al-Habet, S.M.; Rogers, H.J. Rifampicin reduces effectiveness and bioavailability of prednisolone. Br. Med. J. 1983, 286, 923–925. [Google Scholar] [CrossRef]
- Carrie, F.; Roblot, P.; Bouquet, S.; Delon, A.; Roblot, F.; Becq-Giraudon, B. Rifampin-induced nonresponsiveness of giant cell arteritis to prednisone treatment. Arch. Intern. Med. 1994, 154, 1521–1524. [Google Scholar] [CrossRef]
- Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH Consensus Development Panel on the Effect of Corticosteroids for Fetal Maturation on Perinatal Outcomes. JAMA 1995, 273, 413–418. [CrossRef]
- Czeizel, A.E.; Rockenbauer, M. Population-based case-control study of teratogenic potential of corticosteroids. Teratology 1997, 56, 335–340. [Google Scholar] [CrossRef]
- Park-Wyllie, L.; Mazzotta, P.; Pastuszak, A.; Moretti, M.E.; Beique, L.; Hunnisett, L.; Friesen, M.H.; Jacobson, S.; Kasapinovic, S.; Chang, D.; et al. Birth defects after maternal exposure to corticosteroids: Prospective cohort study and meta-analysis of epidemiological studies. Teratology 2000, 62, 385–392. [Google Scholar] [CrossRef]
- Lunghi, L.; Pavan, B.; Biondi, C.; Paolillo, R.; Valerio, A.; Vesce, F.; Patella, A. Use of glucocorticoids in pregnancy. Curr. Pharm. Des. 2010, 16, 3616–3637. [Google Scholar] [CrossRef]
- Pradat, P.; Robert-Gnansia, E.; Di Tanna, G.L.; Rosano, A.; Lisi, A.; Mastroiacovo, P.; All Contributors to the MADRE Database. First trimester exposure to corticosteroids and oral clefts. Birth Defects Res. A Clin. Mol. Teratol. 2003, 67, 968–970. [Google Scholar] [CrossRef]
- Ostensen, M.; Forger, F. Management of RA medications in pregnant patients. Nat. Rev. Rheumatol. 2009, 5, 382–390. [Google Scholar] [CrossRef]
- Middleton, P.G.; Gade, E.J.; Aguilera, C.; MacKillop, L.; Button, B.M.; Coleman, C.; Johnson, B.; Albrechtsen, C.; Edenborough, F.; Rigau, D.; et al. ERS/TSANZ Task Force Statement on the management of reproduction and pregnancy in women with airways diseases. Eur. Respir. J. 2020, 55, 1901208. [Google Scholar] [CrossRef]
- Boone, B.; Lazaroff, S.M.; Wheless, L.; Wolfe, R.M.; Barnado, A. Rates of Pneumocystis jirovecii pneumonia and prophylaxis prescribing patterns in a large electronic health record cohort of patients with systemic lupus erythematosus. Semin. Arthritis Rheum. 2022, 57, 152106. [Google Scholar] [CrossRef] [PubMed]
- Fishman, J.A.; Gans, H.; AST Infectious Diseases Community of Practice. Pneumocystis jiroveci in solid organ transplantation: Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin. Transplant. 2019, 33, e13587. [Google Scholar] [CrossRef] [PubMed]
- Malpica, L.; Moll, S. Practical approach to monitoring and prevention of infectious complications associated with systemic corticosteroids, antimetabolites, cyclosporine, and cyclophosphamide in nonmalignant hematologic diseases. Hematology Am. Soc. Hematol. Educ. Program. 2020, 2020, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Mazurek, G.H.; Jereb, J.A.; Vernon, A.; LoBue, P.; Goldberg, S.; Castro, K.G. Updated Guidelines for Using Interferon Gamma Release Assays to Detect Mycobacterium tuberculosis Infection—United States, 2010; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2010. [Google Scholar]
- Palacios-Gutierrez, J.J.; Rodriguez-Guardado, A.; Arias-Guillen, M.; Alonso-Arias, R.; Palacios-Penedo, S.; Garcia-Garcia, J.M.; Balbin, M.; Perez-Hernandez, D.; Sandoval-Torrientes, M.; Torreblanca-Gil, A.; et al. Clinical and Epidemiological Correlates of Low IFN-Gamma Responses in Mitogen Tube of QuantiFERON Assay in Tuberculosis Infection Screening During the COVID-19 Pandemic: A Population-Based Marker of COVID-19 Mortality? Arch. Bronconeumol. 2022, 58, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Hakimian, S.; Popov, Y.; Rupawala, A.H.; Salomon-Escoto, K.; Hatch, S.; Pellish, R. The conundrum of indeterminate QuantiFERON-TB Gold results before anti-tumor necrosis factor initiation. Biologics 2018, 12, 61–67. [Google Scholar] [CrossRef]
- Kaur, M.; Singapura, P.; Kalakota, N.; Cruz, G.; Shukla, R.; Ahsan, S.; Tansel, A.; Thrift, A.P.; El-Serag, H.B. Factors That Contribute to Indeterminate Results from the QuantiFERON-TB Gold In-Tube Test in Patients with Inflammatory Bowel Disease. Clin. Gastroenterol. Hepatol. 2018, 16, 1616–1621.e1. [Google Scholar] [CrossRef]
- Bass, A.R.; Chakravarty, E.; Akl, E.A.; Bingham, C.O.; Calabrese, L.; Cappelli, L.C.; Johnson, S.R.; Imundo, L.F.; Winthrop, K.L.; Arasaratnam, R.J.; et al. 2022 American College of Rheumatology Guideline for Vaccinations in Patients with Rheumatic and Musculoskeletal Diseases. Arthritis Care Res. 2023, 75, 449–464. [Google Scholar] [CrossRef] [PubMed]
- Altered Immunocompetence. General Best Practice Guidelines for Immunization. Available online: https://www.cdc.gov/vaccines/hcp/acip-recs/general-recs/immunocompetence.html (accessed on 20 November 2023).
- Shingrix. Available online: https://www.cdc.gov/vaccines/vpd/shingles/hcp/shingrix/faqs.html (accessed on 20 November 2023).
