Novel and Emerging LDL-C Lowering Strategies: A New Era of Dyslipidemia Management
Abstract
1. Introduction
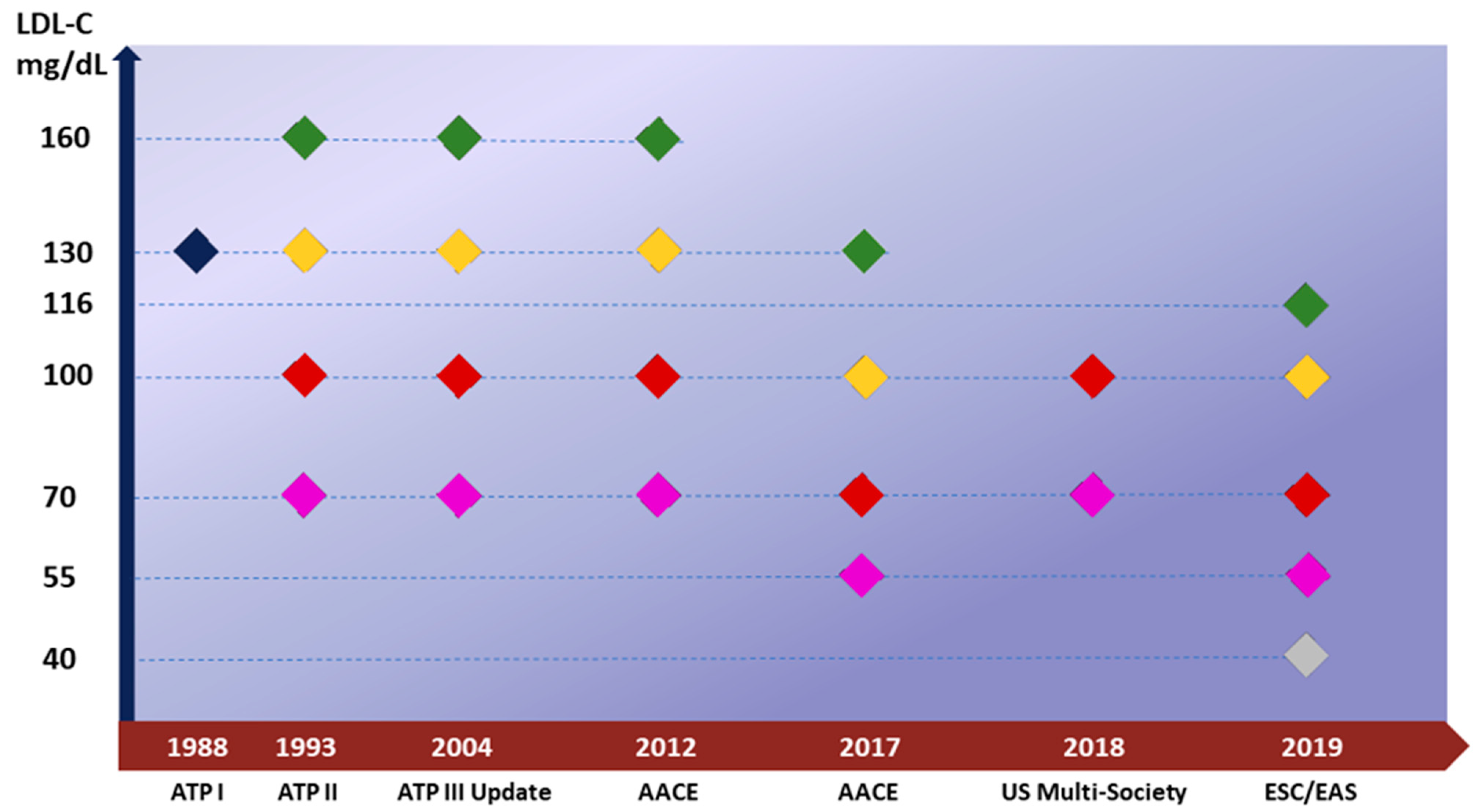
2. Current Recommendation
3. Consolidated Approaches: Certainty and Weakness
4. Innovative Approaches and Investigational Therapies
4.1. Bempedoic Acid
4.2. Novel Strategies for PCSK9 Inhibition
4.2.1. Gene Silencing
4.2.2. Binding Peptides
4.2.3. Gene Editing
4.2.4. Vaccines
4.3. ANGPTL3 Inhibitors
4.4. CETP Inhibitors
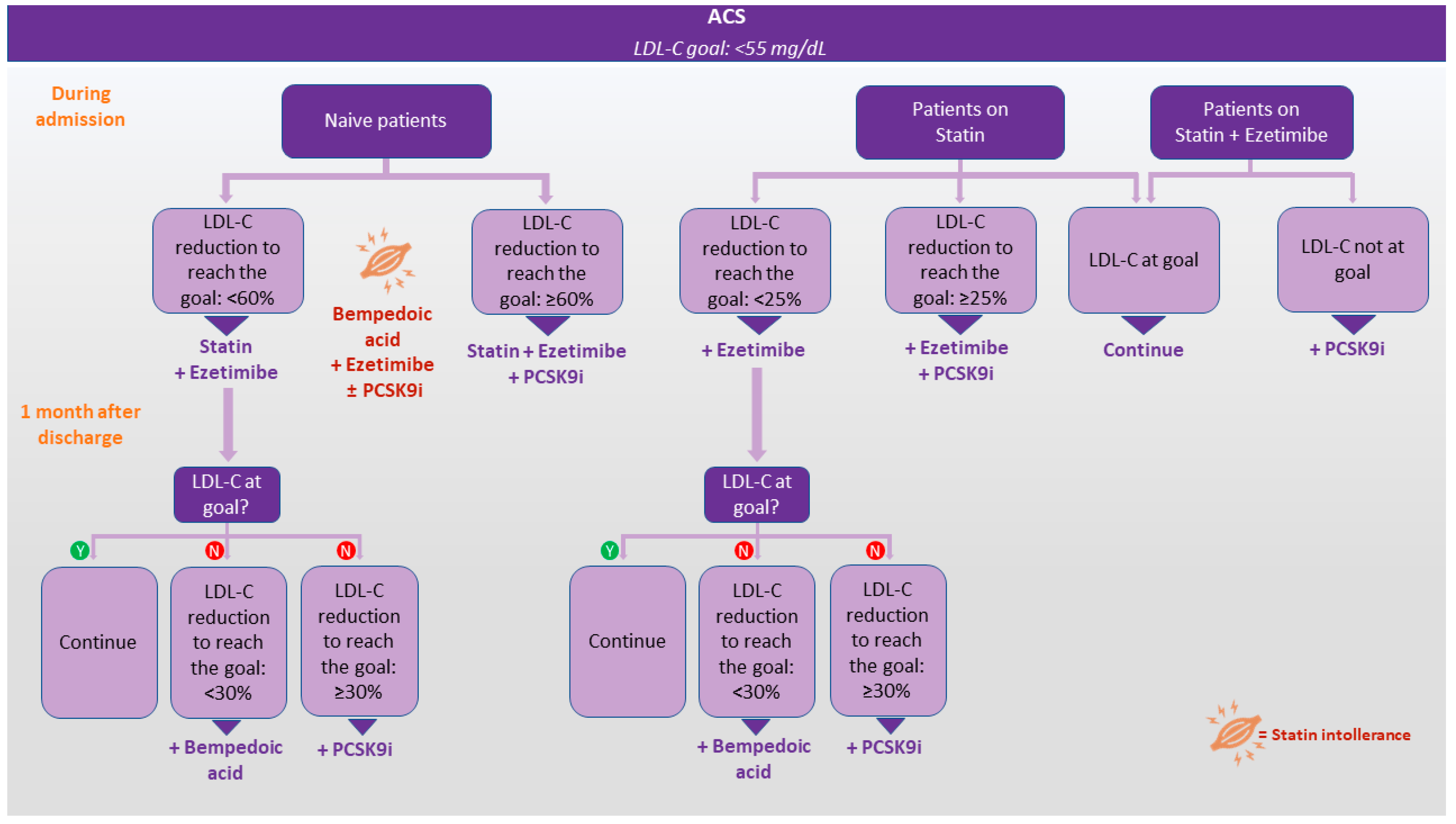
5. Moving to Intensive LDL-C Management
6. Authors’ Opinion
7. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Agnello, F.; Capodanno, D. Anti-inflammatory strategies for atherosclerotic artery disease. Expert Opin. Drug Saf. 2022, 21, 661–672. [Google Scholar] [CrossRef]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk actors, 1990–2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef]
- Emerging Risk Factors Collaboration; Di Angelantonio, E.; Gao, P.; Pennells, L.; Danesh, J. Lipid-related markers and cardiovascular disease prediction. JAMA 2012, 307, 2499–2506. [Google Scholar] [PubMed]
- Cholesterol Treatment Trialists’ (CTT) Collaboration; Baigent, C.; Blackwell, L.; Emberson, J.; Holland, L.E.; Reith, C.; Bhala, N.; Collins, R. Efficacy and safety of more intensive lowering of LDL cholesterol: A meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010, 376, 1670–1681. [Google Scholar] [CrossRef]
- Silverman, M.G.; Ference, B.A.; Im, K.; Wiviott, S.D.; Giugliano, R.P.; Grundy, S.M.; Braunwald, E.; Sabatine, M.S. Association Between Lowering LDL-C and Cardiovascular Risk Reduction Among Different Therapeutic Interventions: A Systematic Review and Meta-Analysis. JAMA 2016, 316, 1289–1297. [Google Scholar] [CrossRef]
- Amarenco, P.; Kim, J.S.; Labreuche, J.; Charles, H.; Abtan, J.; Béjot, Y.; Cabrejo, L.; Cha, J.-K.; Ducrocq, G.; Giroud, M.; et al. A Comparison of Two LDL Cholesterol Targets after Ischemic Stroke. N. Engl. J. Med. 2020, 382, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Guedeney, P.; Claessen, B.E.; Kalkman, D.N.; Aquino, M.; Sorrentino, S.; Giustino, G.; Farhan, S.; Vogel, B.; Sartori, S.; Montalescot, G.; et al. Residual Inflammatory Risk in Patients with Low LDL Cholesterol Levels Undergoing Percutaneous Coronary Intervention. J. Am. Coll. Cardiol. 2019, 73, 2401–2409. [Google Scholar] [CrossRef] [PubMed]
- Peikert, A.; Kaier, K.; Merz, J.; Manhart, L.; Schäfer, I.; Hilgendorf, I.; Hehn, P.; Wolf, D.; Willecke, F.; Sheng, X.; et al. Residual inflammatory risk in coronary heart disease: Incidence of elevated high-sensitive CRP in a real-world cohort. Clin. Res. Cardiol. 2019, 109, 315–323. [Google Scholar] [CrossRef]
- Boekholdt, S.M.; Hovingh, G.K.; Mora, S.; Arsenault, B.J.; Amarenco, P.; Pedersen, T.R.; LaRosa, J.C.; Waters, D.D.; DeMicco, D.A.; Simes, R.J.; et al. Very Low Levels of Atherogenic Lipoproteins and the Risk for Cardiovascular Events: A Meta-Analysis of Statin Trials. J. Am. Coll. Cardiol. 2014, 64, 485–494. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk: The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2018, 73, 3168–3209. [Google Scholar] [CrossRef]
