Patients’ Perceptions of Nusinersen Effects According to Their Responder Status
Abstract
1. Introduction
2. Materials and Methods
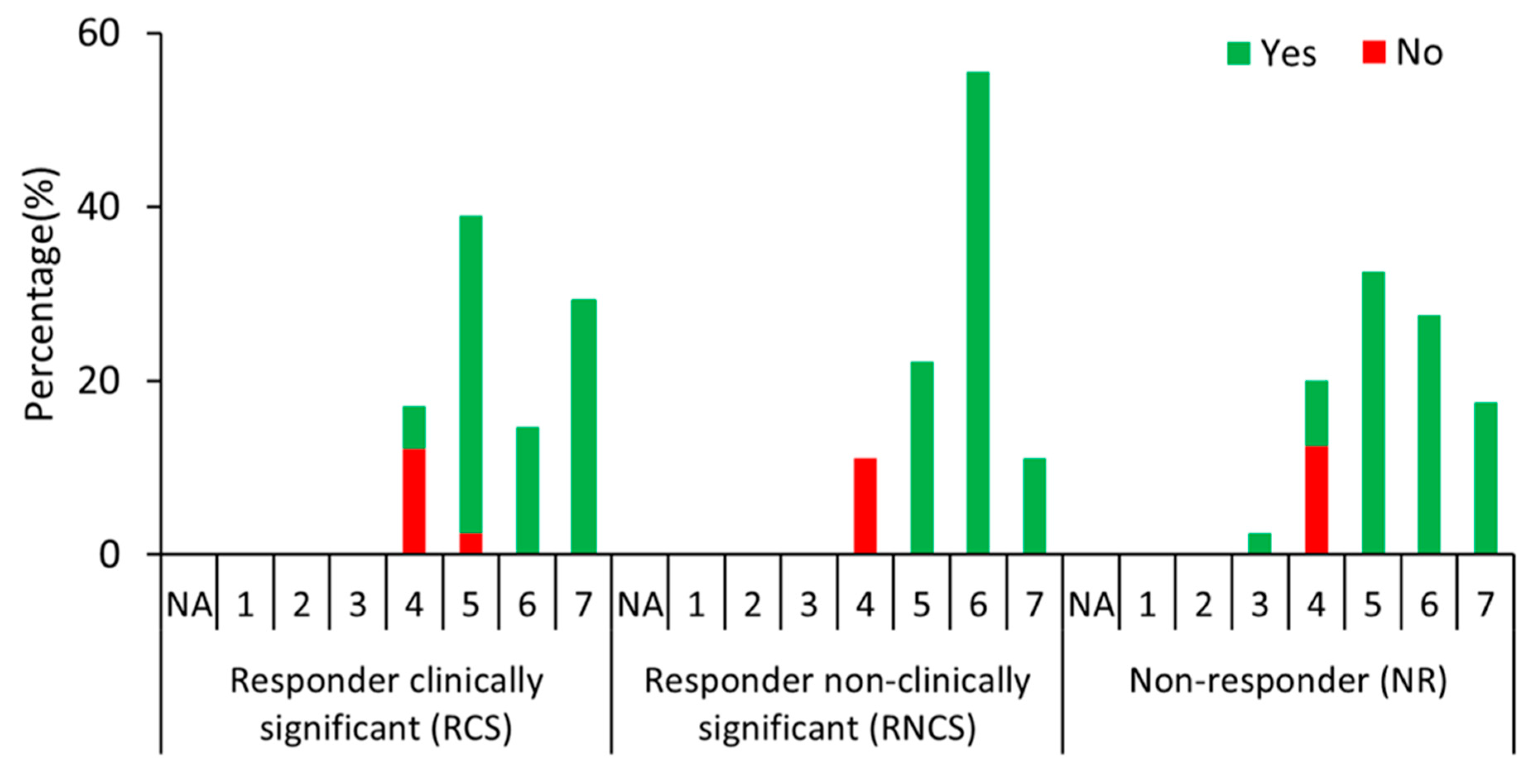
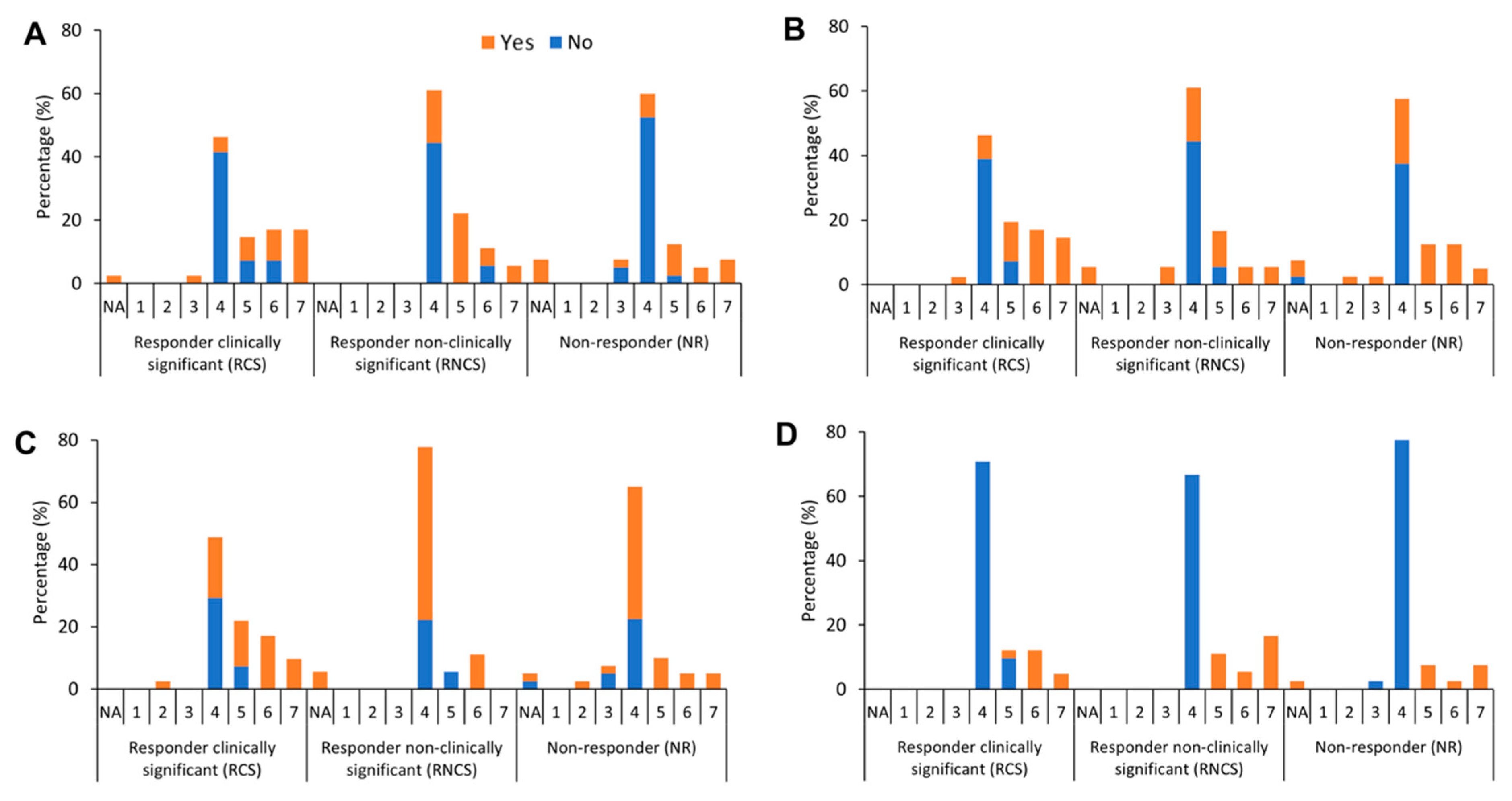
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Annoussamy, M.; Seferian, A.M.; Daron, A.; Péréon, Y.; Cances, C.; Vuillerot, C.; De Waele, L.; Laugel, V.; Schara, U.; Gidaro, T.; et al. Natural history of Type 2 and 3 spinal muscular atrophy: 2-year NatHis-SMA study. Ann. Clin. Transl. Neurol. 2020, 8, 359–373. [Google Scholar] [CrossRef] [PubMed]
- Aragon-Gawinska, K.; Mouraux, C.; Dangouloff, T.; Servais, L. Spinal Muscular Atrophy Treatment in Patients Identified by Newborn Screening—A Systematic Review. Genes 2023, 14, 1377. [Google Scholar] [CrossRef]
- Burghes, A.H.; Beattie, C.E. Spinal muscular atrophy: Why do low levels of survival motor neuron protein make motor neurons sick? Nat. Rev. Neurosci. 2009, 10, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Mercuri, E.; Finkel, R.S.; Muntoni, F.; Wirth, B.; Montes, J.; Main, M.; Mazzone, E.S.; Vitale, M.; Snyder, B.; Quijano-Roy, S.; et al. Diagnosis and management of spinal muscular atrophy: Part 1: Recommendations for diagnosis, rehabilitation, orthopedic and nutritional care. Neuromuscul. Disord. 2018, 28, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Finkel, R.S.; Mercuri, E.; Darras, B.T.; Connolly, A.M.; Kuntz, N.L.; Kirschner, J.; Chiriboga, C.A.; Saito, K.; Servais, L.; Tizzano, E.; et al. Nusinersen versus Sham Control in Infantile-Onset Spinal Muscular Atrophy. N. Engl. J. Med. 2017, 377, 1723–1732. [Google Scholar] [CrossRef] [PubMed]
- Mercuri, E.; Darras, B.T.; Chiriboga, C.A.; Day, J.W.; Campbell, C.; Connolly, A.M.; Iannaccone, S.T.; Kirschner, J.; Kuntz, N.L.; Saito, K.; et al. Nusinersen versus Sham Control in Later-Onset Spinal Muscular Atrophy. N. Engl. J. Med. 2018, 378, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Aragon-Gawinska, K.; Seferian, A.M.; Daron, A.; Gargaun, E.; Vuillerot, C.; Cances, C.; Ropars, J.; Chouchane, M.; Cuppen, I.; Hughes, I.; et al. Nusinersen in patients older than 7 months with spinal muscular atrophy type 1: A cohort study. Neurology 2018, 91, e1312–e1318. [Google Scholar] [CrossRef] [PubMed]
- Audic, F.; de la Banda, M.G.G.; Bernoux, D.; Ramirez-Garcia, P.; Durigneux, J.; Barnerias, C.; Isapof, A.; Cuisset, J.-M.; Cances, C.; Richelme, C.; et al. Effects of nusinersen after one year of treatment in 123 children with SMA type 1 or 2: A French real-life observational study. Orphanet J. Rare Dis. 2020, 15, 148. [Google Scholar] [CrossRef] [PubMed]