- Weinblatt, M.E.; Coblyn, J.S.; Fox, D.A.; Fraser, P.A.; Holdsworth, D.E.; Glass, D.N.; Trentham, D.E. Efficacy of low-dose methotrexate in rheumatoid arthritis. N. Engl. J. Med. 1985, 312, 818–822. [Google Scholar] [CrossRef]
- Andersen, P.A.; West, S.G.; O’Dell, J.R.; Via, C.S.; Claypool, R.G.; Kotzin, B.L. Weekly pulse methotrexate in rheumatoid arthritis. Clinical and immunologic effects in a randomized, double-blind study. Ann. Intern. Med. 1985, 103, 489–496. [Google Scholar] [CrossRef]
- Williams, H.J.; Willkens, R.F.; Samuelson, C.O., Jr.; Alarcon, G.S.; Guttadauria, M.; Yarboro, C.; Polisson, R.P.; Weiner, S.R.; Luggen, M.E.; Billingsley, L.M.; et al. Comparison of low-dose oral pulse methotrexate and placebo in the treatment of rheumatoid arthritis. A controlled clinical trial. Arthritis Rheum. 1985, 28, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.N.; Watts, C.; Edelman, J.; Esdaile, J.; Russell, A.S. A controlled two-centre trial of parenteral methotrexate therapy for refractory rheumatoid arthritis. J. Rheumatol. 1984, 11, 760–763. [Google Scholar]
- Fraenkel, L.; Bathon, J.M.; England, B.R.; St Clair, E.W.; Arayssi, T.; Carandang, K.; Deane, K.D.; Genovese, M.; Huston, K.K.; Kerr, G.; et al. 2021 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Care Res. 2021, 73, 924–939. [Google Scholar] [CrossRef] [PubMed]
- Lower, E.E.; Baughman, R.P. Prolonged use of methotrexate for sarcoidosis. Arch. Intern. Med. 1995, 155, 846–851. [Google Scholar] [CrossRef]
- Mehta, S.; Lightle, A.; Judson, M.A. Renal sarcoidosis. Nephrol. Dial. Transplant. 2023, 38, 803–810. [Google Scholar] [CrossRef]
- Rampon, G.; Henkin, C.; Jorge, V.M.; Almeida, H.L., Jr. Methotrexate-induced mucositis with extra-mucosal involvement after acidental overdose. An. Bras. Dermatol. 2018, 93, 155–156. [Google Scholar] [CrossRef]
- Van Ede, A.E.; Laan, R.F.; Rood, M.J.; Huizinga, T.W.; van de Laar, M.A.; van Denderen, C.J.; Westgeest, T.A.; Romme, T.C.; de Rooij, D.J.; Jacobs, M.J.; et al. Effect of folic or folinic acid supplementation on the toxicity and efficacy of methotrexate in rheumatoid arthritis: A forty-eight week, multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheum. 2001, 44, 1515–1524. [Google Scholar] [CrossRef]
- Pichlmeier, U.; Heuer, K.U. Subcutaneous administration of methotrexate with a prefilled autoinjector pen results in a higher relative bioavailability compared with oral administration of methotrexate. Clin. Exp. Rheumatol. 2014, 32, 563–571. [Google Scholar] [PubMed]
- Herman, R.A.; Veng-Pedersen, P.; Hoffman, J.; Koehnke, R.; Furst, D.E. Pharmacokinetics of low-dose methotrexate in rheumatoid arthritis patients. J. Pharm. Sci. 1989, 78, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Hazlewood, G.S.; Thorne, J.C.; Pope, J.E.; Lin, D.; Tin, D.; Boire, G.; Haraoui, B.; Hitchon, C.A.; Keystone, E.C.; Jamal, S.; et al. The comparative effectiveness of oral versus subcutaneous methotrexate for the treatment of early rheumatoid arthritis. Ann. Rheum. Dis. 2016, 75, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Kintzel, P.E.; Dorr, R.T. Anticancer drug renal toxicity and elimination: Dosing guidelines for altered renal function. Cancer Treat. Rev. 1995, 21, 33–64. [Google Scholar] [CrossRef] [PubMed]
- Basile, C.; Montanaro, A.; Semeraro, A. Should low-dose methotrexate therapy be prescribed to dialysis patients? Nephrol. Dial. Transplant. 2002, 17, 530–531. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Evans, W.E.; Pratt, C.B. Effect of pleural effusion on high-dose methotrexate kinetics. Clin. Pharmacol. Ther. 1978, 23, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Morgan, S.L.; Baggott, J.E.; Vaughn, W.H.; Austin, J.S.; Veitch, T.A.; Lee, J.Y.; Koopman, W.J.; Krumdieck, C.L.; Alarcon, G.S. Supplementation with folic acid during methotrexate therapy for rheumatoid arthritis. A double-blind, placebo-controlled trial. Ann. Intern. Med. 1994, 121, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Davis, L.A.; Polk, B.; Mann, A.; Wolff, R.K.; Kerr, G.S.; Reimold, A.M.; Cannon, G.W.; Mikuls, T.R.; Caplan, L. Folic acid pathway single nucleotide polymorphisms associated with methotrexate significant adverse events in United States veterans with rheumatoid arthritis. Clin. Exp. Rheumatol. 2014, 32, 324–332. [Google Scholar]
- Singh, J.A.; Saag, K.G.; Bridges, S.L., Jr.; Akl, E.A.; Bannuru, R.R.; Sullivan, M.C.; Vaysbrot, E.; McNaughton, C.; Osani, M.; Shmerling, R.H.; et al. 2015 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Care Res 2016, 68, 1–25. [Google Scholar] [CrossRef]
- Hanoodi, M.; Mittal, M. Methotrexate; StatPearls: Treasure Island, FL, USA, 2024. [Google Scholar]
- Steuer, A.; Gumpel, J.M. Methotrexate and trimethoprim: A fatal interaction. Br. J. Rheumatol. 1998, 37, 105–106. [Google Scholar] [CrossRef][Green Version]
- Jeurissen, M.E.; Boerbooms, A.M.; van de Putte, L.B. Pancytopenia and methotrexate with trimethoprim-sulfamethoxazole. Ann. Intern. Med. 1989, 111, 261. [Google Scholar] [CrossRef] [PubMed]
- Maricic, M.; Davis, M.; Gall, E.P. Megaloblastic pancytopenia in a patient receiving concurrent methotrexate and trimethoprim-sulfamethoxazole treatment. Arthritis Rheum. 1986, 29, 133–135. [Google Scholar] [CrossRef]
- Kobrinsky, N.L.; Ramsay, N.K. Acute megaloblastic anemia induced by high-dose trimethoprim-sulfamethoxazole. Ann. Intern. Med. 1981, 94, 780–781. [Google Scholar] [CrossRef]
- Thomas, M.H.; Gutterman, L.A. Methotrexate toxicity in a patient receiving trimethoprim-sulfamethoxazole. J. Rheumatol. 1986, 13, 440–441. [Google Scholar]
- Ouellette, S.; Shah, R.; Razi, S.; Ashforth, G.; Wassef, C. Fatal low-dose methotrexate toxicity: A case report and literature review. Dermatol. Ther. 2022, 35, e15945. [Google Scholar] [CrossRef] [PubMed]