- Aygun, S.; Tokgozoglu, L. Comparison of Current International Guidelines for the Management of Dyslipidemia. J. Clin. Med. 2022, 11, 7249. [Google Scholar] [CrossRef]
- Hsia, J.; MacFadyen, J.G.; Monyak, J.; Ridker, P.M. Cardiovascular Event Reduction and Adverse Events Among Subjects Attaining Low-Density Lipoprotein Cholesterol <50 mg/dL with Rosuvastatin: The JUPITER Trial (Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin). J. Am. Coll. Cardiol. 2011, 57, 1666–1675. [Google Scholar] [CrossRef]
- McCormack, T.; Dent, R.; Blagden, M. Very low LDL-C levels may safely provide additional clinical cardiovascular benefit: The evidence to date. Int. J. Clin. Pract. 2016, 70, 886–897. [Google Scholar] [CrossRef]
- Schubert, J.; Lindahl, B.; Melhus, H.; Renlund, H.; Leosdottir, M.; Yari, A.; Ueda, P.; James, S.; Reading, S.R.; Dluzniewski, P.J.; et al. Low-density lipoprotein cholesterol reduction and statin intensity in myocardial infarction patients and major adverse outcomes: A Swedish nationwide cohort study. Eur. Heart J. 2020, 42, 243–252. [Google Scholar] [CrossRef]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.-M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef]
- Agnello, F.; Mauro, M.S.; Rochira, C.; Landolina, D.; Finocchiaro, S.; Greco, A.; Ammirabile, N.; Raffo, C.; Mazzone, P.M.; Spagnolo, M.; et al. PCSK9 inhibitors: Current status and emerging frontiers in lipid control. Expert Rev. Cardiovasc. Ther. 2023, 1–18. [Google Scholar] [CrossRef]
- Puri, R.; Nicholls, S.J.; Shao, M.; Kataoka, Y.; Uno, K.; Kapadia, S.R.; Tuzcu, E.M.; Nissen, S.E. Impact of Statins on Serial Coronary Calcification During Atheroma Progression and Regression. J. Am. Coll. Cardiol. 2015, 65, 1273–1282. [Google Scholar] [CrossRef]
- Lee, S.-E.; Chang, H.-J.; Sung, J.M.; Park, H.-B.; Heo, R.; Rizvi, A.; Lin, F.Y.; Kumar, A.; Hadamitzky, M.; Kim, Y.J.; et al. Effects of Statins on Coronary Atherosclerotic Plaques: The PARADIGM Study. JACC Cardiovasc. Imaging 2018, 11, 1475–1484. [Google Scholar] [CrossRef]
- Penson, P.E.; Bruckert, E.; Marais, D.; Reiner, Ž.; Pirro, M.; Sahebkar, A.; Bajraktari, G.; Mirrakhimov, E.; Rizzo, M.; Mikhailidis, D.P.; et al. Step-by-step diagnosis and management of the nocebo/drucebo effect in statin-associated muscle symptoms patients: A position paper from the International Lipid Expert Panel(ILEP). J. Cachex- Sarcopenia Muscle 2022, 13, 1596–1622. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Hong, S.-J.; Kang, W.C.; Hong, B.-K.; Lee, J.-Y.; Lee, J.-B.; Cho, H.-J.; Yoon, J.; Lee, S.-J.; Ahn, C.-M.; et al. Rosuvastatin versus atorvastatin treatment in adults with coronary artery disease: Secondary analysis of the randomised LODESTAR trial. BMJ 2023, 383, e075837. [Google Scholar] [CrossRef]
- Toth, P.P.; Phan, B.A.P.; Dayspring, T.D. Ezetimibe therapy: Mechanism of action and clinical update. Vasc. Health Risk Manag. 2012, 8, 415–427. [Google Scholar] [CrossRef]
- Cannon, C.P.; Blazing, M.A.; Giugliano, R.P.; McCagg, A.; White, J.A.; Théroux, P.; Darius, H.; Lewis, B.S.; Ophuis, T.O.; Jukema, J.W.; et al. Ezetimibe Added to Statin Therapy after Acute Coronary Syndromes. N. Engl. J. Med. 2015, 372, 2387–2397. [Google Scholar] [CrossRef]