- Konersman, C.G.; Ewing, E.; Yaszay, B.; Naheedy, J.; Murphy, S.; Skalsky, A. Nusinersen treatment of older children and adults with spinal muscular atrophy. Neuromuscul. Disord. 2021, 31, 183–193. [Google Scholar] [CrossRef]
- Łusakowska, A.; Wójcik, A.; Frączek, A.; Aragon-Gawińska, K.; Potulska-Chromik, A.; Baranowski, P.; Nowak, R.; Rosiak, G.; Milczarek, K.; Konecki, D.; et al. Long-term nusinersen treatment across a wide spectrum of spinal muscular atrophy severity: A real-world experience. Orphanet J. Rare Dis. 2023, 18, 230. [Google Scholar] [CrossRef]
- Maggi, L.; Bello, L.; Bonanno, S.; Govoni, A.; Caponnetto, C.; Passamano, L.; Grandis, M.; Trojsi, F.; Cerri, F.; Ferraro, M.; et al. Nusinersen safety and effects on motor function in adult spinal muscular atrophy type 2 and 3. J. Neurol. Neurosurg. Psychiatry 2020, 91, 1166–1174. [Google Scholar] [CrossRef] [PubMed]
- McMillan, H.J. Nusinersen: Evidence of sustained clinical improvement and lessened fatigue in older ambulatory patients with spinal muscular atrophy. Muscle Nerve 2020, 61, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Pechmann, A.; König, K.; Bernert, G.; Schachtrup, K.; Schara, U.; Schorling, D.; Schwersenz, I.; Stein, S.; Tassoni, A.; Vogt, S.; et al. SMArtCARE—A platform to collect real-life outcome data of patients with spinal muscular atrophy. Orphanet J. Rare Dis. 2019, 14, 18. [Google Scholar] [CrossRef] [PubMed]
- Pechmann, A.; Langer, T.; Schorling, D.; Stein, S.; Vogt, S.; Schara, U.; Kölbel, H.; Schwartz, O.; Hahn, A.; Giese, K.; et al. Evaluation of Children with SMA Type 1 Under Treatment with Nusinersen within the Expanded Access Program in Germany. J. Neuromuscul. Dis. 2018, 5, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Coratti, G.; Cutrona, C.; Pera, M.C.; Bovis, F.; Ponzano, M.; Chieppa, F.; Antonaci, L.; Sansone, V.; Finkel, R.; Pane, M.; et al. Motor function in type 2 and 3 SMA patients treated with Nusinersen: A critical review and meta-analysis. Orphanet J. Rare Dis. 2021, 16, 430. [Google Scholar] [CrossRef] [PubMed]
- Yeo, C.J.J.; Simmons, Z.; De Vivo, D.C.; Darras, B.T. Ethical Perspectives on Treatment Options with Spinal Muscular Atrophy Patients. Ann. Neurol. 2022, 91, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Djordjevic, D.; McFadyen, A.; Anderson, J.A. Ethical challenges and opportunities in the development and approval of novel therapeutics for rare diseases. J. Med. Access 2023, 7, 27550834231177507. [Google Scholar] [CrossRef] [PubMed]
- Stolte, B.; Bois, J.M.; Bolz, S.; Kizina, K.; Totzeck, A.; Schlag, M.; Kleinschnitz, C.; Hagenacker, T. Minimal clinically important differences in functional motor scores in adults with spinal muscular atrophy. Eur. J. Neurol. 2020, 27, 2586–2594. [Google Scholar] [CrossRef] [PubMed]
