Transient Neonatal Myasthenia Gravis as a Common Complication of a Rare Disease: A Systematic Review
Abstract
1. Introduction
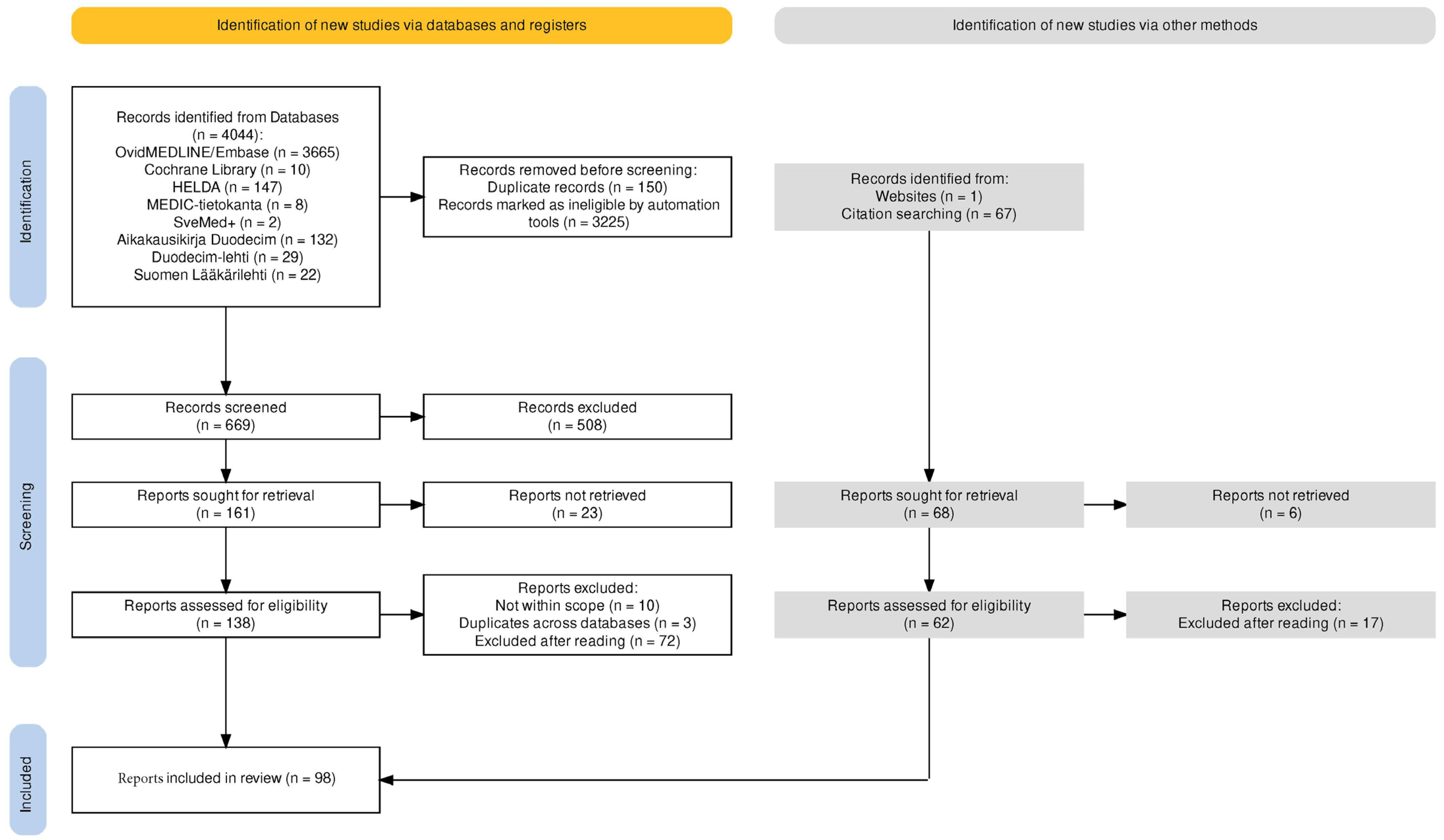
2. Methods
3. Disease Concept
3.1. Classification
3.2. Epidemiology
3.3. Clinical Presentation and Evaluation
3.3.1. Symptoms
3.3.2. Prognosis
3.4. Long-Term Outcome and Persistent Myopathic Sequela
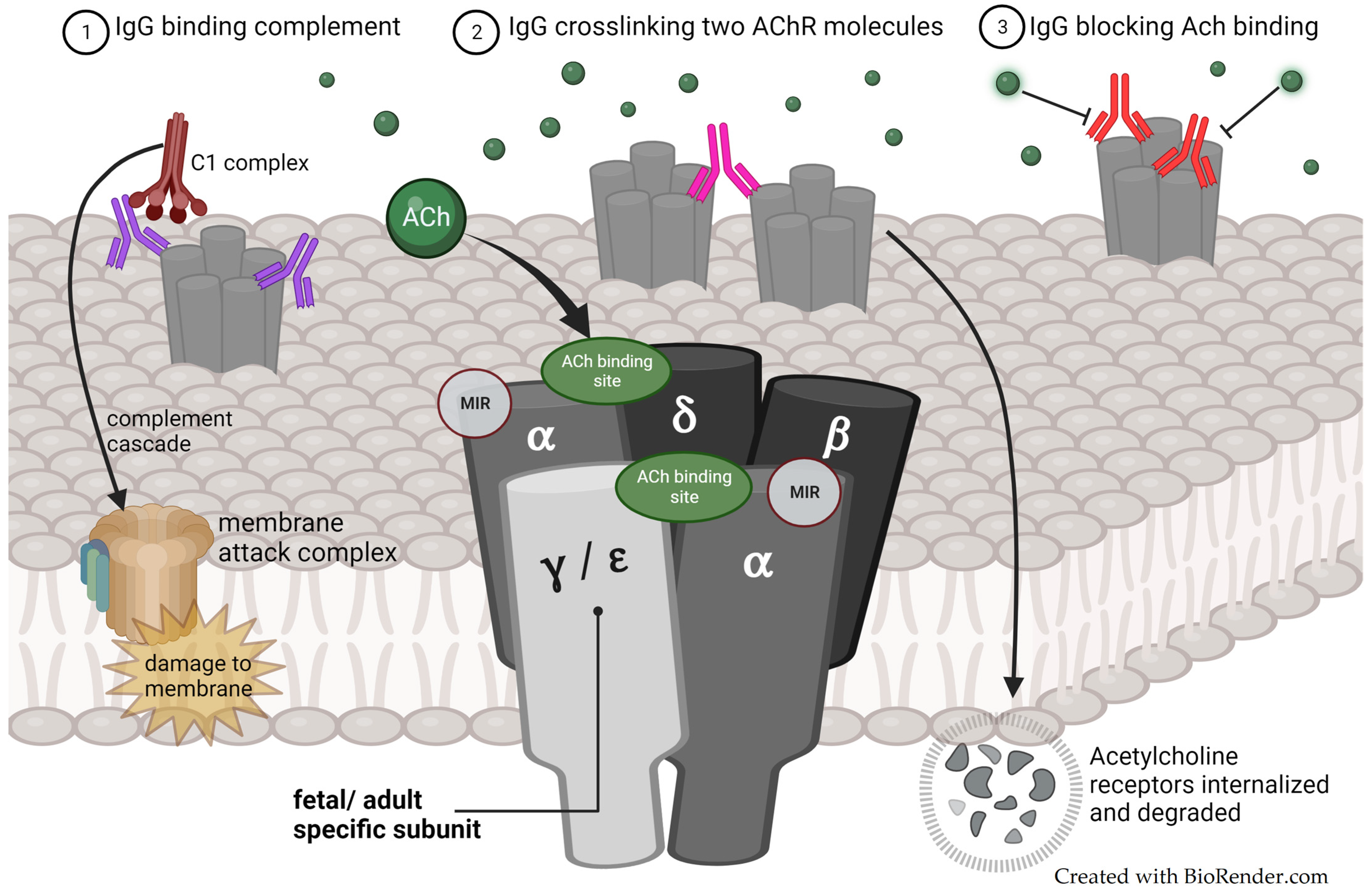
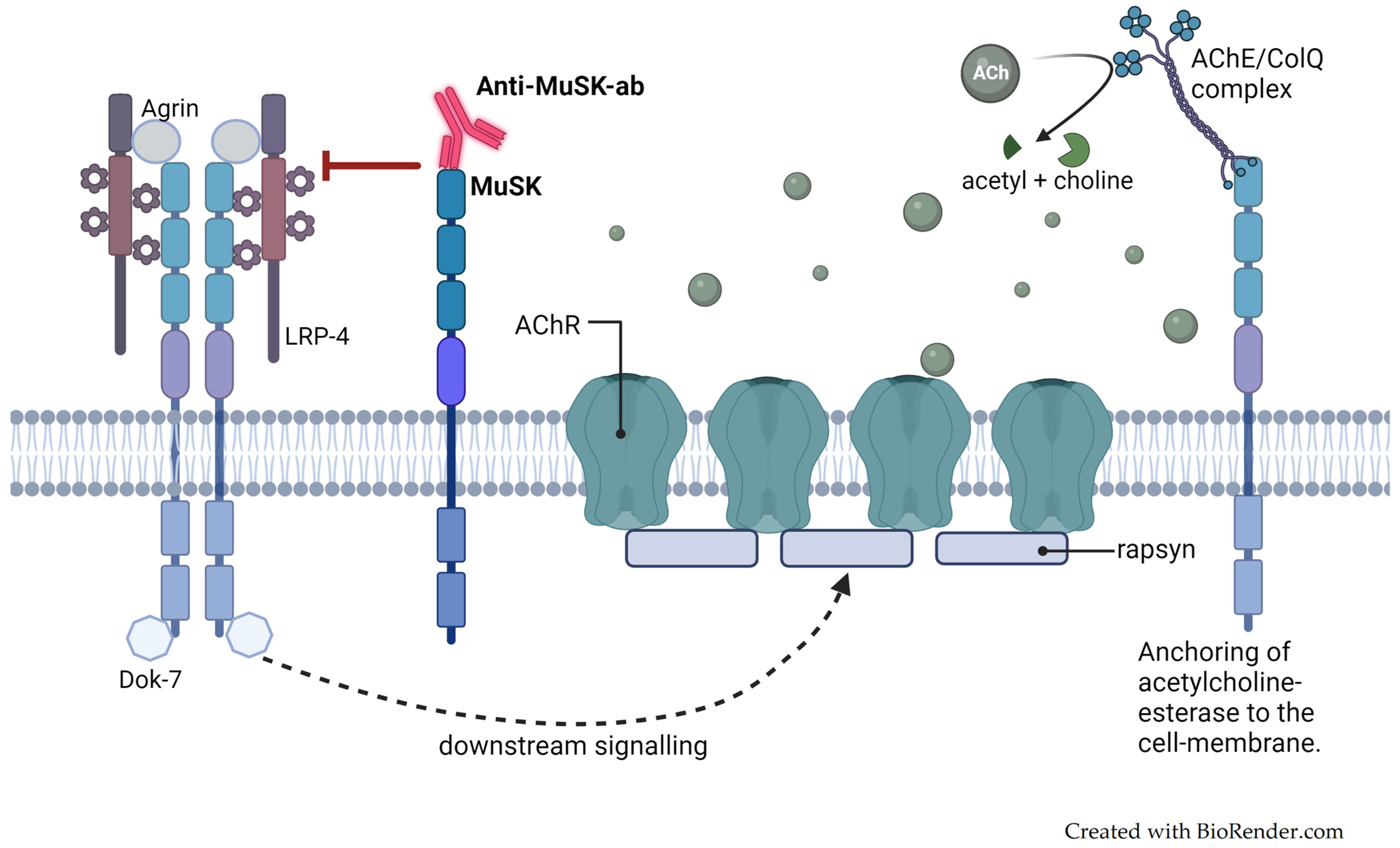
3.5. Pathophysiology
3.5.1. Maternally Produced Autoantibodies
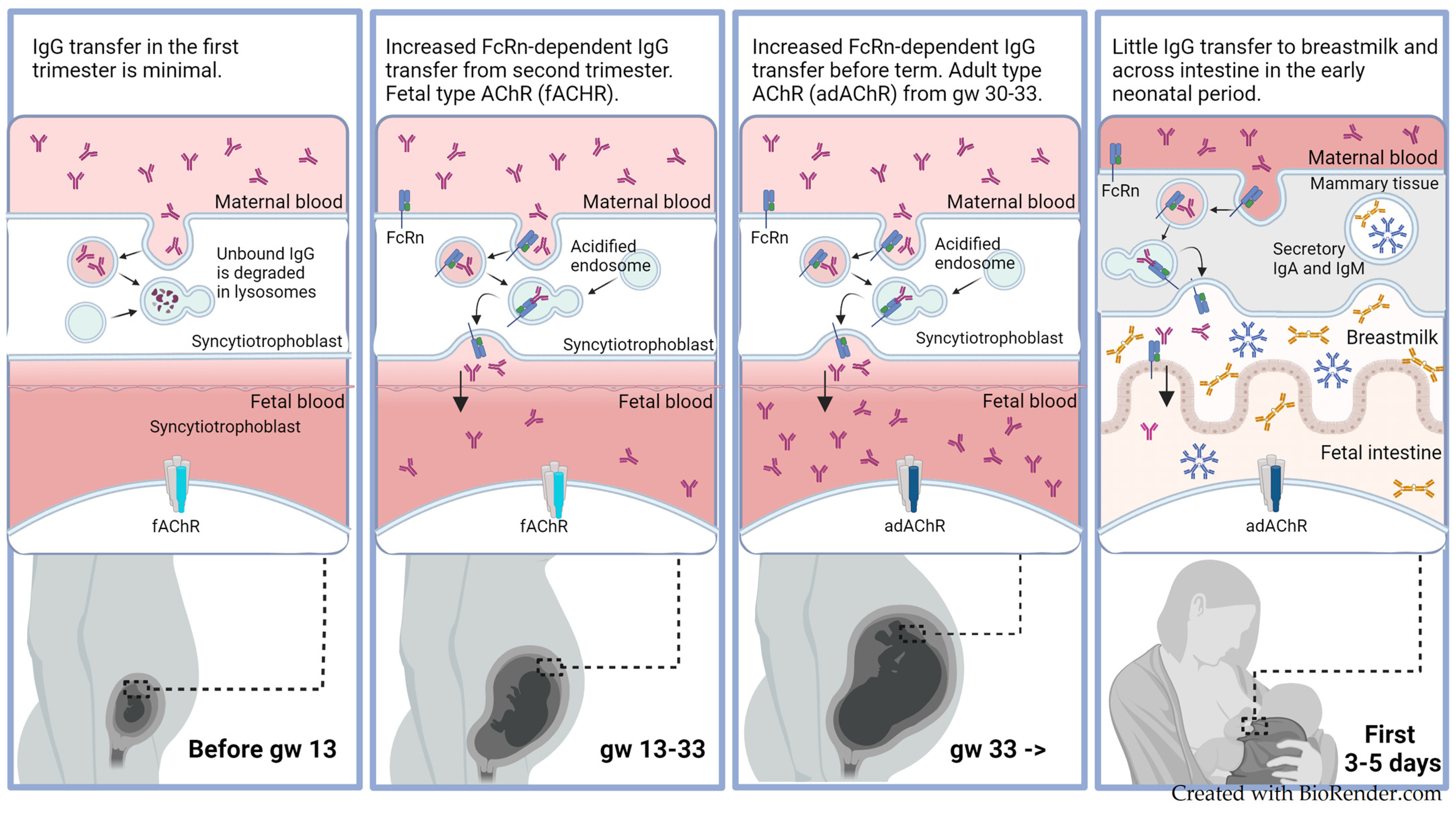
3.5.2. Maternal-to-Fetal IgG Transmission
3.5.3. Fetal- and Adult-Type Acetylcholine Receptors
3.6. Prediction of TNMG
3.7. Diagnostic Procedures
4. Management
4.1. Supportive Treatment
4.2. Pharmacological Treatment
4.3. Immunoglobulins and Plasmapheresis
4.4. Observation of Asymptomatic Newborns
4.5. Special Considerations
4.6. Breastfeeding and TNMG
4.7. Prevention
5. Discussion and Future Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
Appendix A
| Date | Database | Keywords/Search Strategy | Titles Screened | Articles Considered Relevant/Included |
|---|---|---|---|---|
| 5 June 2023 | Cochrane Library | (myasthenia gravis): ti,ab,kw (Word variations searched) | 10 | 2 |
| 13 September 2023 + additional auto-alert until 30 November 2023 (results not shown in table) | Embase <1974 to 12 September 2023> Ovid MEDLINE(R) ALL <1946 to 12 September 2023> |
| 290 | 150 (122 English full texts retrieved) |
| 9 June 2023 | HELDA—Digital Repository of the University of Helsinki (https://helda.helsinki.fi/. Accessed on 9 June 2023) | (myast*) and (neonat* or infan* or offspring* or fetal* or foetal* or foetus* or fetus* or baby* or babies* or prenat* or antenat* or child* or newborn* or laps* or lasten* or vastasyntyn* or vauv*) | 147 | 2 |
| 9 June 2023 | https://researchportal.helsinki.fi/. Accessed on 9 June 2023. | (myast*) and (neonat* or infan* or offspring* or fetal* or foetal* or foetus* or fetus* or baby* or babies* or prenat* or antenat* or child* or newborn* or laps* or lasten* or vastasyntyn* or vauv*) | 29 | 0 |
| 9 June 2023 | Aikakausikirja Duodecim duodecimlehti.fi | (myast*) and (neonat* or infan* or offspring* or fetal* or foetal* or foetus* or fetus* or baby* or babies* or prenat* or antenat* or child* or newborn* or laps* or lasten* or vastasyntyn* or vauv*) | 132 | 2 |
| 14 June 2023 | MEDIC-tietokanta https://www.fimnet.fi/cgi-cug/medic.pl. Accessed on 14 June 2023. | (myast*) and (neonat* or infan* or offspring* or fetal* or foetal* or foetus* or fetus* or baby* or babies* or prenat* or antenat* or child* or newborn* or laps* or lasten* or vastasyntyn* or vauv* or barn* nyfö* spädbarn* foster* fostre* avkomma*) | 8 | 0 |
| 14 June 2023 | Suomen Lääkärilehti kokotekstitietokantana vuodesta 1992 | (myast*) and (neonat* or infan* or offspring* or fetal* or foetal* or foetus* or fetus* or baby* or babies* or prenat* or antenat* or child* or newborn* or laps* or lasten* or vastasyntyn* or vauv* or barn* or nyfö* or spädbarn* or foster* or fostre* avkomma*) | 22 | 2 |
| 14 June 2023 | Duodecim-lehti kokotekstitietokantana vuodesta 1992–2007 | (myast*) and (neonat* or infan* or offspring* or fetal* or foetal* or foetus* or fetus* or baby* or babies* or prenat* or antenat* or child* or newborn* or laps* or lasten* or vastasyntyn* or vauv* or barn* or nyfö* or spädbarn* or foster* or fostre* or avkomma*) | 29 | 2 |
