The Ross Procedure: Imaging, Outcomes and Future Directions in Aortic Valve Replacement
Abstract
1. Introduction
2. Historical Perspective
2.1. Overview of the Development and Evolution of the Ross Procedure
2.2. Milestones and Key Contributors in Advancing the Technique
3. Surgical Technique
Surgeon’s Expertise
4. Patient Selection Criteria
5. Outcomes and Complications
5.1. Analysis of Short-Term and Long-Term Survival Rates
5.2. Evaluation of Postoperative Complications and Their Management
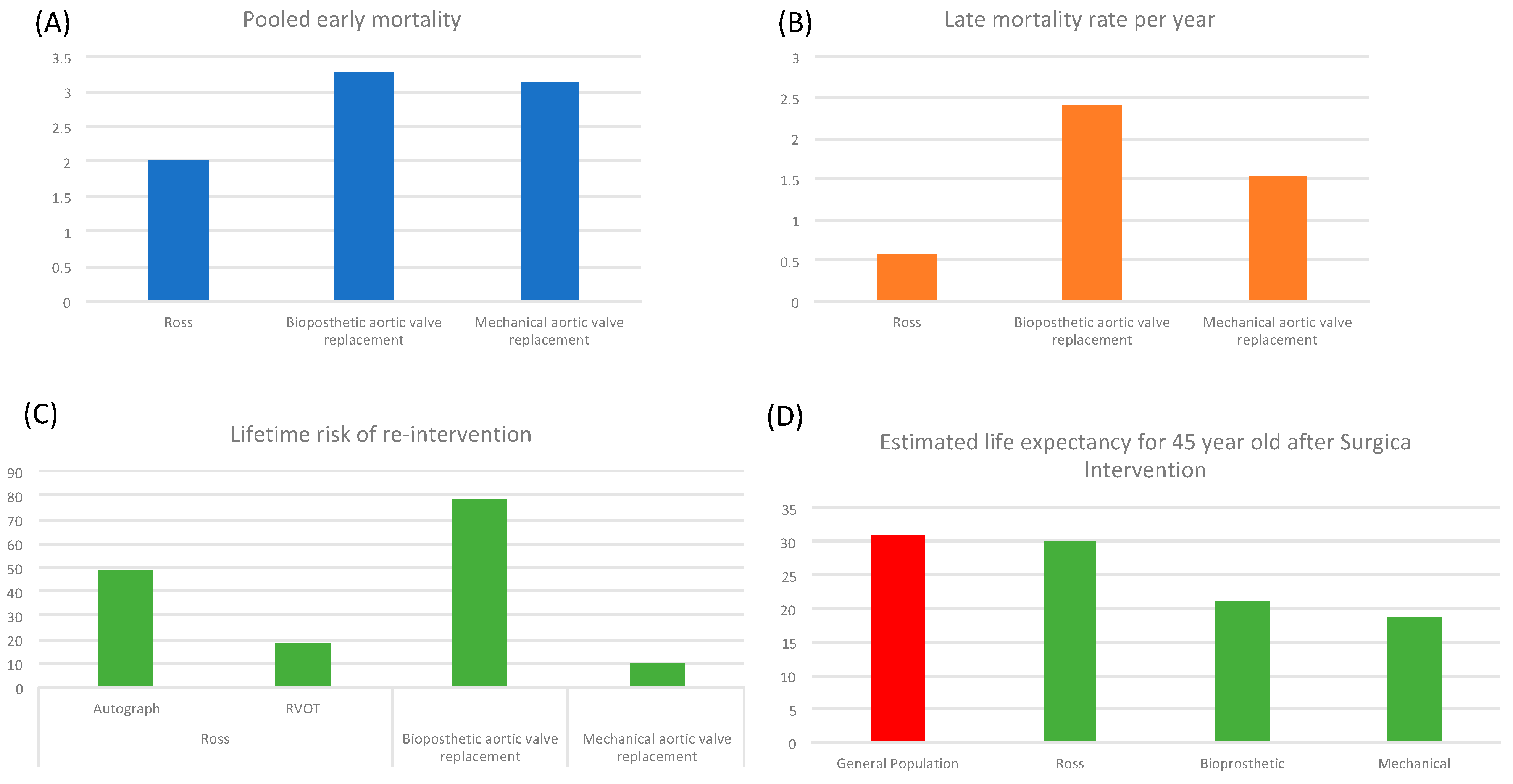
5.3. Comparison of Outcomes with Alternative Procedures
5.4. Comparison of Outcomes with Alternative Procedures Entered in Microsimulation
6. Imaging in Ross Procedure
6.1. Pre-Procedural Imaging
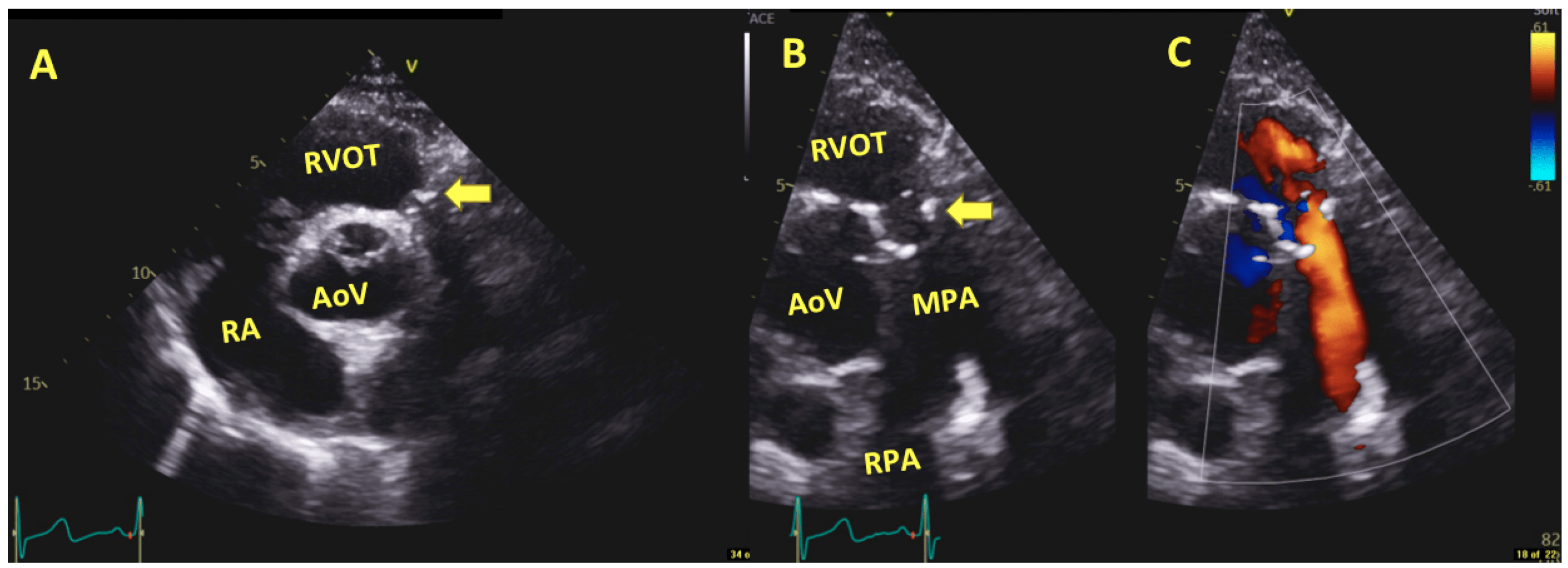
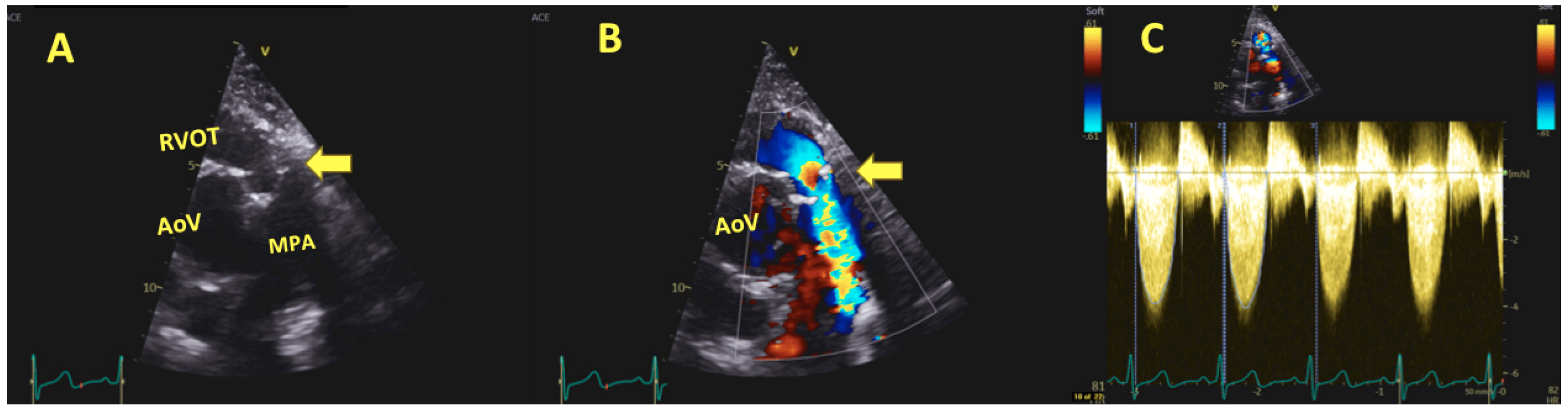
6.2. Intraoperative Imaging
6.3. Post-Operative and Long-Term Follow-Up Imaging
7. Hemodynamic Performance
8. Quality of Life and Functional Outcomes
8.1. Postoperative Quality of Life Measures
8.2. Assessment of Functional Outcomes and Exercise Capacity
8.3. Comparison of Quality of Life and Functional Outcomes with Alternative Procedures
9. Patient Selection and Risk Stratification
The Ross Procedure for the Treatment of Infective Endocarditis
10. Contemporary Techniques and Innovations
11. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Takkenberg, J.J.; Klieverik, L.M.; Schoof, P.H.; van Suylen, R.J.; van Herwerden, L.A.; Zondervan, P.E.; Roos-Hesselink, J.W.; Eijkemans, M.J.; Yacoub, M.H.; Bogers, A.J. The Ross procedure: A systematic review and meta-analysis. Circulation 2009, 119, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Ercan, S.; Koçak, I.; Özkan, F. Valve performance classification in 630 subcoronary Ross patients over 22 years. J. Thorac. Cardiovasc. Surg. 2012, 144, 413–420. [Google Scholar] [CrossRef]