- Schorling, D.C.; Pechmann, A.; Kirschner, J. Advances in Treatment of Spinal Muscular Atrophy—New Phenotypes, New Challenges, New Implications for Care. J. Neuromuscul. Dis. 2020, 7, 1–13. [Google Scholar] [CrossRef]
- Rouault, F.; Christie-Brown, V.; Broekgaarden, R.; Gusset, N.; Henderson, D.; Marczuk, P.; Schwersenz, I.; Bellis, G.; Cottet, C. Disease impact on general well-being and therapeutic expectations of European Type II and Type III spinal muscular atrophy patients. Neuromuscul. Disord. 2017, 27, 428–438. [Google Scholar] [CrossRef]
- McGraw, S.; Qian, Y.; Henne, J.; Jarecki, J.; Hobby, K.; Yeh, W.S. A qualitative study of perceptions of meaningful change in spinal muscular atrophy. BMC Neurol. 2017, 17, 68. [Google Scholar] [CrossRef] [PubMed]
- Mazzone, E.; Bianco, F.; Main, M.; van den Hauwe, M.; Ash, M.; de Vries, R.; Mata, J.F.; Stein, S.; De Sanctis, R.; D’amico, A.; et al. Six minute walk test in type III spinal muscular atrophy: A 12month longitudinal study. Neuromuscul. Disord. 2013, 23, 624–628. [Google Scholar] [CrossRef] [PubMed]
- Montes, J.; McDermott, M.P.; Martens, W.B.; Dunaway, S.; Glanzman, A.M.; Riley, S.; Quigley, J.; Montgomery, M.J.; Sproule, D.; Tawil, R.; et al. Six-Minute Walk Test demonstrates motor fatigue in spinal muscular atrophy. Neurology 2010, 74, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Oskoui, M.; Day, J.W.; Deconinck, N.; Mazzone, E.S.; Nascimento, A.; Saito, K.; Vuillerot, C.; Baranello, G.; Goemans, N.; Kirschner, J.; et al. Two-year efficacy and safety of risdiplam in patients with type 2 or non-ambulant type 3 spinal muscular atrophy (SMA). J. Neurol. 2023, 270, 2531–2546. [Google Scholar] [CrossRef] [PubMed]
- Pane, M.; Palermo, C.; Messina, S.; Sansone, V.A.; Bruno, C.; Catteruccia, M.; Sframeli, M.; Albamonte, E.; Pedemonte, M.; D’Amico, A.; et al. An observational study of functional abilities in infants, children, and adults with type 1 SMA. Neurology 2018, 91, e696–e703. [Google Scholar] [CrossRef] [PubMed]
- Baranello, G.; Gorni, K.; Daigl, M.; Kotzeva, A.; Evans, R.; Hawkins, N.; Scott, D.A.; Mahajan, A.; Muntoni, F.; Servais, L. Prognostic Factors and Treatment-Effect Modifiers in Spinal Muscular Atrophy. Clin. Pharmacol. Ther. 2021, 110, 1435–1454. [Google Scholar] [CrossRef] [PubMed]
- Day, J.W.; Howell, K.; Place, A.; Long, K.; Rossello, J.; Kertesz, N.; Nomikos, G. Advances and limitations for the treatment of spinal muscular atrophy. BMC Pediatr. 2022, 22, 632. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, L.; Scheman, J. Patient global impression of change scores within the context of a chronic pain rehabilitation program. J. Pain 2009, 10, S73. [Google Scholar] [CrossRef]