- Morrone, D.; Weintraub, W.S.; Toth, P.P.; Hanson, M.E.; Lowe, R.S.; Lin, J.; Shah, A.K.; Tershakovec, A.M. Lipid-altering efficacy of ezetimibe plus statin and statin monotherapy and identification of factors associated with treatment response: A pooled analysis of over 21,000 subjects from 27 clinical trials. Atherosclerosis 2012, 223, 251–261. [Google Scholar] [CrossRef]
- Ballantyne, C.M.; Blazing, M.A.; King, T.R.; Brady, W.E.; Palmisano, J. Efficacy and safety of ezetimibe co-administered with simvastatin compared with atorvastatin in adults with hypercholesterolemia. Am. J. Cardiol. 2004, 93, 1487–1494. [Google Scholar] [CrossRef]
- Horton, J.D.; Cohen, J.C.; Hobbs, H.H. PCSK9: A convertase that coordinates LDL catabolism. J. Lipid Res. 2009, 50, S172–S177. [Google Scholar] [CrossRef]
- de Isla, L.P.; Díaz-Díaz, J.L.; Romero, M.J.; Muñiz-Grijalvo, O.; Mediavilla, J.D.; Argüeso, R.; Muñoz-Torrero, J.F.S.; Rubio, P.; Álvarez-Baños, P.; Ponte, P.; et al. Alirocumab and Coronary Atherosclerosis in Asymptomatic Patients with Familial Hypercholesterolemia: The ARCHITECT Study. Circulation 2023, 147, 1436–1443. [Google Scholar] [CrossRef]
- Räber, L.; Ueki, Y.; Otsuka, T.; Losdat, S.; Häner, J.D.; Lonborg, J.; Fahrni, G.; Iglesias, J.F.; van Geuns, R.-J.; Ondracek, A.S.; et al. Effect of Alirocumab Added to High-Intensity Statin Therapy on Coronary Atherosclerosis in Patients with Acute Myocardial Infarction: The PACMAN-AMI Randomized Clinical Trial. JAMA 2022, 327, 1771–1781. [Google Scholar] [CrossRef]
- Grześk, G.; Dorota, B.; Wołowiec, Ł.; Wołowiec, A.; Osiak, J.; Kozakiewicz, M.; Banach, J. Safety of PCSK9 inhibitors. Biomed. Pharmacother. 2022, 156, 113957. [Google Scholar] [CrossRef]
- Feng, X.; Zhang, L.; Xu, S.; Shen, A.-Z. ATP-citrate lyase (ACLY) in lipid metabolism and atherosclerosis: An updated review. Prog. Lipid Res. 2019, 77, 101006. [Google Scholar] [CrossRef]
- Goldberg, A.C.; Leiter, L.A.; Stroes, E.S.G.; Baum, S.J.; Hanselman, J.C.; Bloedon, L.T.; Lalwani, N.D.; Patel, P.M.; Zhao, X.; Duell, P.B. Effect of Bempedoic Acid vs Placebo Added to Maximally Tolerated Statins on Low-Density Lipoprotein Cholesterol in Patients at High Risk for Cardiovascular Disease: The CLEAR Wisdom Randomized Clinical Trial. JAMA 2019, 322, 1780–1788. [Google Scholar] [CrossRef]
- Nissen, S.E.; Lincoff, A.M.; Brennan, D.; Ray, K.K.; Mason, D.; Kastelein, J.J.; Thompson, P.D.; Libby, P.; Cho, L.; Plutzky, J.; et al. Bempedoic Acid and Cardiovascular Outcomes in Statin-Intolerant Patients. N. Engl. J. Med. 2023, 388, 1353–1364. [Google Scholar] [CrossRef]
- Laufs, U.; Ballantyne, C.M.; Banach, M.; Bays, H.; Catapano, A.L.; Duell, P.B.; Goldberg, A.C.; Gotto, A.M.; Leiter, L.A.; Ray, K.K.; et al. Efficacy and safety of bempedoic acid in patients not receiving statins in phase 3 clinical trials. J. Clin. Lipidol. 2022, 16, 286–297. [Google Scholar] [CrossRef]
- Ray, K.K.; Bays, H.E.; Catapano, A.L.; Lalwani, N.D.; Bloedon, L.T.; Sterling, L.R.; Robinson, P.L.; Ballantyne, C.M. Safety and Efficacy of Bempedoic Acid to Reduce LDL Cholesterol. N. Engl. J. Med. 2019, 380, 1022–1032. [Google Scholar] [CrossRef]