| 14 June 2023 | SveMed+ 1977–2019 |
| 2 | 1 |
| Morel et al., 1988 [42] | Gveric-Ahmetasevic et al., 2008 [23] | Gardnerova et al., 1997 [67] | |||
|---|---|---|---|---|---|
| Benign | Poor sucking, hypotonia | Grade 1 | Weak crying and hypomimia. | Grade 1 | Slight hypotonia |
| Grade 2 | Poor sucking and swallowing, and muscular weakness | Grade 2 | Severe hypotonia | ||
| Grade 3 | Suction problems | ||||
| Severe | + respiratory distress needing mechanical ventilation | Grade 3 | Generalized hypotonia with breathing difficulties and respiratory insufficiency | Grade 4 | Assisted ventilation |
References
- Gilhus, N.E.; Hong, Y. Maternal myasthenia gravis represents a risk for the child through autoantibody transfer, immunosuppressive therapy and genetic influence. Eur. J. Neurol. 2018, 25, 1402–1409. [Google Scholar] [CrossRef]
- Gilhus, N.E. Myasthenia Gravis Can Have Consequences for Pregnancy and the Developing Child. Front. Neurol. 2020, 11, 554. [Google Scholar] [CrossRef]
- Ohlraun, S.; Hoffmann, S.; Klehmet, J.; Kohler, S.; Grittner, U.; Schneider, A.; Heuschmann, P.U.; Meisel, A. Impact of myasthenia gravis on family planning: How do women with myasthenia gravis decide and why? Muscle Nerve 2015, 52, 371–379. [Google Scholar] [CrossRef]
- Haddaway, N.R.; Page, M.J.; Pritchard, C.C.; McGuinness, L.A. PRISMA2020: An R package and Shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and Open Synthesis. Campbell Syst. Rev. 2022, 18, e1230. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Rathore, G.; Kang, P.B. Pediatric Neuromuscular Diseases. Pediatr. Neurol. 2023, 149, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Evoli, A. Acquired myasthenia gravis in childhood. Curr. Opin. Neurol. 2010, 23, 536–540. [Google Scholar] [CrossRef]
- Chaudhuri, Z.; Pandey, P.K.; Bhomaj, S.; Chauhan, D.; Rani, L.U. Childhood myasthenia gravis in an infant. Br. J. Ophthalmol. 2002, 86, 704–705. [Google Scholar] [CrossRef]
- Geddes, A.K.; Kidd, H.M. Myasthenia gravis of the newborn. Can. Med. Assoc. J. 1951, 64, 152–156. [Google Scholar]
- Plauché, W.C. Myasthenia gravis in pregnancy: An update. Am. J. Obstet. Gynecol. 1979, 135, 691–697. [Google Scholar] [CrossRef]
- Hoff, J.M.; Loane, M.; Gilhus, N.E.; Rasmussen, S.; Daltveit, A.K. Arthrogryposis multiplexa congenita: An epidemiologic study of nearly 9 million births in 24 EUROCAT registers. Eur. J. Obstet. Gynecol. 2011, 159, 347–350. [Google Scholar] [CrossRef]
- Hoff, J.M.; Daltveit, A.K.; Gilhus, N.E. Artrogryposis multiplex congenita—A rare fetal condition caused by maternal myasthenia gravis. Acta Neurol. Scand. 2006, 113, 26–27. [Google Scholar] [CrossRef] [PubMed]
- Batocchi, A.P.; Majolini, L.; Evoli, A.; Lino, M.M.; Minisci, C.; Tonali, P. Course and treatment of myasthenia gravis during pregnancy. Neurology 1999, 52, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Tjon, J.K.; Tan-Sindhunata, M.B.; Bugiani, M.; Witbreuk, M.M.E.H.; Van Der Sluijs, J.A.; Weiss, M.M.; Van Weissenbruch, M.M.; Van De Pol, L.A.; Buizer, A.I.; Van Doesburg, M.H.M.; et al. Care Pathway for Foetal Joint Contractures, Foetal Akinesia Deformation Sequence, and Arthrogryposis Multiplex Congenita. Fetal Diagn. Ther. 2021, 48, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Oskoui, K.M.; Jacobson, C.L.; Chung, C.W.; Haddad, C.J.; Vincent, C.A.; Kaufmann, C.P.; De Vivo, C.D. Fetal acetylcholine receptor inactivation syndrome and maternal myasthenia gravis. Neurology 2008, 71, 2010–2012. [Google Scholar] [CrossRef] [PubMed]