- Gusset, N.; Stalens, C.; Stumpe, E.; Klouvi, L.; Mejat, A.; Ouillade, M.-C.; de Lemus, M. Understanding European patient expectations towards current therapeutic development in spinal muscular atrophy. Neuromuscul. Disord. 2021, 31, 419–430. [Google Scholar] [CrossRef]
- Pera, M.C.; Coratti, G.; Casiraghi, J.; Bravetti, C.; Fedeli, A.; Strika, M.; Albamonte, E.; Antonaci, L.; Rossi, D.; Pane, M.; et al. Caregivers’ Expectations on Possible Functional Changes following Disease-Modifying Treatment in Type II and III Spinal Muscular Atrophy: A Comparative Study. J. Clin. Med. 2023, 12, 4183. [Google Scholar] [CrossRef]
- Pera, M.C.; Coratti, G.; Forcina, N.; Mazzone, E.S.; Scoto, M.; Montes, J.; Pasternak, A.; Mayhew, A.; Messina, S.; Sframeli, M.; et al. Content validity and clinical meaningfulness of the HFMSE in spinal muscular atrophy. BMC Neurol. 2017, 17, 39. [Google Scholar] [CrossRef]
- Duong, T.; Staunton, H.; Braid, J.; Barriere, A.; Trzaskoma, B.; Gao, L.; Willgoss, T.; Cruz, R.; Gusset, N.; Gorni, K.; et al. A Patient-Centered Evaluation of Meaningful Change on the 32-Item Motor Function Measure in Spinal Muscular Atrophy Using Qualitative and Quantitative Data. Front. Neurol. 2021, 12, 770423. [Google Scholar] [CrossRef] [PubMed]
- Meyer, T.; Maier, A.; Uzelac, Z.; Hagenacker, T.; Günther, R.; Schreiber-Katz, O.; Weiler, M.; Steinbach, R.; Weyen, U.; Koch, J.C.; et al. Treatment expectations and perception of therapy in adult patients with spinal muscular atrophy receiving nusinersen. Eur. J. Neurol. 2021, 28, 2582–2595. [Google Scholar] [CrossRef] [PubMed]
- Bieniaszewska, A.; Sobieska, M.; Steinborn, B.; Gajewska, E. Examination of Upper Limb Function and the Relationship with Gross Motor Functional and Structural Parameters in Patients with Spinal Muscular Atrophy. Biomedicines 2023, 11, 1005. [Google Scholar] [CrossRef] [PubMed]
- Pechmann, A.; Behrens, M.; Dörnbrack, K.; Tassoni, A.; Wenzel, F.; Stein, S.; Vogt, S.; Zöller, D.; Bernert, G.; Hagenacker, T.; et al. Improved upper limb function in non-ambulant children with SMA type 2 and 3 during nusinersen treatment: A prospective 3-years SMArtCARE registry study. Orphanet J. Rare Dis. 2022, 17, 384. [Google Scholar] [CrossRef] [PubMed]