- Cudmore, J.; Seftel, M.; Sisler, J.; Zarychanski, R. Methotrexate and trimethoprim-sulfamethoxazole: Toxicity from this combination continues to occur. Can. Fam. Physician 2014, 60, 53–56. [Google Scholar] [PubMed]
- Mantadakis, E. Pneumocystis jirovecii Pneumonia in Children with Hematological Malignancies: Diagnosis and Approaches to Management. J. Fungi 2020, 6, 331. [Google Scholar] [CrossRef]
- Nazir, H.F.; Elshinawy, M.; AlRawas, A.; Khater, D.; Zadjaly, S.; Wali, Y. Efficacy and Safety of Dapsone Versus Trimethoprim/Sulfamethoxazol for Pneumocystis Jiroveci Prophylaxis in Children with Acute Lymphoblastic Leukemia with a Background of Ethnic Neutropenia. J. Pediatr. Hematol. Oncol. 2017, 39, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Kay, R.; DuBois, R.E. Clindamycin/primaquine therapy and secondary prophylaxis against Pneumocystis carinii pneumonia in patients with AIDS. South. Med. J. 1990, 83, 403–404. [Google Scholar] [CrossRef]
- Curtis, J.R.; Beukelman, T.; Onofrei, A.; Cassell, S.; Greenberg, J.D.; Kavanaugh, A.; Reed, G.; Strand, V.; Kremer, J.M. Elevated liver enzyme tests among patients with rheumatoid arthritis or psoriatic arthritis treated with methotrexate and/or leflunomide. Ann. Rheum. Dis. 2010, 69, 43–47. [Google Scholar] [CrossRef]
- Biancone, L.; Annese, V.; Ardizzone, S.; Armuzzi, A.; Calabrese, E.; Caprioli, F.; Castiglione, F.; Comberlato, M.; Cottone, M.; Danese, S.; et al. Safety of treatments for inflammatory bowel disease: Clinical practice guidelines of the Italian Group for the Study of Inflammatory Bowel Disease (IG-IBD). Dig. Liver Dis. 2017, 49, 338–358. [Google Scholar] [CrossRef] [PubMed]
- Sammaritano, L.R.; Bermas, B.L.; Chakravarty, E.E.; Chambers, C.; Clowse, M.E.B.; Lockshin, M.D.; Marder, W.; Guyatt, G.; Branch, D.W.; Buyon, J.; et al. 2020 American College of Rheumatology Guideline for the Management of Reproductive Health in Rheumatic and Musculoskeletal Diseases. Arthritis Rheumatol. 2020, 72, 529–556. [Google Scholar] [CrossRef] [PubMed]
- Micu, M.C.; Ostensen, M.; Villiger, P.M.; Micu, R.; Ionescu, R. Paternal exposure to antirheumatic drugs-What physicians should know: Review of the literature. Semin. Arthritis Rheum. 2018, 48, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Weber-Schoendorfer, C.; Hoeltzenbein, M.; Wacker, E.; Meister, R.; Schaefer, C. No evidence for an increased risk of adverse pregnancy outcome after paternal low-dose methotrexate: An observational cohort study. Rheumatology 2014, 53, 757–763. [Google Scholar] [CrossRef] [PubMed]
- Beghin, D.; Cournot, M.P.; Vauzelle, C.; Elefant, E. Paternal exposure to methotrexate and pregnancy outcomes. J. Rheumatol. 2011, 38, 628–632. [Google Scholar] [CrossRef] [PubMed]
- Eck, L.K.; Jensen, T.B.; Mastrogiannis, D.; Torp-Pedersen, A.; Askaa, B.; Nielsen, T.K.; Poulsen, H.E.; Jimenez-Solem, E.; Andersen, J.T. Risk of Adverse Pregnancy Outcome After Paternal Exposure to Methotrexate Within 90 Days Before Pregnancy. Obstet. Gynecol. 2017, 129, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Fajardo-Robledo, N.S.; Jacobo-Cuevas, H.; Perez-Guerrero, E.E.; Corona-Sanchez, E.G.; Saldana-Cruz, A.M.; Romero-Tejeda, E.M.; Rodriguez-Jimenez, N.A.; Totsuka-Sutto, S.E.; Lopez-Roa, R.I.; Ponce-Guarneros, J.M.; et al. Therapeutic response to leflunomide in combo therapy and monotherapy is associated to serum teriflunomide (A77 1726) levels. Sci. Rep. 2022, 12, 1877. [Google Scholar] [CrossRef] [PubMed]
- Rozman, B. Clinical pharmacokinetics of leflunomide. Clin. Pharmacokinet. 2002, 41, 421–430. [Google Scholar] [CrossRef]
- Sahoo, D.H.; Bandyopadhyay, D.; Xu, M.; Pearson, K.; Parambil, J.G.; Lazar, C.A.; Chapman, J.T.; Culver, D.A. Effectiveness and safety of leflunomide for pulmonary and extrapulmonary sarcoidosis. Eur. Respir. J. 2011, 38, 1145–1150. [Google Scholar] [CrossRef]
- Leflunomide (Araba) Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020905s022lbl.pdf (accessed on 20 November 2023).
- European Association for the Study of the Liver; Clinical Practice Guideline Panel; EASL Governing Board Representative. EASL Clinical Practice Guidelines: Drug-induced liver injury. J. Hepatol. 2019, 70, 1222–1261. [Google Scholar] [CrossRef]
- Wu, S.; Hoang, H.B.; Yang, J.Z.; Papamatheakis, D.G.; Poch, D.S.; Alotaibi, M.; Lombardi, S.; Rodriguez, C.; Kim, N.H.; Fernandes, T.M. Drug-Drug Interactions in the Management of Patients with Pulmonary Arterial Hypertension. Chest 2022, 162, 1360–1372. [Google Scholar] [CrossRef] [PubMed]
- Skerjanec, A.; Wang, J.; Maren, K.; Rojkjaer, L. Investigation of the pharmacokinetic interactions of deferasirox, a once-daily oral iron chelator, with midazolam, rifampin, and repaglinide in healthy volunteers. J. Clin. Pharmacol. 2010, 50, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Itkonen, M.K.; Tornio, A.; Neuvonen, M.; Neuvonen, P.J.; Niemi, M.; Backman, J.T. Clopidogrel Markedly Increases Plasma Concentrations of CYP2C8 Substrate Pioglitazone. Drug Metab. Dispos. 2016, 44, 1364–1371. [Google Scholar] [CrossRef]
- Chonlahan, J.; Halloran, M.A.; Hammonds, A. Leflunomide and warfarin interaction: Case report and review of the literature. Pharmacotherapy 2006, 26, 868–871. [Google Scholar] [CrossRef] [PubMed]
- Lim, V.; Pande, I. Leflunomide can potentiate the anticoagulant effect of warfarin. BMJ 2002, 325, 1333. [Google Scholar] [CrossRef][Green Version]
- Chambers, C.D.; Johnson, D.L.; Robinson, L.K.; Braddock, S.R.; Xu, R.; Lopez-Jimenez, J.; Mirrasoul, N.; Salas, E.; Luo, Y.J.; Jin, S.; et al. Birth outcomes in women who have taken leflunomide during pregnancy. Arthritis Rheum. 2010, 62, 1494–1503. [Google Scholar] [CrossRef] [PubMed]
- Leflunomide (Arava). Available online: https://mothertobaby.org/fact-sheets/leflunomide-pregnancy/ (accessed on 23 November 2023).