- Ballantyne, C.M.; Laufs, U.; Ray, K.K.; Leiter, L.A.; Bays, H.E.; Goldberg, A.C.; Stroes, E.S.; MacDougall, D.; Zhao, X.; Catapano, A.L. Bempedoic acid plus ezetimibe fixed-dose combination in patients with hypercholesterolemia and high CVD risk treated with maximally tolerated statin therapy. Eur. J. Prev. Cardiol. 2019, 27, 593–603. [Google Scholar] [CrossRef]
- Ray, K.K.; Nicholls, S.J.; Li, N.; Louie, M.J.; Brennan, D.; Lincoff, A.M.; Nissen, S.E. Efficacy and safety of bempedoic acid among patients with and without diabetes: Prespecified analysis of the CLEAR Outcomes randomised trial. Lancet Diabetes Endocrinol. 2023, 12, 19–28. [Google Scholar] [CrossRef]
- Ballantyne, C.M.; Banach, M.; Bays, H.E.; Catapano, A.L.; Laufs, U.; Stroes, E.S.; Robinson, P.; Lei, L.; Ray, K.K. Long-Term Safety and Efficacy of Bempedoic Acid in Patients with Atherosclerotic Cardiovascular Disease and/or Heterozygous Familial Hypercholesterolemia (from the CLEAR Harmony Open-Label Extension Study). Am. J. Cardiol. 2022, 174, 1–11. [Google Scholar] [CrossRef]
- Lloyd-Jones, D.M.; Morris, P.B.; Ballantyne, C.M.; Birtcher, K.K.; Covington, A.M.; DePalma, S.M.; Minissian, M.B.; Orringer, C.E.; Smith, S.C.; Waring, A.A.; et al. 2022 ACC Expert Consensus Decision Pathway on the Role of Nonstatin Therapies for LDL-Cholesterol Lowering in the Management of Atherosclerotic Cardiovascular Disease Risk: A Report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll. Cardiol. 2022, 80, 1366–1418. [Google Scholar] [CrossRef]
- Basiak, M.; Hachula, M.; Kosowski, M.; Okopien, B. Effect of PCSK9 Inhibitors on Hemostasis in Patients with Isolated Hypercholesterolemia. J. Clin. Med. 2022, 11, 2542. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, K.; White, S.; Borodovsky, A.; Bettencourt, B.R.; Strahs, A.; Clausen, V.; Wijngaard, P.; Horton, J.D.; Taubel, J.; Brooks, A.; et al. A Highly Durable RNAi Therapeutic Inhibitor of PCSK9. N. Engl. J. Med. 2017, 376, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Ray, K.K.; Landmesser, U.; Leiter, L.A.; Kallend, D.; Dufour, R.; Karakas, M.; Hall, T.; Troquay, R.P.; Turner, T.; Visseren, F.L.; et al. Inclisiran in Patients at High Cardiovascular Risk with Elevated LDL Cholesterol. N. Engl. J. Med. 2017, 376, 1430–1440. [Google Scholar] [CrossRef] [PubMed]
- Ray, K.K.; Wright, R.S.; Kallend, D.; Koenig, W.; Leiter, L.A.; Raal, F.J.; Bisch, J.A.; Richardson, T.; Jaros, M.; Wijngaard, P.L.; et al. Two Phase 3 Trials of Inclisiran in Patients with Elevated LDL Cholesterol. N. Engl. J. Med. 2020, 382, 1507–1519. [Google Scholar] [CrossRef] [PubMed]
- Ray, K.K.; Troquay, R.P.T.; Visseren, F.L.J.; Leiter, L.A.; Wright, R.S.; Vikarunnessa, S.; Talloczy, Z.; Zang, X.; Maheux, P.; Lesogor, A.; et al. Long-term efficacy and safety of inclisiran in patients with high cardiovascular risk and elevated LDL cholesterol (ORION-3): Results from the 4-year open-label extension of the ORION-1 trial. Lancet Diabetes Endocrinol. 2023, 11, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Raal, F.J.; Kallend, D.; Ray, K.K.; Turner, T.; Koenig, W.; Wright, R.S.; Wijngaard, P.L.; Curcio, D.; Jaros, M.J.; Leiter, L.A.; et al. Inclisiran for the Treatment of Heterozygous Familial Hypercholesterolemia. N. Engl. J. Med. 2020, 382, 1520–1530. [Google Scholar] [CrossRef] [PubMed]