- Slayter, J.; Casey, L.; O’Connell, C. Patient Reported Outcome Measures in Adult Spinal Muscular Atrophy: A Scoping Review and Graphical Visualization of the Evidence. J. Neuromuscul. Dis. 2023, 10, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Shieh, P.B.; Kuntz, N.L.; Dowling, J.J.; Müller-Felber, W.; Bönnemann, C.G.; Seferian, A.M.; Servais, L.; Smith, B.K.; Muntoni, F.; Blaschek, A.; et al. Safety and efficacy of gene replacement therapy for X-linked myotubular myopathy (ASPIRO): A multinational, open-label, dose-escalation trial. Lancet Neurol. 2023, 22, 1125–1139. [Google Scholar] [CrossRef]
- Mercuri, E.; Sansone, V. Nusinersen in adults with spinal muscular atrophy: New challenges. Lancet Neurol. 2020, 19, 283–284. [Google Scholar] [CrossRef] [PubMed]
- Kuzma-Kozakiewicz, M.; Andersen, P.M.; Ciecwierska, K.; Vázquez, C.; Helczyk, O.; Loose, M.; Uttner, I.; Ludolph, A.C.; Lulé, D. An observational study on quality of life and preferences to sustain life in locked-in state. Neurology 2019, 93, e938–e945. [Google Scholar] [CrossRef]
- Duong, T.; Krosschell, K.J.; James, M.K.; Nelson, L.; Alfano, L.N.; Eichinger, K.; Mazzone, E.; Rose, K.; Lowes, L.P.; Mayhew, A.; et al. Consensus Guidelines for Improving Quality of Assessment and Training for Neuromuscular Diseases. Front. Genet. 2021, 12, 735936. [Google Scholar] [CrossRef]
- Servais, L.; Yen, K.; Guridi, M.; Lukawy, J.; Vissière, D.; Strijbos, P. Stride Velocity 95th Centile: Insights into Gaining Regulatory Qualification of the First Wearable-Derived Digital Endpoint for use in Duchenne Muscular Dystrophy Trials. J. Neuromuscul. Dis. 2022, 9, 335–346. [Google Scholar] [CrossRef] [PubMed]
| Total (n = 99) | RCS (n = 41) | RNCS (n = 18) | NR (n = 40) | Raw p-Value | |
|---|---|---|---|---|---|
| Age at diagnosis (y); | 2.36; | 2.36; | 1.77; | 3.7; | 0.47 |
| [Median (min–max)] | (0.01–28.5) | (0.01–28.5) | (0.49–16.5) | (0.23–22.0) | |
| Age of first symptoms (y); | 1.25; | 1.42; | 1.0; | 1.33; | 0.29 |
| [Median(min–max)] | (0.04–16.0) | (0.08–12.0) | (0.33–7.0) | (0.04–16.0) | |
| Age at treatment initiation (y); | 11.16; | 5.20; | 12.3; | 18.1; | |
| [Median(min–max)] | (0.39–57.4) | (0.39–47.8) | (1.41–48.8) | (0.83–57.4) | RCS < NR, p adjusted ≤ 0.001 |
| Sex (% male) | 51.5% | 48.8% | 50.0% | 55.0% | 0.85 |
| SMN2 copy, n | |||||
| 2 SMN2 copies | 9.0 | 5.0 | 2.0 | 2.0 | 0.13 |
| 3 SMN2 copies | 62.0 | 31.0 | 10.0 | 21.0 | |
| 4 SMN2 copies | 20.0 | 3.0 | 6.0 | 11.0 | |
| >4 SMN2 copies | 3.0 | 1.0 | 0.0 | 2.0 | |
| Unknown | 5.0 | 1.0 | 0.0 | 4.0 | |
| Patient type, n | |||||
| SMA Type 1 from 3 to 24 months | 6.0 | 4.0 | 0.0 | 2.0 | |
| SMA Type 1 from 24 months | 5.0 | 0.0 | 3.0 | 2.0 | 0.17 |
| SMA Type 2 from 6 to 24 months | 2.0 | 1.0 | 0.0 | 1.0 | |
| SMA Type 2 from 24 months | 32.0 | 11.0 | 8.0 | 13.0 | |
| SMA Type 3 from 24 months | 11.0 | 7.0 | 0.0 | 4.0 | |