- Clinical Pharmacology and Biopharmaceuticas Reviews. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/98/20905_arava_biopharmr.pdf (accessed on 23 November 2023).
- Soares, G.; Rajabi-Estarabadi, A.; Nouri, K. Medicines and Therapies Associated with Skin Cancer. In Skin Cancer: A Comprehensive Guide; Nouri, K., Ed.; McGraw-Hill Education: New York, NY, USA, 2023. [Google Scholar]
- Pasadhika, S.; Rosenbaum, J.T. Ocular Sarcoidosis. Clin. Chest Med. 2015, 36, 669–683. [Google Scholar] [CrossRef]
- Azathioprine (Imuran). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/016324s039lbl.pdf (accessed on 25 November 2023).
- Azathioprine. Lexicomp Online. Waltham, MA: UpToDate, Inc. Available online: https://online.lexi.com. (accessed on 25 November 2023).
- Dean, L. Azathioprine Therapy and TPMT and NUDT15 Genotype. In Medical Genetics Summarie; Pratt, V.M., Scott, S.A., Pirmohamed, M., Esquivel, B., Kattman, B.L., Malheiro, A.J., Eds.; National Center for Biotechnology Information: Bethesda, MD, USA, 2012. [Google Scholar]
- Relling, M.V.; Schwab, M.; Whirl-Carrillo, M.; Suarez-Kurtz, G.; Pui, C.H.; Stein, C.M.; Moyer, A.M.; Evans, W.E.; Klein, T.E.; Antillon-Klussmann, F.G.; et al. Clinical Pharmacogenetics Implementation Consortium Guideline for Thiopurine Dosing Based on TPMT and NUDT15 Genotypes: 2018 Update. Clin. Pharmacol. Ther. 2019, 105, 1095–1105. [Google Scholar] [CrossRef]
- Plasmeijer, E.I.; Sachse, M.M.; Gebhardt, C.; Geusau, A.; Bouwes Bavinck, J.N. Cutaneous squamous cell carcinoma (cSCC) and immunosurveillance—The impact of immunosuppression on frequency of cSCC. J. Eur. Acad. Dermatol. Venereol. 2019, 33 (Suppl. 8), 33–37. [Google Scholar] [CrossRef]
- Farraye, F.A.; Melmed, G.Y.; Lichtenstein, G.R.; Kane, S.V. ACG Clinical Guideline: Preventive Care in Inflammatory Bowel Disease. Am. J. Gastroenterol. 2017, 112, 241–258. [Google Scholar] [CrossRef]
- Pernia, S.; DeMaagd, G. The New Pregnancy and Lactation Labeling Rule. Pharm. Ther. 2016, 41, 713–715. [Google Scholar]
- Damas, O.M.; Deshpande, A.R.; Avalos, D.J.; Abreu, M.T. Treating Inflammatory Bowel Disease in Pregnancy: The Issues We Face Today. J. Crohns Colitis 2015, 9, 928–936. [Google Scholar] [CrossRef]
- Nguyen, G.C.; Seow, C.H.; Maxwell, C.; Huang, V.; Leung, Y.; Jones, J.; Leontiadis, G.I.; Tse, F.; Mahadevan, U.; van der Woude, C.J.; et al. The Toronto Consensus Statements for the Management of Inflammatory Bowel Disease in Pregnancy. Gastroenterology 2016, 150, 734–757 e731. [Google Scholar] [CrossRef]
- Angelberger, S.; Reinisch, W.; Messerschmidt, A.; Miehsler, W.; Novacek, G.; Vogelsang, H.; Dejaco, C. Long-term follow-up of babies exposed to azathioprine in utero and via breastfeeding. J. Crohns Colitis 2011, 5, 95–100. [Google Scholar] [CrossRef]
- Van Assche, G.; Dignass, A.; Reinisch, W.; van der Woude, C.J.; Sturm, A.; De Vos, M.; Guslandi, M.; Oldenburg, B.; Dotan, I.; Marteau, P.; et al. The second European evidence-based Consensus on the diagnosis and management of Crohn’s disease: Special situations. J. Crohns Colitis 2010, 4, 63–101. [Google Scholar] [CrossRef]
- Norgard, B.M.; Magnussen, B.; Larsen, M.D.; Friedman, S. Reassuring results on birth outcomes in children fathered by men treated with azathioprine/6-mercaptopurine within 3 months before conception: A nationwide cohort study. Gut 2017, 66, 1761–1766. [Google Scholar] [CrossRef] [PubMed]
- Resman-Targoff, B.H. Systemic Lupus Erythematosus. In DiPiro’s Pharmacotherapy: A Pathophysiologic Approach, 12th ed.; DiPiro, J.T., Yee, G.C., Haines, S.T., Nolin, T.D., Ellingrod, V.L., Posey, L.M., Eds.; McGraw Hill: New York, NY, USA, 2023. [Google Scholar]
- Daniel, E.; Thorne, J.E.; Newcomb, C.W.; Pujari, S.S.; Kacmaz, R.O.; Levy-Clarke, G.A.; Nussenblatt, R.B.; Rosenbaum, J.T.; Suhler, E.B.; Foster, C.S.; et al. Mycophenolate mofetil for ocular inflammation. Am. J. Ophthalmol. 2010, 149, 423–432.e2. [Google Scholar] [CrossRef] [PubMed]
- Petri, M. Pregnancy and Systemic Lupus Erythematosus. Best. Pract. Res. Clin. Obstet. Gynaecol. 2020, 64, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Mycophenolate Mofetil (Cellcept). Available online: https://www.gene.com/download/pdf/cellcept_prescribing.pdf (accessed on 26 November 2023).