- Sharabiani, M.T.; Dorobantu, D.M.; Mahani, A.S.; Turner, M.; Tometzki, A.J.P.; Angelini, G.D.; Parry, A.J.; Caputo, M.; Stoica, S.C. Aortic Valve Replacement and the Ross Operation in Children and Young Adults. J. Am. Coll. Cardiol. 2016, 67, 2858–2870. [Google Scholar] [CrossRef] [PubMed]
- Mookhoek, A.; Charitos, E.I.; Hazekamp, M.G.; Bogers, A.J.; Hörer, J.; Lange, R.; Hetzer, R.; Sachweh, J.S.; Riso, A.; Stierle, U.; et al. Ross Procedure in Neonates and Infants: A European Multicenter Experience. Ann. Thorac. Surg. 2015, 100, 2278–2284. [Google Scholar] [CrossRef] [PubMed]
- Stulak, J.M.; Burkhart, H.M.; Sundt, T.M.; Connolly, H.M.; Suri, R.M.; Schaff, H.V.; Dearani, J.A. Spectrum and Outcome of Reoperations after the Ross Procedure. Circulation 2010, 122, 1153–1158. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; O’Gara, P.T.; et al. 2020 ACC/AHA Guideline for the Management of Patients with Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, e35–e71. [Google Scholar] [CrossRef] [PubMed]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. ESC/EACTS Scientific Document Group. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef] [PubMed]
- Mazine, A.; Rocha, R.V.; El-Hamamsy, I.; Ouzounian, M.; Yanagawa, B.; Bhatt, D.L.; Verma, S.; Friedrich, J.O. Ross Procedure vs Mechanical Aortic Valve Replacement in Adults: A Systematic Review and Me-ta-analysis. JAMA Cardiol. 2018, 3, 978–987. [Google Scholar] [CrossRef]
- El-Hamamsy, I.; Toyoda, N.; Itagaki, S.; Stelzer, P.; Varghese, R.; Williams, E.E.; Erogova, N.; Adams, D.H. Propensity-Matched Comparison of the Ross Procedure and Prosthetic Aortic Valve Re-placement in Adults. J. Am. Coll. Cardiol. 2022, 79, 805–815. [Google Scholar] [CrossRef]
- Ross, D. Replacement of aortic and mitral valves with a pulmonary autograft. Lancet 1967, 2, 956–958. [Google Scholar] [CrossRef]