| SMA Type 3 from 36 months | 44.0 | 18.0 | 7.0 | 18.0 | |
| Ambulatory status, n | |||||
| Non-ambulant | 58.0 | 20.0 | 13.0 | 25.0 | 0.30 |
| Loss of ambulation in progress | 9.0 | 6.0 | 0.0 | 3.0 | |
| Ambulant | 32.0 | 15.0 | 5.0 | 12.0 | |
| AE, n | |||||
| Hernia/fractures | 12.0% | 43.0% | 0% | 0% | |
| Spinal surgery a | 52.0% | 29.0% | 88.0% | 40.0% | 0.06 |
| Hospitalization in ICU | 12.0% | 14.0% | 12.0% | 10.0% | |
| NIV implementation | 8.0% | 14.0% | 0% | 10.0% | |
| Two AEs | 4.0% | 0.0% | 0.0% | 10.0% | |
| Other | 12.0% | 0.0% | 0.0% | 30.0% | |
| Physiotherapy (% with physiotherapy) | 78.8% | 92.7% | 61.1% | 72.5% | RCS > NR, RNCS < NR, p adjusted = 0.01 |
| Contractures (% with contracture) | |||||
| Left wrist/hand | 15.2% | 2.4% | 27.8% | 22.5% | RCS < RNCS, p adjusted = 0.01 |
| Right wrist/hand | 15.2% | 0.0% | 33.3% | 22.5% | RNCS > NR, p adjusted = 0.001 |
| Left elbow | 23.2% | 14.6% | 33.3% | 27.5% | 0.21 |
| Right elbow | 23.2% | 14.6% | 33.3% | 27.5% | 0.21 |
| Left shoulder | 10.1% | 2.4% | 22.2% | 12.5% | 0.06 |
| Right shoulder | 10.1% | 2.4% | 22.2% | 12.5% | 0.06 |
| Left hip | 41.4% | 31.7% | 61.1% | 42.5% | 0.11 |
| Right hip | 41.4% | 31.7% | 61.1% | 42.5% | 0.11 |
| Left knee | 52.5% | 51.2% | 50.0% | 55.0% | 0.92 |
| Right knee | 51.5% | 51.2% | 44.4% | 55.0% | 0.76 |
| Left ankle | 48.5% | 36.6% | 55.6% | 57.5% | 0.14 |
| Right ankle | 44.4% | 31.7% | 50.0% | 55.0% | 0.09 |
| Neck | 10.1% | 0.0% | 22.2% | 15.0% | 0.13 |
| Scoliosis (% with scoliosis) | 64.6% | 56.1% | 83.3% | 65.0% | 0.13 |
| All spinal surgery b (% with spinal surgery) | 27.3% | 9.8% | 38.9% | 40.0% | RCS < RNCS, RCS < NR, p adjusted = 0.03 |
| Ventilatory assistance (% with assistance) | 23.2% | 14.6% | 44.4% | 22.5% | RCS < RNCS, RNCS > NR, p adjusted = 0.04 |
| % with diurnal assistance | 23.0% | 17.0% | 37.0% | 12.5% | 0.45 |
| % with nocturnal assistance | 87.0% | 100.0% | 87.0% | 78.0% | 0.45 |
| Feeding assistance (% with assistance) | 7.10% | 4.90% | 5.60% | 10.0% | 0.64 |
| Assessment Type | Age Category | RCS (n = 41) | RNCS (n = 18) | NR (n = 40) |
|---|---|---|---|---|
| CHOP-INTEND (Δ) | Children (<18 y) | 16 ± 4.74 (n = 5) | 2.0 1.0 (n = 3) | −2.0 ± 2.0 (n = 3) |
| Adult (≥18 y) | – | – | – | |
| HINE-2 (Δ) | Children (<18 y) | – | – | −0.50 ± 0.71 (n = 2) |
| Adult (≥18 y) | – | – | – | |
| HFMSE (Δ) | Children (<18 y) | 8.4 ± 4.5 (n = 22) | 1.6 ± 0.52 (n = 10) | −1.82 ± 2.14 (n = 11) |
| Adult (≥18 y) | 7.6 ± 5.4 (n = 5) | 2.0 (n = 1) | −1.54 ± 1.94 (n = 13) | |
| MFM32 (Δ) | Children (<18 y) | – | 2.0 (n = 1) | −6.0 (n = 1) |
| Adult (≥18 y) | – | – | – | |