- Mok, C.C. Therapeutic monitoring of the immuno-modulating drugs in systemic lupus erythematosus. Expert Rev. Clin. Immunol. 2017, 13, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Anders, H.J.; Saxena, R.; Zhao, M.H.; Parodis, I.; Salmon, J.E.; Mohan, C. Lupus nephritis. Nat. Rev. Dis. Primers 2020, 6, 7. [Google Scholar] [CrossRef]
- Pieper, A.K.; Buhle, F.; Bauer, S.; Mai, I.; Budde, K.; Haffner, D.; Neumayer, H.H.; Querfeld, U. The effect of sevelamer on the pharmacokinetics of cyclosporin A and mycophenolate mofetil after renal transplantation. Nephrol. Dial. Transplant. 2004, 19, 2630–2633. [Google Scholar] [CrossRef] [PubMed]
- Naderer, O.J.; Dupuis, R.E.; Heinzen, E.L.; Wiwattanawongsa, K.; Johnson, M.W.; Smith, P.C. The influence of norfloxacin and metronidazole on the disposition of mycophenolate mofetil. J. Clin. Pharmacol. 2005, 45, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Borrows, R.; Chusney, G.; Loucaidou, M.; James, A.; Van Tromp, J.; Cairns, T.; Griffith, M.; Hakim, N.; McLean, A.; Palmer, A.; et al. The magnitude and time course of changes in mycophenolic acid 12-hour predose levels during antibiotic therapy in mycophenolate mofetil-based renal transplantation. Ther. Drug. Monit. 2007, 29, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Naesens, M.; Kuypers, D.R.; Streit, F.; Armstrong, V.W.; Oellerich, M.; Verbeke, K.; Vanrenterghem, Y. Rifampin induces alterations in mycophenolic acid glucuronidation and elimination: Implications for drug exposure in renal allograft recipients. Clin. Pharmacol. Ther. 2006, 80, 509–521. [Google Scholar] [CrossRef]
- Annapandian, V.M.; Fleming, D.H.; Mathew, B.S.; John, G.T. Mycophenolic acid area under the curve recovery time following rifampicin withdrawal. Indian J. Nephrol. 2010, 20, 51–53. [Google Scholar] [CrossRef] [PubMed]
- Kuypers, D.R.; Verleden, G.; Naesens, M.; Vanrenterghem, Y. Drug interaction between mycophenolate mofetil and rifampin: Possible induction of uridine diphosphate-glucuronosyltransferase. Clin. Pharmacol. Ther. 2005, 78, 81–88. [Google Scholar] [CrossRef]
- Bergan, S.; Brunet, M.; Hesselink, D.A.; Johnson-Davis, K.L.; Kunicki, P.K.; Lemaitre, F.; Marquet, P.; Molinaro, M.; Noceti, O.; Pattanaik, S.; et al. Personalized Therapy for Mycophenolate: Consensus Report by the International Association of Therapeutic Drug Monitoring and Clinical Toxicology. Ther Drug Monit 2021, 43, 150–200. [Google Scholar] [CrossRef]
- Lake, D.F.; Briggs, A.D. Immunopharmacology. In Katzung’s Basic & Clinical Pharmacology, 16th ed.; Vanderah, T.W., Ed.; McGraw-Hill: New York, NY, 2024. [Google Scholar]
- Kafaja, T.K.; Anwar, S.; Furst, D.E. Nonsteroidal Anti-Inflammatory Drugs, Disease-Modifying Antirheumatic Drugs, Nonopioid Analgesics, & Drugs Used in Gout. In Katzung’s Basic & Clinical Pharmacology, 16th ed.; Vanderah, T.W., Ed.; McGraw-Hill: New York, NY, USA, 2024. [Google Scholar]
- Marmor, M.F.; Kellner, U.; Lai, T.Y.; Melles, R.B.; Mieler, W.F.; American Academy of Ophthalmology. Recommendations on Screening for Chloroquine and Hydroxychloroquine Retinopathy (2016 Revision). Ophthalmology 2016, 123, 1386–1394. [Google Scholar] [CrossRef]
- Tonnesmann, E.; Stroehmann, I.; Kandolf, R.; Wolburg, H.; Strach, K.; Musshoff, F.; Tiemann, K.; Lewalter, T. Cardiomyopathy caused by longterm treatment with chloroquine: A rare disease, or a rare diagnosis? J. Rheumatol. 2012, 39, 1099–1103. [Google Scholar] [CrossRef][Green Version]
- Youngster, I.; Arcavi, L.; Schechmaster, R.; Akayzen, Y.; Popliski, H.; Shimonov, J.; Beig, S.; Berkovitch, M. Medications and glucose-6-phosphate dehydrogenase deficiency: An evidence-based review. Drug Saf. 2010, 33, 713–726. [Google Scholar] [CrossRef] [PubMed]
- Manzo, C.; Gareri, P.; Castagna, A. Psychomotor Agitation Following Treatment with Hydroxychloroquine. Drug Saf. Case Rep. 2017, 4, 6. [Google Scholar] [CrossRef] [PubMed]
- Mascolo, A.; Berrino, P.M.; Gareri, P.; Castagna, A.; Capuano, A.; Manzo, C.; Berrino, L. Neuropsychiatric clinical manifestations in elderly patients treated with hydroxychloroquine: A review article. Inflammopharmacology 2018, 26, 1141–1149. [Google Scholar] [CrossRef] [PubMed]
- Shojania, K.; Koehler, B.E.; Elliott, T. Hypoglycemia induced by hydroxychloroquine in a type II diabetic treated for polyarthritis. J. Rheumatol. 1999, 26, 195–196. [Google Scholar] [PubMed]
- Cansu, D.U.; Korkmaz, C. Hypoglycaemia induced by hydroxychloroquine in a non-diabetic patient treated for RA. Rheumatology 2008, 47, 378–379. [Google Scholar] [CrossRef]
- Melles, R.B.; Marmor, M.F. The risk of toxic retinopathy in patients on long-term hydroxychloroquine therapy. JAMA Ophthalmol. 2014, 132, 1453–1460. [Google Scholar] [CrossRef]
- Hundal, R.S.; Petersen, K.F.; Mayerson, A.B.; Randhawa, P.S.; Inzucchi, S.; Shoelson, S.E.; Shulman, G.I. Mechanism by which high-dose aspirin improves glucose metabolism in type 2 diabetes. J. Clin. Invest. 2002, 109, 1321–1326. [Google Scholar] [CrossRef]
- Baron, S.H. Salicylates as hypoglycemic agents. Diabetes Care 1982, 5, 64–71. [Google Scholar] [CrossRef]
- Leden, I. Digoxin-hydroxychloroquine interaction? Acta Med. Scand 1982, 211, 411–412. [Google Scholar] [CrossRef]
- Tett, S.E. Clinical pharmacokinetics of slow-acting antirheumatic drugs. Clin. Pharmacokinet. 1993, 25, 392–407. [Google Scholar] [CrossRef]
- Fehrenbach, H.; Zissel, G.; Goldmann, T.; Tschernig, T.; Vollmer, E.; Pabst, R.; Muller-Quernheim, J. Alveolar macrophages are the main source for tumour necrosis factor-alpha in patients with sarcoidosis. Eur. Respir. J. 2003, 21, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Zissel, G.; Muller-Quernheim, J. Sarcoidosis: Historical perspective and immunopathogenesis (Part I). Respir. Med. 1998, 92, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Utz, J.P.; Limper, A.H.; Kalra, S.; Specks, U.; Scott, J.P.; Vuk-Pavlovic, Z.; Schroeder, D.R. Etanercept for the treatment of stage II and III progressive pulmonary sarcoidosis. Chest 2003, 124, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Judson, M.A.; Baughman, R.P.; Costabel, U.; Drent, M.; Gibson, K.F.; Raghu, G.; Shigemitsu, H.; Barney, J.B.; Culver, D.A.; Hamzeh, N.Y.; et al. Safety and efficacy of ustekinumab or golimumab in patients with chronic sarcoidosis. Eur. Respir. J. 2014, 44, 1296–1307. [Google Scholar] [CrossRef] [PubMed]