- Hacohen, W.Y.; Jacobson, P.L.; Byrne, P.S.; Norwood, P.F.; Lall, P.A.; Robb, P.S.; Dilena, P.R.; Fumagalli, P.M.; Born, P.A.; Clarke, P.D.; et al. Fetal acetylcholine receptor inactivation syndrome: A myopathy due to maternal antibodies. Neurol. Neuroimmunol. Neuroinflamm. 2015, 2, e57. [Google Scholar] [CrossRef] [PubMed]
- Allen, N.M.; O’Rahelly, M.; Eymard, B.; Chouchane, M.; Hahn, A.; Kearns, G.; Kim, D.S.; Byun, S.Y.; Nguyen, C.E.; Schara-Schmidt, U.; et al. The emerging spectrum of foetal acetylcholine receptor antibody-associated disorders (FARAD). Brain 2023, 146, 4233–4246. [Google Scholar] [CrossRef]
- Boldingh, M.I.; Maniaol, A.H.; Brunborg, C.; Dekker, L.; Heldal, A.T.; Lipka, A.F.; Popperud, T.H.; Niks, E.H.; Verschuuren, J.J.G.M.; Tallaksen, C.M.E. Geographical Distribution of Myasthenia Gravis in Northern Europe—Results from a Population-Based Study from Two Countries. Neuroepidemiology 2015, 44, 221–231. [Google Scholar] [CrossRef]
- Gilhus, N.E. Treatment considerations in myasthenia gravis for the pregnant patient. Expert Rev. Neurother. 2023, 23, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Nicholls-Dempsey, L.; Czuzoj-Shulman, N.; Abenhaim, H.A. Maternal and neonatal outcomes among pregnant women with myasthenia gravis. J. Perinat. Med. 2020, 48, 793–798. [Google Scholar] [CrossRef]
- Kumar, L.; Kachhadia, M.P.; Kaur, J.; Patel, H.; Noor, K.; Gohel, R.G.; Kaur, P.; Raiyani, S.; Gohel, V.A.; Vasavada, A.M. Choices and Challenges with Treatment of Myasthenia Gravis in Pregnancy: A Systematic Review. Cureus 2023, 15, e42772. [Google Scholar] [CrossRef]
- Banner, H.; Niles, K.M.; Ryu, M.; Sermer, M.; Bril, V.; Murphy, K.E. Myasthenia Gravis in pregnancy: Systematic review and case series. Obstet. Med. 2022, 5, 108–117. [Google Scholar] [CrossRef]
- Gveric-Ahmetasevic, S.; Colic, A.; Elvedji-Gasparovic, V.; Gveric, T.; Vukelic, V. Can neonatal myasthenia gravis be predicted? J. Perinat. Med. 2008, 36, 503. [Google Scholar] [CrossRef]
- Hoff, J.M.; Daltveit, A.K.; Gilhus, N.E. Myasthenia gravis: Consequences for pregnancy, delivery, and the newborn. Neurology 2003, 61, 1362–1366. [Google Scholar] [CrossRef]
- O’Connor, L.; Malmeström, C.; Da Silva Rodrigues, R.; Brauner, S.; Wikström, A.K.; Punga, A.R. Pregnancy outcomes for women with myasthenia gravis and their newborns: A nationwide register-based cohort study. Eur. J. Neurol. 2023, 31, e16100. [Google Scholar] [CrossRef]
- Djelmis, J.; Sostarko, M.; Mayer, D.; Ivanisevic, M. Myasthenia gravis in pregnancy: Report on 69 cases. Eur. J. Obstet. Gynecol. 2002, 104, 21–25. [Google Scholar] [CrossRef]
- Hoff, J.M.; Daltveit, A.K.; Gilhus, N.E. Asymptomatic myasthenia gravis influences pregnancy and birth. Eur. J. Neurol. 2004, 11, 559–562. [Google Scholar] [CrossRef]
- Kochhar, P.K.; Schumacher, R.E.; Sarkar, S. Transient neonatal myasthenia gravis: Refining risk estimate for infants born to women with myasthenia gravis. J. Perinatol. 2021, 41, 2279–2283. [Google Scholar] [CrossRef]
- Gomella, T.L.; Cunningham, M.D. Neonatology, 7th ed.; McGraw-Hill Prof Med/Tech: Irvine, CA, USA, 2013. [Google Scholar]