| MFM20 (Δ) | Children (<18 y) | 8.67 ± 3.2 (n = 3) | – | – |
| Adult (≥18 y) | – | – | – | |
| RULM (Δ) | Children (<18 y) | 5.0 ± 2.8 (n = 2) | 1.0 ± 0.0 (n = 2) | −1.0 ± 1.4 (n = 2) |
| Adult (≥18 y) | 3.0 ± 1.4 (n = 2) | 1.0 ± 0.0 (n = 1) | −1.0 ± 1.0 (n = 5) | |
| 6MWT (Δ) | Children (<18 y) | 91.0 (n = 1) | – | −8.0 (n = 1) |
| Adult (≥18 y) | 86.0 (n = 1) | – | −13.0 ± 18.4 (n = 2) |
| Questions (n = 21) | Symptom/Difficulty before Treatment Initiation? (Baseline Question) | RCS (n = 41) | RNCS (n = 18) | NR (n = 40) | Raw p-Value |
|---|---|---|---|---|---|
| Mean Rank | |||||
| Change in tremor | Yes | 33.8 (n = 32) | 40.5 (n = 12) | 29.3 (n = 22) | 0.23 |
| No | – (n = 9) | – (n = 6) | – (n = 18) | ||
| Change in balance while sitting | YES | 26.2 (n = 18) | 19.8 (n = 9) | 18.5 (n = 16) | 0.16 |
| No | 32.5 (n = 23) | 28.7 (n = 9) | 24.5 (n = 24) | RCS > NR, p adjusted = 0.04 | |
| Change in balance while standing/walking | Yes | 43.2 (n = 34) | 35.1 (n = 14) | 35.9 (n = 29) | 0.32 |
| No | – (n = 7) | – (n = 4) | – (n = 11) | ||
| Change in fatigue | Yes | 22.0 (n = 19) | 26.2 (n = 9) | 17.8 (n = 14) | 0.22 |
| No | 28.6 (n = 22) | 31.7 (n = 9) | 28.4 (n = 26) | 0.76 | |
| Change in function involving hand and wrist muscles | Yes | 32.7 (n = 23) | 29.9 (n = 11) | 23.4 (n = 22) | 0.13 |
| No | 24.6 (n = 18) | 23.2 (n = 7) | 18.9 (n = 18) | 0.07 | |
| Change in function involving arm muscles | Yes | 35.1 (n = 22) | 21.9 (n = 9) | 23.7 (n = 24) | RCS > NR, p adjusted = 0.04 |
| No | 24.4 (n = 19) | 23.4 (n = 9) | 19.7 (n = 16) | 0.14 | |
| Change in function involving shoulder muscles | Yes | 42.7 (n = 26) | 26.4 (n = 13) | 29.4 (n = 28) | RCS > RNCS, p adjusted = 0.02 RCS > NR, p adjusted = 0.02 |
| No | 18.9 (n = 15) | 18.9 (n = 5) | 12.5 (n = 12) | RCS > NR, p adjusted = 0.04 | |
| Change in ability to eat | Yes | 17.7 (n = 12) | 18.3 (n = 7) | 15.7 (n = 14) | 0.79 |
| No | 33.5 (n = 29) | 33.5 (n = 11) | 33.5 (n = 26) | 1.00 | |
| Change in appetite | Yes | – (n = 9) | – (n = 6) | – (n = 10) | |
| No | 40.1 (n = 32) | 38.1 (n = 12) | 34.5 (n = 30) | 0.24 | |
| Change in aspiration | Yes | – (n = 10) | – (n = 5) | – (n = 6) | |
| No | 40.7 (n = 31) | 39.5 (n = 13) | 38.4 (n = 34) | 0.55 | |
| Change in swallowing | Yes | – (n = 9) | – (n = 4) | – (n = 11) | |
| No | 37.5 (n = 32) | 37.5 (n = 14) | 38.8 (n = 29) | 0.76 | |
| Change in chewing | Yes | 15.5 (n = 11) | 14.6 (n = 6) | 12.1 (n = 10) | 0.59 |
| No | 36.0 (n = 30) | 36.0 (n = 12) | 37.2 (n = 30) | 0.87 | |
| Change in loudness of voice | Yes | – (n = 8) | – (n = 6) | – (n = 8) | |
| No | 42.1 (n = 33) | 37.5 (n = 12) | 36.4 (n = 32) | RCS > NR, p adjusted = 0.04 | |