- Raal, F.; Durst, R.; Bi, R.; Talloczy, Z.; Maheux, P.; Lesogor, A.; Kastelein, J.J. Efficacy, Safety, and Tolerability of Inclisiran in Patients with Homozygous Familial Hypercholesterolemia: Results from the ORION-5 Randomized Clinical Trial. Circulation 2024, 149, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Gareri, C.; Polimeni, A.; Giordano, S.; Tammè, L.; Curcio, A.; Indolfi, C. Antisense Oligonucleotides and Small Interfering RNA for the Treatment of Dyslipidemias. J. Clin. Med. 2022, 11, 3884. [Google Scholar] [CrossRef] [PubMed]
- Graham, M.J.; Lemonidis, K.M.; Whipple, C.P.; Subramaniam, A.; Monia, B.P.; Crooke, S.T.; Crooke, R.M. Antisense inhibition of proprotein convertase subtilisin/kexin type 9 reduces serum LDL in hyperlipidemic mice. J. Lipid Res. 2007, 48, 763–767. [Google Scholar] [CrossRef] [PubMed]
- van Poelgeest, E.P.; Swart, R.M.; Betjes, M.G.; Moerland, M.; Weening, J.J.; Tessier, Y.; Hodges, M.R.; Levin, A.A.; Burggraaf, J. Acute Kidney Injury During Therapy with an Antisense Oligonucleotide Directed Against PCSK9. Am. J. Kidney Dis. 2013, 62, 796–800. [Google Scholar] [CrossRef]
- Knöchel, J.; Nilsson, C.; Carlsson, B.; Wernevik, L.; Hofherr, A.; Gennemark, P.; Jansson-Löfmark, R.; Isaksson, R.; Rydén-Bergsten, T.; Hamrén, B.; et al. A case-study of model-informed drug development of a novel PCSK9 anti sense oligonucleotide. Part 1: First time in man to phase II. CPT Pharmacomet. Syst. Pharmacol. 2022, 11, 1569–1577. [Google Scholar] [CrossRef]
- Ma, N.; Fan, L.; Dong, Y.; Xu, X.; Yu, C.; Chen, J.; Ren, J. New PCSK9 inhibitor miR-552-3p reduces LDL-C via enhancing LDLR in high fat diet-fed mice. Pharmacol. Res. 2021, 167, 105562. [Google Scholar] [CrossRef]
- Khoshnejad, M.; Patel, A.; Wojtak, K.; Kudchodkar, S.B.; Humeau, L.; Lyssenko, N.N.; Rader, D.J.; Muthumani, K.; Weiner, D.B. Development of Novel DNA-Encoded PCSK9 Monoclonal Antibodies as Lipid-Lowering Therapeutics. Mol. Ther. 2018, 27, 188–199. [Google Scholar] [CrossRef]
- Ballantyne, C.M.; Banka, P.; Mendez, G.; Garcia, R.; Rosenstock, J.; Rodgers, A.; Mendizabal, G.; Mitchel, Y.; Catapano, A.L. Phase 2b Randomized Trial of the Oral PCSK9 Inhibitor MK-0616. J. Am. Coll. Cardiol. 2023, 81, 1553–1564. [Google Scholar] [CrossRef] [PubMed]
- Dixon, D.L.; Trankle, C.; Buckley, L.; Parod, E.; Carbone, S.; Van Tassell, B.W.; Abbate, A. A review of PCSK9 inhibition and its effects beyond LDL receptors. J. Clin. Lipidol. 2016, 10, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.G.; Mazzola, A.M.; Braun, M.C.; Platt, C.; Vafai, S.B.; Kathiresan, S.; Rohde, E.; Bellinger, A.M.; Khera, A.V. Efficacy and Safety of an Investigational Single-Course CRISPR Base-Editing Therapy Targeting PCSK9 in Nonhuman Primate and Mouse Models. Circulation 2023, 147, 242–253. [Google Scholar] [CrossRef] [PubMed]
- VERVE-101: CRISPR-Based Gene Editing Therapy Shows Promise in Reducing LDL-C and PCSK9 Levels in Patients with HeFH—American College of Cardiology. Available online: https://www.acc.org/Latest-in-Cardiology/Articles/2023/11/08/20/14/sun-445pm-heart1-aha-2023 (accessed on 10 December 2023).