- Al Halees, Z.; Awad, M.M.; Pieters, F.; Shahid, M.S.; Al Amri, M.A. Six-year follow-up of a pulmonary autograft in the mitral position: The Ross II procedure. J. Thorac. Cardiovasc. Surg. 1999, 117, 614–616. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kumar, N.; Gallo, R.; Gometza, B.; Al-Halees, Z.; Duran, C.M. Pulmonary autograft for aortic valve replacement in rheumatic disease—An ideal solution? J. Heart Valve Dis. 1994, 3, 384–387. [Google Scholar] [PubMed]
- Kholaif, N.; Mohamed, T.I.; Alharbi, I.S.; Aljenedil, S.A.; AlHumaidan, H.; Al-Ashwal, A.; Almahfouz, A.; Algorashi, S.; Almasood, A.; Baqal, O.J. Management and clinical outcomes of patients with homozygous familial hypercholesteremia in Saudi Arabia. Monaldi Arch Chest Dis. 2023, 93. [Google Scholar] [CrossRef] [PubMed]
- Kabbani, S.; Jamil, H.; Nabhani, F.; Hamoud, A.; Katan, K.; Sabbagh, N.; Koudsi, A.; Kabbani, L.; Hamed, G. Analysis of 92 mitral pulmonary autograft replacement (Ross II) operations. J. Thorac. Cardiovasc. Surg. 2007, 134, 902–908.e7. [Google Scholar] [CrossRef] [PubMed]
- El-Hamamsy, I.; Eryigit, Z.; Stevens, L.-M.; Sarang, Z.; George, R.; Clark, L.; Melina, G.; Takkenberg, J.J.; Yacoub, M.H. Long-term outcomes after autograft versus homograft aortic root replacement in adults with aortic valve disease: A randomised controlled trial. Lancet 2010, 376, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Elkins, R.C.; Thompson, D.M.; Lane, M.M.; Elkins, C.C.; Peyton, M.D. Ross operation: 16-year experience. J. Thorac. Cardiovasc. Surg. 2008, 136, 623–630.e5. [Google Scholar] [CrossRef] [PubMed]
- Stelzer, P. The Ross Procedure: State of the Art 2011. Semin. Thorac. Cardiovasc. Surg. 2011, 23, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Pergola, V.; Di Salvo, G.; Fadel, B.; Galzerano, D.; Al-Shaid, M.; Al-Admawi, M.; Al Amri, M.; Al-Ahmadi, M.; Al-Halees, Z. The long term results of the Ross procedure: The importance of candidate selection. Int. J. Cardiol. 2020, 320, 35–41. [Google Scholar] [CrossRef]