| Change in capacity for continuous conversation | Yes | – (n = 8) | – (n = 6) | – (n = 5) | |
| No | 42.6 (n = 33) | 39.0 (n = 12) | 39.0 (n = 35) | 0.26 | |
| Change in quality of sleep | Yes | 18.5 (n = 19) | 22.3 (n = 6) | 14.6 (n = 10) | 0.24 |
| No | 33.4 (n = 22) | 32.0 (n = 12) | 32.0 (n = 30) | 0.82 | |
| Change in need for nocturnal ventilation | Yes | – (n = 6) | – (n = 6) | – (n = 9) | |
| No | 40.1 (n = 35) | 42.3 (n = 12) | 37.8 (n = 31) | 0.35 | |
| Change in diurnal ventilation | Yes | – (n = 2) | – (n = 3) | – (n = 4) | |
| No | 45.9 (n = 39) | 46.0 (n = 15) | 44.8 (n = 36) | 0.78 | |
| Change in cough | Yes | 23.0 (n = 15) | 21.6 (n = 8) | 17.7 (n = 17) | 0.39 |
| No | 29.5 (n = 26) | 29.5 (n = 10) | 30.8 (n = 23) | 0.77 | |
| Change in frequency of respiratory infections | Yes | 18.9 (n = 13) | 19.2 (n = 8) | 18.9 (n = 16) | 0.99 |
| No | 30.8 (n = 28) | 33.0 (n = 10) | 31.7 (n = 24) | 0.78 | |
| Change in the frequency of hospitalization due to respiratory infection | Yes | – (n = 7) | – (n = 5) | – (n = 10) | |
| No | 39.0 (n = 34) | 39.0 (n = 13) | 39.0 (n = 30) | 1.00 | |
| Change in recurrent disease-related pain | Yes | 17.7 (n = 16) | 18.8 (n = 3) | 13.1 (n = 12) | 0.33 |
| No | 34.0 (n = 25) | 34.0 (n = 15) | 35.2 (n = 28) | 0.93 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lilien, C.; Vrscaj, E.; Thapaliya, G.; Deconinck, N.; De Waele, L.; Duong, T.; Haberlová, J.; Kumhera, M.; Peirens, G.; Szabo, L.; et al. Patients’ Perceptions of Nusinersen Effects According to Their Responder Status. J. Clin. Med. 2024, 13, 3418. https://doi.org/10.3390/jcm13123418
Lilien C, Vrscaj E, Thapaliya G, Deconinck N, De Waele L, Duong T, Haberlová J, Kumhera M, Peirens G, Szabo L, et al. Patients’ Perceptions of Nusinersen Effects According to Their Responder Status. Journal of Clinical Medicine. 2024; 13(12):3418. https://doi.org/10.3390/jcm13123418
Chicago/Turabian StyleLilien, Charlotte, Eva Vrscaj, Gita Thapaliya, Nicolas Deconinck, Liesbeth De Waele, Tina Duong, Jana Haberlová, Markéta Kumhera, Geertrui Peirens, Lena Szabo, and et al. 2024. "Patients’ Perceptions of Nusinersen Effects According to Their Responder Status" Journal of Clinical Medicine 13, no. 12: 3418. https://doi.org/10.3390/jcm13123418
APA StyleLilien, C., Vrscaj, E., Thapaliya, G., Deconinck, N., De Waele, L., Duong, T., Haberlová, J., Kumhera, M., Peirens, G., Szabo, L., Tahon, V., Tang, W. J., Benmhammed, N., Médard, L., & Servais, L. (2024). Patients’ Perceptions of Nusinersen Effects According to Their Responder Status. Journal of Clinical Medicine, 13(12), 3418. https://doi.org/10.3390/jcm13123418


.png)