- Zhou, Q.; Yin, W.; Zhu, J.; Duan, W.; Li, Y.; Jin, W.; Yang, H. Risk factors associated with adverse pregnancy outcomes and postpartum exacerbation in women with myasthenia gravis. Am. J. Reprod. Immunol. 2022, 88, e13641. [Google Scholar] [CrossRef]
- Namba, T.; Brown, S.B.; Grob, D. Neonatal myasthenia gravis: Report of two cases and review of the literature. Pediatrics 1970, 45, 488–504. [Google Scholar] [CrossRef]
- Iijima, S. Clinical and pathophysiologic relevance of autoantibodies in neonatal myasthenia gravis. Pediatr. Neonatol. 2021, 62, 581–590. [Google Scholar] [CrossRef]
- Keller, R.; Johnson, K.; Hussain, N.; Campbell, W.; Townsel, C. Seronegative Maternal Ocular Myasthenia Gravis and Delayed Transient Neonatal Myasthenia Gravis. Am. J. Perinatol. Rep. 2016, 6, e133–e136. [Google Scholar] [CrossRef]
- Pyzik, M.; Kozicky, L.K.; Gandhi, A.K.; Blumberg, R.S. The therapeutic age of the neonatal Fc receptor. Nat. Rev. Immunol. 2023, 23, 415–432. [Google Scholar] [CrossRef]
- Kalamida, D.; Poulas, K.; Avramopoulou, V.; Fostieri, E.; Lagoumintzis, G.; Lazaridis, K.; Sideri, A.; Zouridakis, M.; Tzartos, S.J. Muscle and neuronal nicotinic acetylcholine receptors. FEBS J. 2007, 274, 3799–3845. [Google Scholar] [CrossRef]
- Eymard, B.; Vauthier, D.; Dommergues, M.; Chatenoud, L. P4.49 Preventive therapy of severe neonatal myasthenia gravis during pregnancy. Neuromuscul. Disord. 2011, 21, 719. [Google Scholar] [CrossRef]
- Rieder, A.A.; Conley, S.F.; Rowe, L. Pediatric myasthenia gravis and velopharyngeal incompetence. Int. J. Pediatr. Otorhinolaryngol. 2004, 68, 747–752. [Google Scholar] [CrossRef]
- Jeannet, P.Y.; Marcoz, J.P.; Kuntzer, T.; Roulet-Perez, E. Isolated facial and bulbar paresis: A persistent manifestation of neonatal myasthenia gravis. Neurology 2008, 70, 237–238. [Google Scholar] [CrossRef]
- Behin, A.; Mayer, M.; Kassis-Makhoul, B.; Jugie, M.; Espil-Taris, C.; Ferrer, X.; Chatenoud, L.; Laforet, P.; Eymard, B. Severe neonatal myasthenia due to maternal anti-MuSK antibodies. Neuromuscul. Disord. 2008, 18, 443–446. [Google Scholar] [CrossRef]
- Ahlsten, G.; Lefvert, A.K.; Osterman, P.O.; Stålberg, E.; Säfwenberg, J. Follow-up study of muscle function in children of mothers with myasthenia gravis during pregnancy. J. Child Neurol. 1992, 7, 264–269. [Google Scholar] [CrossRef]
- Polizzi, A.; Huson, S.M.; Vincent, A. Teratogen update: Maternal myasthenia gravis as a cause of congenital arthrogryposis. Teratology 2000, 62, 332–341. [Google Scholar] [CrossRef]
- Morel, E.; Eymard, B.; Vernet-der Garabedian, B.; Pannier, C.; Dulac, O.; Bach, J.F. Neonatal myasthenia gravis: A new clinical and immunologic appraisal on 30 cases. Neurology 1988, 38, 138–142. [Google Scholar] [CrossRef]
- Chang Hoftman, A.; Hernandez, M.I.; Lee, K.-W.; Stiehm, E.R. Newborn Illnesses Caused by Transplacental Antibodies. Adv. Pediatr. 2008, 55, 271–304. [Google Scholar] [CrossRef]
- Podciechowski, L.; Brocka-Nitecka, U.; Dabrowska, K.; Bielak, A.; Hadacz, B.; Wilczynski, J. Pregnancy complicated by Myasthenia gravis—Twelve years experience. Neuro Endocrinol. Lett. 2005, 26, 603–608. [Google Scholar]
- Sisman, J.; Ceri, A.; Nafday, S.M. Seronegative neonatal myasthenia gravis in one of the twins. Indian Pediatr. 2004, 41, 938–940. [Google Scholar]