- AlHalees, Z. Chapter 20-The Pulmonary Autograft for Aortic Valve Replacement. In Operative Cardiac Surgery, 6th ed.; Spray, T.L., Acker, M.A., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 227–230. [Google Scholar]
- Mazine, A.; David, T.E.; Stoklosa, K.; Chung, J.; Lafreniere-Roula, M.; Ouzounian, M. Improved Outcomes Following the Ross Procedure Compared with Bioprosthetic Aortic Valve Replacement. J. Am. Coll. Cardiol. 2022, 79, 993–1005. [Google Scholar] [CrossRef]
- Ouzounian, M.; Mazine, A.; David, T.E. The Ross procedure is the best operation to treat aortic stenosis in young and middle-aged adults. J. Thorac. Cardiovasc. Surg. 2017, 154, 778–782. [Google Scholar] [CrossRef]
- Al-Halees, Z.; Kumar, N.; Gallo, R.; Gometza, B.; Duran, C.M. Pulmonary autograft for aortic valve replacement in rheumatic disease: A caveat. Ann. Thorac. Surg. 1995, 60 (Suppl. 2), S172–S176, discussion S176. [Google Scholar] [CrossRef] [PubMed]
- David, T.E.; Ouzounian, M.; David, C.M.; Lafreniere-Roula, M.; Manlhiot, C. Late results of the Ross procedure. J. Thorac. Cardiovasc. Surg. 2019, 157, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Chauvette, V.; Lefebvre, L.; Chamberland, M.; Williams, E.E.; El-Hamamsy, I. Contemporary Review of the Ross Procedure. Struct. Heart 2021, 5, 11–23. [Google Scholar] [CrossRef]
- Stelzer, P.; Mejia, J.; Varghese, R. Operative risks of the Ross procedure. J. Thorac. Cardiovasc. Surg. 2021, 161, 905–915.e3. [Google Scholar] [CrossRef] [PubMed]
- Ryan, W.H.; Squiers, J.J.; Harrington, K.B.; Goodenow, T.; Rawitscher, C.; Schaffer, J.M.; DiMaio, J.M.; Brinkman, W.T. Long-term outcomes of the Ross procedure in adults. Ann. Cardiothorac. Surg. 2021, 10, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Alsoufi, B.; Al-Halees, Z.; Manlhiot, C.; McCrindle, B.W.; Al-Ahmadi, M.; Sallehuddin, A.; Canver, C.C.; Bulbul, Z.; Joufan, M.; Fadel, B. Mechanical valves versus the Ross procedure for aortic valve replacement in children: Propensity-adjusted comparison of long-term outcomes. J. Thorac. Cardiovasc. Surg. 2009, 137, 362–370.e9. [Google Scholar] [CrossRef] [PubMed]