- Toth, S.; Pella, D.; Fedacko, J. Vaccines Targeting PSCK9 for the Treatment of Hyperlipidemia. Cardiol. Ther. 2020, 9, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Galabova, G.; Brunner, S.; Winsauer, G.; Juno, C.; Wanko, B.; Mairhofer, A.; Lührs, P.; Schneeberger, A.; von Bonin, A.; Mattner, F.; et al. Peptide-Based Anti-PCSK9 Vaccines—An Approach for Long-Term LDLc Management. PLoS ONE 2014, 9, e114469. [Google Scholar] [CrossRef] [PubMed]
- Kobiyama, K.; Saigusa, R.; Ley, K. Vaccination against atherosclerosis. Curr. Opin. Immunol. 2019, 59, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Sahebkar, A.; Momtazi-Borojeni, A.A.; Banach, M. PCSK9 vaccine: So near, yet so far! Eur. Heart J. 2021, 42, 4007–4010. [Google Scholar] [CrossRef] [PubMed]
- Landlinger, C.; Pouwer, M.G.; Juno, C.; Van Der Hoorn, J.W.; Pieterman, E.J.; Jukema, J.W.; Staffler, G.; Princen, H.M.; Galabova, G. The AT04A vaccine against proprotein convertase subtilisin/kexin type 9 reduces total cholesterol, vascular inflammation, and atherosclerosis in APOE*3Leiden.CETP mice. Eur. Heart J. 2017, 38, 2499–2507. [Google Scholar] [CrossRef]
- Momtazi-Borojeni, A.A.; Jaafari, M.R.; Banach, M.; Gorabi, A.M.; Sahraei, H.; Sahebkar, A. Pre-Clinical Evaluation of the Nanoliposomal antiPCSK9 Vaccine in Healthy Non-Human Primates. Vaccines 2021, 9, 749. [Google Scholar] [CrossRef]
- Gaudet, D.; Gipe, D.A.; Pordy, R.; Ahmad, Z.; Cuchel, M.; Shah, P.K.; Chyu, K.-Y.; Sasiela, W.J.; Chan, K.-C.; Brisson, D.; et al. ANGPTL3 Inhibition in Homozygous Familial Hypercholesterolemia. N. Engl. J. Med. 2017, 377, 296–297. [Google Scholar] [CrossRef]
- Rosenson, R.S.; Burgess, L.J.; Ebenbichler, C.F.; Baum, S.J.; Stroes, E.S.; Ali, S.; Khilla, N.; Hamlin, R.; Pordy, R.; Dong, Y.; et al. Evinacumab in Patients with Refractory Hypercholesterolemia. N. Engl. J. Med. 2020, 383, 2307–2319. [Google Scholar] [CrossRef]
- Rosenson, R.S.; Burgess, L.J.; Ebenbichler, C.F.; Baum, S.J.; Stroes, E.S.G.; Ali, S.; Khilla, N.; McGinniss, J.; Gaudet, D.; Pordy, R. Longer-Term Efficacy and Safety of Evinacumab in Patients with Refractory Hypercholesterolemia. JAMA Cardiol. 2023, 8, 1070–1076. [Google Scholar] [CrossRef]
- Raal, F.J.; Rosenson, R.S.; Reeskamp, L.F.; Hovingh, G.K.; Kastelein, J.J.; Rubba, P.; Ali, S.; Banerjee, P.; Chan, K.-C.; Gipe, D.A.; et al. Evinacumab for Homozygous Familial Hypercholesterolemia. N. Engl. J. Med. 2020, 383, 711–720. [Google Scholar] [CrossRef]
- Raal, F.J.; Rosenson, R.S.; Reeskamp, L.F.; Kastelein, J.J.; Rubba, P.; Duell, P.B.; Koseki, M.; Stroes, E.; Ali, S.; Banerjee, P.; et al. The Long-Term Efficacy and Safety of Evinacumab in Patients with Homozygous Familial Hypercholesterolemia. JACC Adv. 2023, 2, 100648. [Google Scholar] [CrossRef]
- Wiegman, A.; Greber-Platzer, S.; Ali, S.; Reijman, M.D.; Brinton, E.A.; Charng, M.-J.; Srinivasan, S.; Baker-Smith, C.; Baum, S.; Brothers, J.A.; et al. Evinacumab for Pediatric Patients with Homozygous Familial Hypercholesterolemia. Circulation 2024, 149, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Graham, M.J.; Lee, R.G.; Brandt, T.A.; Tai, L.-J.; Fu, W.; Peralta, R.; Yu, R.; Hurh, E.; Paz, E.; McEvoy, B.W.; et al. Cardiovascular and Metabolic Effects of ANGPTL3 Antisense Oligonucleotides. N. Engl. J. Med. 2017, 377, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Gaudet, D.; Karwatowska-Prokopczuk, E.; Baum, S.J.; Hurh, E.; Kingsbury, J.; Bartlett, V.J.; Figueroa, A.L.; Piscitelli, P.; Singleton, W.; Witztum, J.L.; et al. Vupanorsen, an N-acetyl galactosamine-conjugated antisense drug to ANGPTL3 mRNA, lowers triglycerides and atherogenic lipoproteins in patients with diabetes, hepatic steatosis, and hypertriglyceridaemia. Eur. Heart J. 2020, 41, 3936–3945. [Google Scholar] [CrossRef] [PubMed]
- Bergmark, B.A.; Marston, N.A.; Bramson, C.R.; Curto, M.; Ramos, V.; Jevne, A.; Kuder, J.F.; Park, J.-G.; Murphy, S.A.; Verma, S.; et al. Effect of Vupanorsen on Non–High-Density Lipoprotein Cholesterol Levels in Statin-Treated Patients with Elevated Cholesterol: TRANSLATE-TIMI 70. Circulation 2022, 145, 1377–1386. [Google Scholar] [CrossRef] [PubMed]
- Pfizer and Lonis Announce Discontinuation of Vupanorsen Clinical Development Program|Pfizer. Available online: https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-ionis-announce-discontinuation-vupanorsen (accessed on 3 December 2023).