- Vincent, A.; Beeson, D.; Lang, B. Molecular targets for autoimmune and genetic disorders of neuromuscular transmission. Eur. J. Biochem. 2000, 267, 6717–6728. [Google Scholar] [CrossRef]
- Gilhus, N.E.; Tzartos, S.; Evoli, A.; Palace, J.; Burns, T.M.; Verschuuren Jan, J.G.M. Myasthenia gravis. Nat. Rev. Dis. Primers 2019, 5, 30. [Google Scholar] [CrossRef]
- Saxena, A.; Stevens, J.; Cetin, H.; Koneczny, I.; Webster, R.; Lazaridis, K.; Tzartos, S.; Vrolix, K.; Nogales-Gadea, G.; Machiels, B.; et al. Characterization of an anti-fetal AChR monoclonal antibody isolated from a myasthenia gravis patient. Sci. Rep. 2017, 7, 14426. [Google Scholar] [CrossRef]
- Rubin, J.E.; Ramamurthi, R.J. The Role of Sugammadex in Symptomatic Transient Neonatal Myasthenia Gravis: A Case Report. A & A Case Rep. 2017, 9, 271–273. [Google Scholar]
- Inoue, K.-i.; Tsugawa, J.; Fukae, J.; Fukuhara, K.; Kawano, H.; Fujioka, S.; Tsuboi, Y. Myasthenia Gravis with Anti-Muscle-Specific Tyrosine Kinase Antibody during Pregnancy and Risk of Neonatal Myasthenia Gravis: A Case Report and Review of the Literature. Case Rep. Neurol. 2020, 12, 114–120. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Min, J.-H.; Han, S.-H.; Han, J. Transient neonatal myasthenia gravis due to a mother with ocular onset of anti-muscle specific kinase myasthenia gravis. Neuromuscul. Disord. 2017, 27, 655–657. [Google Scholar] [CrossRef]
- Harada, Y.; Bettin, M.; Juel, V.C.; Massey, J.M.; Sanders, D.B. Pregnancy in Seronegative Myasthenia Gravis: A Single-Center Case Series. J. Clin. Neuromuscul. Dis. 2023, 25, 85–88. [Google Scholar] [CrossRef]
- Reuner, U.; Kamin, G.; Ramantani, G.; Reichmann, H.; Dinger, J. Transient neonatal Lambert-Eaton syndrome. J. Neurol. 2008, 255, 1827–1828. [Google Scholar] [CrossRef]
- Tzartos, S.J.; Efthimiadis, A.; Morel, E.; Eymard, B.; Bach, J.F. Neonatal myasthenia gravis: Antigenic specificities of antibodies in sera from mothers and their infants. Clin. Exp. Immunol. 1990, 80, 376–380. [Google Scholar] [CrossRef]
- Madi, A.; Bransburg-Zabary, S.; Maayan-Metzger, A.; Dar, G.; Ben-Jacob, E.; Cohen, I.R. Tumor-associated and disease-associated autoantibody repertoires in healthy colostrum and maternal and newborn cord sera. J. Immunol. 2015, 194, 5272–5281. [Google Scholar] [CrossRef] [PubMed]
- Moise, K.J.; Oepkes, D.; Lopriore, E.; Bredius, R.G.M. Targeting neonatal Fc receptor: Potential clinical applications in pregnancy. Ultrasound Obstet. Gynecol. 2022, 60, 167–175. [Google Scholar] [CrossRef]
- Palmeira, P.; Quinello, C.; Silveira-Lessa, A.L.; Zago, C.A.; Carneiro-Sampaio, M. IgG Placental Transfer in Healthy and Pathological Pregnancies. Clin. Dev. Immunol. 2012, 2012, 1–13. [Google Scholar] [CrossRef]
- Lefvert, A.K.; Osterman, P.O. Newborn infants to myasthenic mothers: A clinical study and an investigation of acetylcholine receptor antibodies in 17 children. Neurology 1983, 33, 133–138. [Google Scholar] [CrossRef]
- Cetin, H.; Beeson, D.; Vincent, A.; Webster, R. The Structure, Function, and Physiology of the Fetal and Adult Acetylcholine Receptor in Muscle. Front. Mol. Neurosci. 2020, 13, 581097. [Google Scholar] [CrossRef]
- Vincent, A.; Jacobson, L.; Plested, P.; Polizzi, A.; Tang, T.; Riemersma, S.; Newland, C.; Ghorazian, S.; Farrar, J.; MacLennan, C.; et al. Antibodies affecting ion channel function in acquired neuromyotonia, in seropositive and seronegative myasthenia gravis, and in antibody-mediated arthrogryposis multiplex congenita. Ann. N. Y. Acad. Sci. 1998, 841, 482–496. [Google Scholar] [CrossRef]