- Aboud, A.; Charitos, E.I.; Fujita, B.; Stierle, U.; Reil, J.-C.; Voth, V.; Liebrich, M.; Andreas, M.; Holubec, T.; Bening, C.; et al. Long-Term Outcomes of Patients Undergoing the Ross Procedure. J. Am. Coll. Cardiol. 2021, 77, 1412–1422. [Google Scholar] [CrossRef]
- Laudito, A.; Brook, M.M.; Suleman, S.; Bleiweis, M.S.; Thompson, L.D.; Hanley, F.L.; Reddy, V. The Ross procedure in children and young adults: A word of caution. J. Thorac. Cardiovasc. Surg. 2001, 122, 147–153. [Google Scholar] [CrossRef]
- Gebauer, R.; Cerny, S. A modification of the Ross procedure to prevent pulmonary autograft dilatation. Eur. J. Cardio-Thoracic Surg. 2009, 36, 195–197. [Google Scholar] [CrossRef]
- Starnes, V.A.; Bowdish, M.E.; Cohen, R.G.; Baker, C.J.; Elsayed, R.S. The Ross procedure utilizing the pulmonary autograft inclusion technique in adults. JTCVS Tech. 2021, 10, 372–376. [Google Scholar] [CrossRef]
- Kenny, L.A.; Austin, C.; Golesworthy, T.; Venugopal, P.; Alphonso, N. Personalized External Aortic Root Support (PEARS) for Aortic Root Aneurysm. Oper. Tech. Thorac. Cardiovasc. Surg. 2021, 26, 290–305. [Google Scholar] [CrossRef]
- Williams, E.; El-Hamamsy, I. Commentary: The Ross procedure in a graft: A word of caution. JTCVS Tech. 2021, 10, 377–378. [Google Scholar] [CrossRef] [PubMed]
- Etnel, J.R.; Grashuis, P.; Huygens, S.A.; Pekbay, B.; Papageorgiou, G.; Helbing, W.A.; Roos-Hesselink, J.W.; Bogers, A.J.; Mokhles, M.M.; Takkenberg, J.J. The Ross Procedure: A Systematic Review, Meta-Analysis, and Microsimulation. Circ. Cardiovasc. Qual. Outcomes 2018, 11, e004748. [Google Scholar] [CrossRef] [PubMed]