- Watts, G.F.; Schwabe, C.; Scott, R.; Gladding, P.; Sullivan, D.; Baker, J.; Clifton, P.; Hamilton, J.; Given, B.; Martin, J.S.; et al. Abstract 15751: Pharmacodynamic Effect of ARO-ANG3, an Investigational RNA Interference Targeting Hepatic Angiopoietin-like Protein 3, in Patients with Hypercholesterolemia. Circulation 2020, 142, A15751. [Google Scholar] [CrossRef]
- Watts, G.F.; Gaudet, D.; Altamirano, D.; Hegele, R.; Ballantyne, C.M.; Nicholls, S.; Chang, T.; Alagarsamy, S.; Fu, R.; Martin, J.S.; et al. Abstract 17120: ARO-ANG3, an Investigational RNAi Therapeutic, Silences the Expression of ANGPTL3 and Decreases Atherogenic Lipoproteins in Patients with Mixed Dyslipidemia: ARCHES-2 Study Results. Circulation 2023, 148, A17120. [Google Scholar] [CrossRef]
- Inazu, A.; Jiang, X.C.; Haraki, T.; Yagi, K.; Kamon, N.; Koizumi, J.; Mabuchi, H.; Takeda, R.; Takata, K.; Moriyama, Y. Genetic cholesteryl ester transfer protein deficiency caused by two prevalent mutations as a major determinant of increased levels of high density lipoprotein cholesterol. J. Clin. Investig. 1994, 94, 1872–1882. [Google Scholar] [CrossRef]
- Inazu, A.; Brown, M.L.; Hesler, C.B.; Agellon, L.B.; Koizumi, J.; Takata, K.; Maruhama, Y.; Mabuchi, H.; Tall, A.R. Increased High-Density Lipoprotein Levels Caused by a Common Cholesteryl-Ester Transfer Protein Gene Mutation. N. Engl. J. Med. 1990, 323, 1234–1238. [Google Scholar] [CrossRef] [PubMed]
- Barter, P.J.; Caulfield, M.; Eriksson, M.; Grundy, S.M.; Kastelein, J.J.P.; Komajda, M.; Lopez-Sendon, J.; Mosca, L.; Tardif, J.-C.; Waters, D.D.; et al. Effects of Torcetrapib in Patients at High Risk for Coronary Events. N. Engl. J. Med. 2007, 357, 2109–2122. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Dietz, J.D.; Xia, C.; Knight, D.R.; Loging, W.T.; Smith, A.H.; Yuan, H.; Perry, D.A.; Keiser, J. Torcetrapib Induces Aldosterone and Cortisol Production by an Intracellular Calcium-Mediated Mechanism Independently of Cholesteryl Ester Transfer Protein Inhibition. Endocrinology 2009, 150, 2211–2219. [Google Scholar] [CrossRef] [PubMed]
- Simic, B.; Hermann, M.; Shaw, S.G.; Bigler, L.; Stalder, U.; Dörries, C.; Besler, C.; Lüscher, T.F.; Ruschitzka, F. Torcetrapib impairs endothelial function in hypertension. Eur. Heart J. 2011, 33, 1615–1624. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, G.G.; Olsson, A.G.; Abt, M.; Ballantyne, C.M.; Barter, P.J.; Brumm, J.; Chaitman, B.R.; Holme, I.M.; Kallend, D.; Leiter, L.A.; et al. Effects of Dalcetrapib in Patients with a Recent Acute Coronary Syndrome. N. Engl. J. Med. 2012, 367, 2089–2099. [Google Scholar] [CrossRef] [PubMed]
- Lincoff, A.M.; Nicholls, S.J.; Riesmeyer, J.S.; Barter, P.J.; Brewer, H.B.; Fox, K.A.A.; Gibson, C.M.; Granger, C.; Menon, V.; Montalescot, G.; et al. Evacetrapib and Cardiovascular Outcomes in High-Risk Vascular Disease. N. Engl. J. Med. 2017, 376, 1933–1942. [Google Scholar] [CrossRef] [PubMed]
- The HPS3/TIMI55–REVEAL Collaborative Group. Effects of Anacetrapib in Patients with Atherosclerotic Vascular Disease. N. Engl. J. Med. 2017, 377, 1217–1227. [Google Scholar] [CrossRef]
- Ballantyne, C.M.; Ditmarsch, M.; Kastelein, J.J.; Nelson, A.J.; Kling, D.; Hsieh, A.; Curcio, D.L.; Maki, K.C.; Davidson, M.H.; Nicholls, S.J. Obicetrapib plus ezetimibe as an adjunct to high-intensity statin therapy: A randomized phase 2 trial. J. Clin. Lipidol. 2023, 17, 491–503. [Google Scholar] [CrossRef]
- Cohen, J.C.; Boerwinkle, E.; Mosley, T.H.; Hobbs, H.H. Sequence Variations in PCSK9, Low LDL, and Protection against Coronary Heart Disease. N. Engl. J. Med. 2006, 354, 1264–1272. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, G.G.; Steg, P.G.; Szarek, M.; Bhatt, D.L.; Bittner, V.A.; Diaz, R.; Edelberg, J.M.; Goodman, S.G.; Hanotin, C.; Harrington, R.A.; et al. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome. N. Engl. J. Med. 2018, 379, 2097–2107. [Google Scholar] [CrossRef]
- Sabatine, M.S.; Giugliano, R.P.; Keech, A.C.; Honarpour, N.; Wiviott, S.D.; Murphy, S.A.; Kuder, J.F.; Wang, H.; Liu, T.; Wasserman, S.M.; et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N. Engl. J. Med. 2017, 376, 1713–1722. [Google Scholar] [CrossRef]
- O’donoghue, M.L.; Giugliano, R.P.; Wiviott, S.D.; Atar, D.; Keech, A.C.; Kuder, J.F.; Im, K.; Murphy, S.A.; Flores-Arredondo, J.H.; López, J.A.G.; et al. Long-Term Evolocumab in Patients with Established Atherosclerotic Cardiovascular Disease. Circulation 2022, 146, 1109–1119. [Google Scholar] [CrossRef] [PubMed]
- Ference, B.A.; Ginsberg, H.N.; Graham, I.; Ray, K.K.; Packard, C.J.; Bruckert, E.; Hegele, R.A.; Krauss, R.M.; Raal, F.J.; Schunkert, H.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2017, 38, 2459–2472. [Google Scholar] [CrossRef]
- Ray, K.K.; Haq, I.; Bilitou, A.; Manu, M.C.; Burden, A.; Aguiar, C.; Arca, M.; Connolly, D.L.; Eriksson, M.; Ferrières, J.; et al. Treatment gaps in the implementation of LDL cholesterol control among high- and very high-risk patients in Europe between 2020 and 2021: The multinational observational SANTORINI study. Lancet Reg. Health Eur. 2023, 29, 100624. [Google Scholar] [CrossRef] [PubMed]