- Jackson, J.M. TNF- alpha inhibitors. Dermatol. Ther. 2007, 20, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Stagaki, E.; Mountford, W.K.; Lackland, D.T.; Judson, M.A. The treatment of lupus pernio: Results of 116 treatment courses in 54 patients. Chest 2009, 135, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Judson, M.A.; Adelstein, E.; Fish, K.M.; Feustel, P.J.; Yucel, R.; Preston, S.; Vancavage, R.; Chopra, A.; Steckman, D.A. Outcomes of prednisone-tapering regimens for cardiac sarcoidosis: A retrospective analysis demonstrating a benefit of infliximab. Respir. Med. 2022, 203, 107004. [Google Scholar] [CrossRef] [PubMed]
- Chaiyanarm, S.; Satiraphan, P.; Apiraksattaykul, N.; Jitprapaikulsan, J.; Owattanapanich, W.; Rungjirajittranon, T.; Nanthasi, W. Infliximab in neurosarcoidosis: A systematic review and meta-analysis. Ann. Clin. Transl. Neurol. 2024, 11, 466–476. [Google Scholar] [CrossRef]
- Fritz, D.; Timmermans, W.M.C.; van Laar, J.A.M.; van Hagen, P.M.; Siepman, T.A.M.; van de Beek, D.; Brouwer, M.C. Infliximab treatment in pathology-confirmed neurosarcoidosis. Neurol. Neuroimmunol Neuroinflamm 2020, 7, e847. [Google Scholar] [CrossRef]
- Loomba, R.; Liang, T.J. Hepatitis B Reactivation Associated with Immune Suppressive and Biological Modifier Therapies: Current Concepts, Management Strategies, and Future Directions. Gastroenterology 2017, 152, 1297–1309. [Google Scholar] [CrossRef]
- Lan, J.L.; Chen, Y.M.; Hsieh, T.Y.; Chen, Y.H.; Hsieh, C.W.; Chen, D.Y.; Yang, S.S. Kinetics of viral loads and risk of hepatitis B virus reactivation in hepatitis B core antibody-positive rheumatoid arthritis patients undergoing anti-tumour necrosis factor alpha therapy. Ann. Rheum. Dis. 2011, 70, 1719–1725. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, M.S.; Friedman, L.N. Tuberculosis and Biologic Therapies: Anti-Tumor Necrosis Factor-α and Beyond. Clin. Chest. Med. 2019, 40, 721–739. [Google Scholar] [CrossRef] [PubMed]
- Ai, J.W.; Zhang, S.; Ruan, Q.L.; Yu, Y.Q.; Zhang, B.Y.; Liu, Q.H.; Zhang, W.H. The Risk of Tuberculosis in Patients with Rheumatoid Arthritis Treated with Tumor Necrosis Factor-α Antagonist: A Metaanalysis of Both Randomized Controlled Trials and Registry/Cohort Studies. J. Rheumatol. 2015, 42, 2229–2237. [Google Scholar] [CrossRef] [PubMed]
- Atiqi, S.; Hooijberg, F.; Loeff, F.C.; Rispens, T.; Wolbink, G.J. Immunogenicity of TNF-Inhibitors. Front. Immunol. 2020, 11, 312. [Google Scholar] [CrossRef] [PubMed]
- Garces, S.; Demengeot, J.; Benito-Garcia, E. The immunogenicity of anti-TNF therapy in immune-mediated inflammatory diseases: A systematic review of the literature with a meta-analysis. Ann. Rheum. Dis. 2013, 72, 1947–1955. [Google Scholar] [CrossRef] [PubMed]
- Askling, J.; Baecklund, E.; Granath, F.; Geborek, P.; Fored, M.; Backlin, C.; Bertilsson, L.; Cöster, L.; Jacobsson, L.T.; Lindblad, S.; et al. Anti-tumour necrosis factor therapy in rheumatoid arthritis and risk of malignant lymphomas: Relative risks and time trends in the Swedish Biologics Register. Ann. Rheum. Dis. 2009, 68, 648–653. [Google Scholar] [CrossRef]
- Yanai, H.; Shuster, D.; Calabrese, E.; Mlynarsky, L.; Tumuluri, S.; Cohen, R.D. The incidence and predictors of lupus-like reaction in patients with IBD treated with anti-TNF therapies. Inflamm. Bowel. Dis. 2013, 19, 2778–2786. [Google Scholar] [CrossRef]
- Vermeire, S.; Noman, M.; Van Assche, G.; Baert, F.; Van Steen, K.; Esters, N.; Joossens, S.; Bossuyt, X.; Rutgeerts, P. Autoimmunity associated with anti-tumor necrosis factor alpha treatment in Crohn’s disease: A prospective cohort study. Gastroenterology 2003, 125, 32–39. [Google Scholar] [CrossRef]
- Choi, S.J.; Ahn, S.M.; Oh, J.S.; Hong, S.; Lee, C.K.; Yoo, B.; Ye, B.D.; Yang, S.K.; Park, S.H.; Kim, Y.G. Anti-tumor necrosis factor-induced lupus in patients with inflammatory bowel disease: A hospital-based cohort study from Korea. Therap. Adv. Gastroenterol. 2021, 14, 1–9. [Google Scholar] [CrossRef]
- Feuerstein, J.D.; Cheifetz, A.S. Miscellaneous adverse events with biologic agents (excludes infection and malignancy). Gastroenterol. Clin. North Am. 2014, 43, 543–563. [Google Scholar] [CrossRef]
- Schiff, M.H.; Burmester, G.R.; Kent, J.D.; Pangan, A.L.; Kupper, H.; Fitzpatrick, S.B.; Donovan, C. Safety analyses of adalimumab (HUMIRA) in global clinical trials and US postmarketing surveillance of patients with rheumatoid arthritis. Ann. Rheum. Dis. 2006, 65, 889–894. [Google Scholar] [CrossRef]
- Burmester, G.R.; Panaccione, R.; Gordon, K.B.; McIlraith, M.J.; Lacerda, A.P. Adalimumab: Long-term safety in 23,458 patients from global clinical trials in rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis and Crohn’s disease. Ann. Rheum. Dis. 2013, 72, 517–524. [Google Scholar] [CrossRef]
- Kumar, N.; Abboud, H. Iatrogenic CNS demyelination in the era of modern biologics. Mult. Scler. 2019, 25, 1079–1085. [Google Scholar] [CrossRef]
- Murdaca, G.; Spano, F.; Puppo, F. Selective TNF-alpha inhibitor-induced injection site reactions. Expert Opin. Drug. Saf. 2013, 12, 187–193. [Google Scholar] [CrossRef]
- Aby, E.S.; Lake, J.R.; Vaughn, B.P. The Impact of Biologics for the Management of Inflammatory Bowel Disease on Liver Enzymes. Clin. Liver Dis. 2020, 16, 212–217. [Google Scholar] [CrossRef]
- Infliximab. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012. [Google Scholar]