- Licht, C.; Model, P.; Kribs, A.; Herkenrath, P.; Michalk, D.V.; Haupt, W.F.; Gohring, U.J.; Roth, B. Transient neonatal myasthenia gravis. Nervenarzt 2002, 73, 774–778. [Google Scholar] [CrossRef]
- Tanacan, A.; Fadiloglu, E.; Ozten, G.; Gunes, A.C.; Orgul, G.; Beksac, M.S. Myasthenia gravis and pregnancy: Retrospective evaluation of 27 pregnancies in a tertiary center and comparison with previous studies. Ir. J. Med. Sci. 2019, 188, 1261–1267. [Google Scholar] [CrossRef]
- Verspyck, E.; Mandelbrot, L.; Dommergues, M.; Huon, C.; Woimant, F.; Baumann, C.; Garabedian, B.V.-D. Myasthenia gravis with polyhydramnios in the fetus of an asymptomatic mother. Prenat. Diagn. 1993, 13, 539–542. [Google Scholar] [CrossRef]
- Elias, S.B.; Butler, I.; Appel, S.H. Neonatal myasthenia gravis in the infant of a myasthenic mother in remission. Ann. Neurol. 1979, 6, 72–75. [Google Scholar] [CrossRef]
- Ohta, M.; Matsubara, F.; Hayashi, K.; Nakao, K.; Nishitani, H. Acetylcholine receptor antibodies in infants of mothers with myasthenia gravis. Neurology 1981, 31, 1019–1022. [Google Scholar] [CrossRef]
- Wassenberg, M.; Hahn, A.; Mück, A.; Krämer, H.H. Maternal immunoglobulin treatment can reduce severity of fetal acetylcholine receptor antibody-associated disorders (FARAD). Neurol. Res. Pract. 2023, 5, 58. [Google Scholar] [CrossRef]
- Gardnerova, F.M.; Eymard, F.B.; Morel, F.E.; Faltin, F.M.; Zajac, F.J.; Sadovsky, F.O.; Tripon, F.P.; Domergue, F.M.; Vernet-Der Garabedian, F.B.; Bach, F.J. The Fetal/Adult Acetylcholine Receptor Antibody Ratio in Mothers with Myasthenia Gravis as a Marker for Transfer of the Disease to the Newborn. Neurology 1997, 48, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Gilhus, N.E.; Verschuuren, J.J. Myasthenia gravis: Subgroup classification and therapeutic strategies. Lancet Neurol. 2015, 14, 1023–1036. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, M.; Arck, P.C. Vertically Transferred Immunity in Neonates: Mothers, Mechanisms and Mediators. Front. Immunol. 2020, 11, 555. [Google Scholar] [CrossRef]
- Vernet-Der Garabedian, B.; Lacokova, M.; Eymard, B.; Morel, E.; Faltin, M.; Zajac, J.; Sadovsky, O.; Dommergues, M.; Tripon, P.; Bach, J.F. Association of neonatal myasthenia gravis with antibodies against the fetal acetylcholine receptor. J. Clin. Investig. 1994, 94, 555–559. [Google Scholar] [CrossRef]
- Norwood, F.; Dhanjal, M.; Hill, M.; James, N.; Jungbluth, H.; Kyle, P.; O’Sullivan, G.; Palace, J.; Robb, S.; Williamson, C.; et al. Myasthenia in pregnancy: Best practice guidelines from a UK multispecialty working group. J. Neurol. Neurosurg. Psychiatry 2014, 85, 538–543. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Recommendations on Antenatal Care for a Positive Pregnancy Experience; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- Merz, W.M.; Fischer-Betz, R.; Hellwig, K.; Lamprecht, G.; Gembruch, U. Pregnancy and Autoimmune Disease. Dtsch. Arztebl. Int. 2022, 119, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Cimpoca-Raptis, B.A.; Ciobanu, A.M.; Gica, N.; Peltecu, G.; Mitrea, D.; Panaitescu, A.M. Fetal Surveillance in Pregnancies with Myasthenia Gravis. Medicina 2021, 57, 1277. [Google Scholar] [CrossRef]