- Ray, K.K.; Reeskamp, L.F.; Laufs, U.; Banach, M.; Mach, F.; Tokgözoğlu, L.S.; Connolly, D.L.; Gerrits, A.J.; Stroes, E.S.G.; Masana, L.; et al. Combination lipid-lowering therapy as first-line strategy in very high-risk patients. Eur. Heart J. 2022, 43, 830–833. [Google Scholar] [CrossRef]
- Agnello, F.; Finocchiaro, S.; Laudani, C.; Legnazzi, M.; Mauro, M.S.; Rochira, C.; Scalia, L.; Capodanno, D. A review of polypills for the prevention of atherosclerotic cardiovascular disease. Am. Heart J. 2023, 266, 74–85. [Google Scholar] [CrossRef]
- Siniawski, D.; Masson, G.; Masson, W.; Barbagelata, L.; Destaville, J.; Lynch, S.; Vitagliano, L.; Parodi, J.B.; Berton, F.; Indavere, A.; et al. Residual cardiovascular risk, use of standard care treatments, and achievement of treatment goals in patients with cardiovascular disease. Int. J. Cardiol. Cardiovasc. Risk Prev. 2023, 18, 200198. [Google Scholar] [CrossRef]
- Yang, L.; Yue, Q.; Fang, F.; Zhang, Y.; Liu, P.; Zhang, Z.; Wang, G.; Chen, S.; Wu, S.; Yang, X. Effect of dual residual risk of cholesterol and inflammation on all-cause mortality in patients with cardiovascular disease. Cardiovasc. Diabetol. 2023, 22, 96. [Google Scholar] [CrossRef]
- Greco, A.; Scilletta, S.; Faro, D.C.; Agnello, F.; Mauro, M.S.; Laudani, C.; Occhipinti, G.; Spagnolo, M.; Rochira, C.; Finocchiaro, S.; et al. Eligibility to Intensified Antithrombotic Regimens for Secondary Prevention in Patients Who Underwent Percutaneous Coronary Intervention. Am. J. Cardiol. 2023, 199, 7–17. [Google Scholar] [CrossRef] [PubMed]
| Drug | Mechanism of Action | Administration | Strength | Weakness | Adverse Reaction |
|---|---|---|---|---|---|
| Statin | Inhibition of HMG-CoA reductase | Oral tablets, once per day | LDL-C reduction ≥ 50% Strong evidence for ASCVD risk reduction Available as generic Available in FDC and polypill | Low adherence Muscle toxicity Nocebo effect | Muscle toxicity (myalgia, myopathy, myositis, rhabdomyolysis), transaminase elevation with rare risk for liver toxicity, risk for new-onset diabetes |
| Ezetimibe | Cholesterol absorption inhibitor | Oral tablets, once per day | Moderate evidence of secondary prevention Well tolerated Available as generic Available in FDC | Modest LDL-C reduction | Arthralgia, diarrhea, URTI |
| Evolocumab Alirocumab | Humanized monoclonal antibody against PCSK9 | Subcutaneous injection, once a month | LDL reduction ≈60% Strong evidence for ASCVD risk reduction | High cost Injection | Injection-site reactions, nasopharyngitis, influenza |
| Inclisiran | siRNA against PCK9 mRNA | Subcutaneous injection, once on six months | LDL-C reduction ≥50% | High cost Injection No ASCVD outcomes | Injection-site reactions, arthralgia, UTI, diarrhea, bronchitis, pain in extremity, dyspnea |
| Evinacumab | Humanized monoclonal antibody against ANGPTL3 | Endovenous infusion, once a month | +Statins: 47% (currently only approved for HoFH) | High cost Injection | Flu-like symptoms, nasopharyngitis, dizziness, rhinorrhea, nausea |
| Bempedoic Acid | ACL inhibitor | Oral tablets, once per day | Well tolerated Available in fixed-dose combination | Modest LDL-C reduction | Hyperuricemia, gout, cholelithiasis, URTI, muscle spasms, back pain, abdominal pain, pain in extremity, bronchitis, anemia, elevated liver enzymes |
| Target | Strategy | Agent | Phase | LDL Reduction | Administration |
|---|---|---|---|---|---|
| PCSK9 | ASO | AZD 8233 | II | 68% | Subcutaneous injection, once a month Oral potential |
| Adnectine | LIB003 | II | ≥50% | Subcutaneous injection, once a month | |
| Cyclic peptide | MK-0616 | II | 60.9% | Oral tablets, once per day | |
| Small molecules | NYXPCSK9i | I | 57% total cholesterol | Oral tablets, once per day | |
| Vaccine | epitope vaccine AT04A | I | 13% | Subcutaneous injection, once a year | |
| CRISP-Cas 9 | VERVE-101 | I | 60% | For life | |
| ANGPTL3 | ASO | AKCEA-ANGPTL3-RX | I–II | 33% | Subcutaneous injection, once a month. |
| siRNA | ARO-ANG3 | I | 42% | Subcutaneous injection, once a month | |
| CETP | CETP inhibition | Obicetrapib | II | 45% | Oral tablets, once per day |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agnello, F.; Ingala, S.; Laterra, G.; Scalia, L.; Barbanti, M. Novel and Emerging LDL-C Lowering Strategies: A New Era of Dyslipidemia Management. J. Clin. Med. 2024, 13, 1251. https://doi.org/10.3390/jcm13051251
Agnello F, Ingala S, Laterra G, Scalia L, Barbanti M. Novel and Emerging LDL-C Lowering Strategies: A New Era of Dyslipidemia Management. Journal of Clinical Medicine. 2024; 13(5):1251. https://doi.org/10.3390/jcm13051251
Chicago/Turabian StyleAgnello, Federica, Salvatore Ingala, Giulia Laterra, Lorenzo Scalia, and Marco Barbanti. 2024. "Novel and Emerging LDL-C Lowering Strategies: A New Era of Dyslipidemia Management" Journal of Clinical Medicine 13, no. 5: 1251. https://doi.org/10.3390/jcm13051251
APA StyleAgnello, F., Ingala, S., Laterra, G., Scalia, L., & Barbanti, M. (2024). Novel and Emerging LDL-C Lowering Strategies: A New Era of Dyslipidemia Management. Journal of Clinical Medicine, 13(5), 1251. https://doi.org/10.3390/jcm13051251