- Reff, M.E.; Carner, K.; Chambers, K.S.; Chinn, P.C.; Leonard, J.E.; Raab, R.; Newman, R.A.; Hanna, N.; Anderson, D.R. Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood 1994, 83, 435–445. [Google Scholar] [CrossRef]
- Cohen, S.B.; Emery, P.; Greenwald, M.W.; Dougados, M.; Furie, R.A.; Genovese, M.C.; Keystone, E.C.; Loveless, J.E.; Burmester, G.R.; Cravets, M.W.; et al. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: Results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum. 2006, 54, 2793–2806. [Google Scholar] [CrossRef]
- Sell, S.; Max, E.E. Immunology, Immunopathology, and Immunity, 6th ed.; ASM Press: Washington, DC, USA, 2001. [Google Scholar]
- Hunninghake, G.W.; Crystal, R.G. Mechanisms of hypergammaglobulinemia in pulmonary sarcoidosis. Site of increased antibody production and role of T lymphocytes. J. Clin. Invest. 1981, 67, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Liote, H.; Liote, F.; Seroussi, B.; Mayaud, C.; Cadranel, J. Rituximab-induced lung disease: A systematic literature review. Eur. Respir. J. 2010, 35, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Ramachandrappa, S.; Gorrigan, R.J.; Clark, A.J.; Chan, L.F. The melanocortin receptors and their accessory proteins. Front. Endocrinol. 2013, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Laiho, L.; Murray, J.F. The Multifaceted Melanocortin Receptors. Endocrinology 2022, 163, bqac083. [Google Scholar] [CrossRef]
- Wang, W.; Guo, D.Y.; Lin, Y.J.; Tao, Y.X. Melanocortin Regulation of Inflammation. Front. Endocrinol. 2019, 10, 683. [Google Scholar] [CrossRef] [PubMed]
- Mirsaeidi, M.; Baughman, R.P. Repository Corticotropin Injection for the Treatment of Pulmonary Sarcoidosis: A Narrative Review. Pulm. Ther. 2022, 8, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Judson, M.A. Quality of Life in Sarcoidosis. Semin. Respir Crit. Care Med. 2017, 38, 546–558. [Google Scholar] [CrossRef] [PubMed]
| Compounds | Anti-Inflammatory Potency | Equivalent Dose (mg) |
|---|---|---|
| Cortisone | 0.8 | 25 |
| Hydrocortisone | 1 | 20 |
| Prednisolone | 4 | 5 |
| Prednisone | 4 | 5 |
| Methylprednisolone | 5 | 4 |
| Triamcinolone | 5 | 4 |
| Betamethasone | 25 | 0.75 |
| Dexamethasone | 25 | 0.75 |
| Monitoring Parameter | Monitoring Time Frame | Reference |
|---|---|---|
| Body weight | Baseline, frequently. | [6] |
| Height | Baseline, annually. | [6,7] |
| Blood pressure | Baseline, frequently. | [5,6] |
| HbA1C | Baseline, every 3~6 months. | [6] |
| Blood glucose | Baseline, frequently. | [6] |
| CBC | Baseline, frequently. | [6] |
| Lipid profile | Baseline, one month after initiation of glucocorticoid therapy, then every 6–12 months. | [5,6,8] |
| Bone-mineral density | Baseline, every 1–2 years. | [7] |
| Fracture history | Baseline, then at routine follow up visits. | [6] |
| Joint pain | Baseline, then at routine follow up visits. | [6,9] |
| Infection | Baseline, then at routine follow up visits. | [5,6] |
| Eye exam | Baseline, then annually or as recommended by an ophthalmologist. | [5,6] |
| Healthy lifestyle inventory and education | Baseline documentation of patient’s lifestyle and awareness. After initial counseling, reinforce healthy lifestyle choices at routine follow up visits. | [6] |
| Perceived fatigue | Baseline, then at routine follow up visits. | [6,10] |
| Adrenal insufficiency | Measure serum cortisol or perform an ACTH stimulation test in patients with symptoms of adrenal insufficiency (or withdrawal) who have been tapered to a low dose or off corticosteroids. | [6] |
| Anginal symptoms (cardiovascular events) | Baseline, at routine follow up visits, educate the patient concerning these symptoms. | [6,11] |
| CYP450 3A4 Inhibitors | CYP450 3A4 Inducers | ||
|---|---|---|---|
| INCREASED GLUCOCORTICOID EFFECTIVENESS INCREASED GLUCOCORTICOID SIDE EFFECT RISK | DECREASED GLUCOCORTICOID EFFECTIVENESS DECREASED GLUCOCORTICOID SIDE EFFECT RISK | ||
| Moderate Effect | Strong Effect | Moderate Effect | Strong Effect |
| Diltiazem Verapamil Erythromycin Fluconazole Isavuconazole Cyclosporine Dronedarone | Clarithromycin Erythromycin Itraconazole Ketoconazole Voriconazole Posaconazole Ritonavir Indinavir Darunavir Nelfinavir Saquinavir | Rifapentine Rifabutin Efavirenz Bosentan | Phenobarbital Phenytoin Fosphenytoin Primidone Rifampicin Rifampin Carbamazepine Eslicarbazepine Lumacaftor Lumacaftor-ivacaftor |
| CrCl | Methotrexate Dose |
|---|---|
| CrCl > 60 mL/min | No dose adjustment necessary. |
| 46 ≤ CrCl < 60 mL/min | 65% of normal dose. |
| 31 ≤ CrCl < 45 mL/min | 50% of normal dose. |
| CrCl < 30 mL/min | Avoid use. |
| Drug | Dosage Form | Dosing | Side Effects | Contraindications per US or Canadian Label | Renal Dose Adjustment Required | Hepatic Dose Adjustment Required | PGx Dose Adjustment Required |
|---|---|---|---|---|---|---|---|
| Prednisone (FDA approved as “systemic rheumatic disorders”) | Oral | Varies. 5–30 mg daily in single or divided doses. Higher dose may be needed for severe diseases. | gastritis, nausea and other GI effects, osteoporosis, weight gain, diabetes, hypertension, fluid retention, hyperglycemia, skin atrophy, impaired wound healing, depression, mood change, adrenocortical insufficiency with inappropriate tapering, Cushing syndrome, decreased growth in children, myopathy, glaucoma, cataract, risk of infection | Herpes simplex of the eye, measles, or chickenpox (except for short term or emergency), peptic ulcer, diverticulitis, viral or bacterial infections not controlled by anti-infective treatment. | No | No | No |
| Methotrexate | Oral, SC | 5-25 mg/week Split dosing for ≥15 mg for oral dosing. Split dosing in not needed for SC. | Mouth sores, bone marrow suppression, hepatotoxicity, nausea and other GI effects, hair loss, pneumonitis, photosensitivity | Pregnancy, severe hepatic insufficiency, alcohol use dialysis, chronic pleural effusion | Yes | Yes | No |