- Fisher, P.G. 50 Years Ago in The Journal of Pediatrics: Neonatal Myasthenia Gravis. J. Pediatr. 2016, 171, 201. [Google Scholar] [CrossRef]
- Hays, R.M.; Michaud, L.J. Neonatal myasthenia gravis: Specific advantages of repetitive stimulation over edrophonium testing. Pediatr. Neurol. 1988, 4, 245–247. [Google Scholar] [CrossRef] [PubMed]
- Massey, J.M.; De Jesus-Acosta, C. Pregnancy and myasthenia gravis. Contin. Lifelong Learn. Neurol. 2014, 20, 115–127. [Google Scholar] [CrossRef]
- Tagher, R.J.; Baumann, R.; Desai, N. Failure of intravenously administered immunoglobulin in the treatment of neonatal myasthenia gravis. J. Pediatr. 1999, 134, 233–235. [Google Scholar] [CrossRef]
- Donat, J.F.; Donat, J.R.; Lennon, V.A. Exchange transfusion in neonatal myasthenia gravis. Neurology 1981, 31, 911–912. [Google Scholar] [CrossRef]
- Varner, M. Myasthenia Gravis and Pregnancy. Clin. Obstet. Gynecol. 2013, 56, 372–381. [Google Scholar] [CrossRef]
- Bardhan, M.; Dogra, H.; Samanta, D. Neonatal Myasthenia Gravis. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2023. [Google Scholar]
- Ferrero, S.; Esposito, F.; Biamonti, M.; Bentivoglio, G.; Ragni, N. Myasthenia gravis during Pregnancy. Expert Rev. Neurother. 2008, 8, 979–988. [Google Scholar] [CrossRef]
- Boldingh, M.I.; Maniaol, A.H.; Brunborg, C.; Weedon-Fekjær, H.; Verschuuren, J.J.G.M.; Tallaksen, C.M.E. Increased risk for clinical onset of myasthenia gravis during the postpartum period. Breastfeeding reduced the risk. Neurology 2016, 87, 2139–2145. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.; Yasawy, Z.M. Myasthaenia Gravis: Clinical management issues before, during and after pregnancy. Sultan Qaboos Univ. Med. J. 2017, 17, e259–e267. [Google Scholar] [CrossRef] [PubMed]
- Bassan, H.; Muhlbaur, B.; Tomer, A.; Spirer, Z. High-dose intravenous immunoglobulin in transient neonatal myasthenia gravis. Pediatr. Neurol. 1998, 18, 181–183. [Google Scholar] [CrossRef] [PubMed]
- Hurley, W.L.; Theil, P.K. Perspectives on Immunoglobulins in Colostrum and Milk. Nutrients 2011, 3, 442–474. [Google Scholar] [CrossRef]
- Pentšuk, N.; Van Der Laan, J.W. An interspecies comparison of placental antibody transfer: New insights into developmental toxicity testing of monoclonal antibodies. Birth Defects Res. Part B Dev. Reprod. Toxicol. 2009, 86, 328–344. [Google Scholar] [CrossRef] [PubMed]
- Iyengar, L.; Selvaraj, R.J. Intestinal Absorption of Immunoglobulins by Newborn Infants. Arch. Dis. Child 1972, 47, 411–414. [Google Scholar] [CrossRef][Green Version]
- Shah, U.; Dickinson, B.L.; Blumberg, R.S.; Simister, N.E.; Lencer, W.I.; Walker, A.W. Distribution of the IgG Fc Receptor, FcRn, in the Human Fetal Intestine. Pediatr. Res. 2003, 53, 295–301. [Google Scholar] [CrossRef]
- Rio-Aige, K.; Azagra-Boronat, I.; Castell, M.; Selma-Royo, M.; Collado, M.C.; Rodríguez-Lagunas, M.J.; Pérez-Cano, F.J. The Breast Milk Immunoglobulinome. Nutrients 2021, 13, 1810. [Google Scholar] [CrossRef]
- Brenner, T.; Shahin, R.; Steiner, I.; Abramsky, O. Presence of Anti-Acetylcholine Receptor Antibodies in Human Milk: Possible Correlation with Neonatal Myasthenia Gravis. Autoimmunity 1992, 12, 315–316. [Google Scholar] [CrossRef]
- Carr, S.R.; Gilchrist, J.M.; Abuelo, D.N.; Clark, D. Treatment of antenatal myasthenia gravis. Obstet. Gynecol. 1991, 78, 485–489. [Google Scholar] [CrossRef]