| Leflunomide | Oral | 10~20 mg daily | Mouth sores, bone marrow suppression, hepatotoxicity, nausea and other GI effects, hair loss, peripheral neuropathy, increased blood pressure | Pregnancy Severe hepatic insufficiency Alcohol use | No | Yes | No |
| Hydroxychloroquine | Oral | 5 mg/kg/day with a maximum of 400 mg daily given in divided doses | Retinopathy, QT prolongation, psoriasis, nausea, and other GI effects | No | No | No | |
| Azathioprine | Oral | 50~250 mg daily in divided doses | Bone marrow suppression, nausea and other GI effects, hepatotoxicity | No (manufacturer) Yes (experts) | No | Yes | |
| Mycophenolate Mofetil | Oral tablet, capsule, and suspension | Start with 500 mg BID. Max maintenance dose 1500 mg BID. Do so slowly to avoid GI side effects | Bone marrow suppression, nausea and other GI effects, fever, arthralgia, myalgias, liver, hematological, dermatological toxicity, malignancy, hypertension, John-Cunningham (JC) virus associated Progressive Multifocal Leukoencephalopathy (PML) | Pregnancy | No | No | No |
| Infliximab | IV | Induction: 3–5 mg/kg at week 0, 2, 6. Maintenance: 3–5 mg/kg every 4–8 weeks after induction. | Serious infection, malignancy, lymphoma, heart failure, demyelinating disease, autoimmune disorder (e.g., lupus-like syndrome, fever), reactivation of latent infections such as Hepatitis B, Tuberculosis, infusion related reactions (e.g., angioedema, bronchospasm) | Severe heart failure | No | No | No |
| Adalimumab | SC | 40 mg every week or every other week | Serious infection, malignancy, lymphoma, heart failure, demyelinating disease, autoimmune disorder (e.g., lupus-like syndrome, fever), reactivation of latent infections such as Hepatitis B, Tuberculosis, injection site reaction | Severe heart failure | No | No | No |
| Rituximab | IV | 1 gram two weeks apart (week 0 and 2). Repeat every 6 months if clinically needed. | Serious infection, PML, reactivation of Hepatitis B, infusion related reactions (e.g., angioedema, bronchospasm), flushing, hypertension, edema, pruritis, hematologic side effects (anemia, neutropenia, hypogammaglobulinemia, leucopenia, thrombocytopenia), dyspnea | Severe, active infection, PML, hypersensitivity or anaphylactic reaction to murine proteins | No | No | No |
| Repository corticotropin injection (FDA approved) | SC | 40–80 units twice weekly | Same as glucocorticoids Hyperpigmentation | Same as glucocorticoid assumed | No | No | No |
| Drug | Safe to Administer Non-Live Vaccine, Influenza Vaccine | Safe to Administer live or Live-Attenuated Vaccine | Safe to Use during Pregnancy | Safe to Use during Breastfeeding | Drug to be Avoided for Concomitant Use | Cautions |
|---|---|---|---|---|---|---|
| Prednisone (FDA approved as “systemic rheumatic disorders”) | Yes | Depends on dose | Yes | Yes | Use steroid sparing agents as possible to avoid long term side effects of glucocorticoids. | |
| Methotrexate | Yes, hold for 2 weeks after vaccination if possible. | Hold 4 weeks prior and 4 weeks after | No | No | Sulfamethoxazole/ trimethoprim | Folic acid supplement daily (1~4 mg daily) recommended. Leucovorin rescue in case of toxicity. |
| Leflunomide | Yes | Hold 4 weeks prior and 4 weeks after | No | No | Enterohepatic recycling occurs: Accelerated clearance process with cholestyramine or charcoal needed in case of toxicity or unplanned pregnancy | |
| Hydroxychloroquine | Yes | Yes | Yes | Yes | Yearly eye exam | |
| Azathioprine | Yes | Hold 4 weeks prior and 4 weeks after | Yes | Yes | Allopurinol Febuxostat | TPMT and/or NUDT15 deficiency |
| Mycophenolate Mofetil | Yes | Hold 4 weeks prior and 4 weeks after | No | No | Avoid use with azathioprine (↑ risk myelosuppression) Oral suspension formulation useful for patients with swallowing issues | |
| Infliximab | Yes | Hold 1 dose prior and 4 weeks after | OK 1st and 2nd trimester Hold for 3rd trimester | Yes | Other immunosuppressive biologic DMARD or Janus Kinase inhibitors | Monitor for anaphylaxis, severe infusion reaction. Consider antibody formation if efficacy wanes. |
| Adalimumab | Yes | Hold 1 dose prior and 4 weeks after | OK 1st and 2nd trimester Hold for 3rd trimester | Yes | Other immunosuppressive biologic DMARD or Janus Kinase inhibitors | |
| Rituximab | Yes | Hold 6 months prior and 4 weeks after | Discontinue at conception unless life or organ threatening condition | Yes | Other immunosuppressive biologic DMARD or Janus Kinase inhibitors | Monitor for anaphylaxis, severe infusion reaction. Consider antibody formation if efficacy wanes. |
| Repository corticotropin injection (FDA approved) | No specific recommendationSame as glucocorticoid assumed | No specific recommendation Same as glucocorticoid assumed | No specific recommendation Same as glucocorticoid assumed | No specific recommendationSame as glucocorticoid assumed | No specific recommendation Same as glucocorticoid assumed | Same as glucocorticoid assumed |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, S.; Judson, M.A. Clinical Pharmacology in Sarcoidosis: How to Use and Monitor Sarcoidosis Medications. J. Clin. Med. 2024, 13, 1250. https://doi.org/10.3390/jcm13051250
Kwon S, Judson MA. Clinical Pharmacology in Sarcoidosis: How to Use and Monitor Sarcoidosis Medications. Journal of Clinical Medicine. 2024; 13(5):1250. https://doi.org/10.3390/jcm13051250
Chicago/Turabian StyleKwon, Sooyeon, and Marc A. Judson. 2024. "Clinical Pharmacology in Sarcoidosis: How to Use and Monitor Sarcoidosis Medications" Journal of Clinical Medicine 13, no. 5: 1250. https://doi.org/10.3390/jcm13051250
APA StyleKwon, S., & Judson, M. A. (2024). Clinical Pharmacology in Sarcoidosis: How to Use and Monitor Sarcoidosis Medications. Journal of Clinical Medicine, 13(5), 1250. https://doi.org/10.3390/jcm13051250