- Roth, T.C.; Raths, J.; Carboni, G.; Rösler, K.; Schmid, R.A. Effect of pregnancy and birth on the course of myasthenia gravis before or after transsternal radical thymectomy. Eur. J. Cardio-Thorac. Surg. 2006, 29, 231–235. [Google Scholar] [CrossRef]
- Hoff, J.M.; Daltveit, A.K.; Gilhus, N.E. Myasthenia gravis in pregnancy and birth: Identifying risk factors, optimising care. Eur. J. Neurol. 2007, 14, 38–43. [Google Scholar] [CrossRef]
- Su, M.; Liu, X.; Wang, L.; Song, J.; Zhou, Z.; Luo, S.; Zhao, C. Risk factors for pregnancy-related clinical outcome in myasthenia gravis: A systemic review and meta-analysis. Orphanet J. Rare Dis. 2022, 17, 52. [Google Scholar] [CrossRef]
- Roy, S.; Nanovskaya, T.; Patrikeeva, S.; Cochran, E.; Parge, V.; Guess, J.; Schaeck, J.; Choudhury, A.; Ahmed, M.; Ling, L.E. M281, an anti-FcRn antibody, inhibits IgG transfer in a human ex vivo placental perfusion model. Am. J. Obstet. Gynecol. 2019, 220, 498.e491–498.e499. [Google Scholar] [CrossRef]
- Coutinho, E.; Jacobson, L.; Shock, A.; Smith, B.; Vernon, A.; Vincent, A. Inhibition of Maternal-to-Fetal Transfer of IgG Antibodies by FcRn Blockade in a Mouse Model of Arthrogryposis Multiplex Congenita. Neurol. Neuroimmunol. Neuroinflamm. 2021, 8, e1011. [Google Scholar] [CrossRef] [PubMed]
- Gamez, J.; Salvado, M.; Casellas, M.; Manrique, S.; Castillo, F. Intravenous immunoglobulin as monotherapy for myasthenia gravis during pregnancy. J. Neurol. Sci. 2017, 383, 118–122. [Google Scholar] [CrossRef]
- Marson, P.; Gervasi, M.T.; Tison, T.; Colpo, A.; De Silvestro, G. Therapeutic apheresis in pregnancy: General considerations and current practice. Transfus Apher. Sci. 2015, 53, 256–261. [Google Scholar] [CrossRef] [PubMed]
| Severity Grade | Symptoms | Relevant Treatment Options |
|---|---|---|
| Very mild | Fluctuating mild hypotonia. Oral feeding is possible. | Close observation and breastfeeding support. |
| Mild | Persistent or intermittent hypotonia with feeding difficulties. | Consider low-dose acetylcholine-esterase inhibitor before feedings based on the result of the pharmacological challenge test. |
| Moderate | Oral feeding is inadequate but there is no respiratory distress. | Nasogastric tube feedings. Acetylcholine-esterase inhibitor. Consider IVIG. |
| Severe | Respiratory distress. | Acetylcholine-esterase inhibitor regularly. Respiratory support. IVIG and/or TPE. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lindroos, J.L.V.; Bjørk, M.-H.; Gilhus, N.E. Transient Neonatal Myasthenia Gravis as a Common Complication of a Rare Disease: A Systematic Review. J. Clin. Med. 2024, 13, 1136. https://doi.org/10.3390/jcm13041136
Lindroos JLV, Bjørk M-H, Gilhus NE. Transient Neonatal Myasthenia Gravis as a Common Complication of a Rare Disease: A Systematic Review. Journal of Clinical Medicine. 2024; 13(4):1136. https://doi.org/10.3390/jcm13041136
Chicago/Turabian StyleLindroos, Jenny Linnea Victoria, Marte-Helene Bjørk, and Nils Erik Gilhus. 2024. "Transient Neonatal Myasthenia Gravis as a Common Complication of a Rare Disease: A Systematic Review" Journal of Clinical Medicine 13, no. 4: 1136. https://doi.org/10.3390/jcm13041136
APA StyleLindroos, J. L. V., Bjørk, M.-H., & Gilhus, N. E. (2024). Transient Neonatal Myasthenia Gravis as a Common Complication of a Rare Disease: A Systematic Review. Journal of Clinical Medicine, 13(4), 1136. https://doi.org/10.3390/jcm13041136