- Etnel, J.R.; Huygens, S.A.; Grashuis, P.; Pekbay, B.; Papageorgiou, G.; Hesselink, J.W.R.; Bogers, A.J.; Takkenberg, J.J. Bioprosthetic Aortic Valve Replacement in Nonelderly Adults: A Systematic Review, Meta-Analysis, Microsimulation. Circ. Cardiovasc. Qual. Outcomes 2019, 12, e005481. [Google Scholar] [CrossRef]
- Korteland, N.M.; Etnel, J.R.G.; Arabkhani, B.; Mokhles, M.M.; Mohamad, A.; Roos-Hesselink, J.W.; Bogers, A.J.J.C.; Takkenberg, J.J.M. Mechanical aortic valve replacement in non-elderly adults: Meta-analysis and microsimulation. Eur. Hear. J. 2017, 38, 3370–3377. [Google Scholar] [CrossRef] [PubMed]
- Al Halees, Z. Commentary: The pulmonary autograft, too valuable to repeal. JTCVS Tech. 2021, 10, 415–416. [Google Scholar] [CrossRef] [PubMed]
- Goldstone, A.B.; Woo, Y.J. Valve-sparing reoperations for failed pulmonary autografts. JTCVS Tech. 2021, 10, 408–412. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Kuno, T.; Toyoda, N.; Fujisaki, T.; Takagi, H.; Itagaki, S.; Ibrahim, M.; Ouzounian, M.; El-Hamamsy, I. Ross Procedure Versus Mechanical Versus Bioprosthetic Aortic Valve Replacement: A Network Meta-Analysis. J. Am. Heart Assoc. 2023, 12, e8066. [Google Scholar] [CrossRef]
- Aicher, D.; Holz, A.; Feldner, S.; Köllner, V.; Schäfers, H.-J. Quality of life after aortic valve surgery: Replacement versus reconstruction. J. Thorac. Cardiovasc. Surg. 2011, 142, e19–e24. [Google Scholar] [CrossRef]
- Fadel, B.M.; Mohty, D.; Husain, A.; Dahdouh, Z.; Al-Admawi, M.; Pergola, V.; Di Salvo, G. The Various Hemodynamic Profiles of the Patent Ductus Arteriosus in Adults. Echocardiography 2015, 32, 1172–1178. [Google Scholar] [CrossRef]
- Baumgartner, H.; De Backer, J.; Babu-Narayan, S.V.; Budts, W.; Chessa, M.; Diller, G.-P.; lung, B.; Kluin, J.; Lang, I.M.; Meijboom, F.; et al. 2020 ESC Guidelines for the management of adult congenital heart disease. Eur. Heart J. 2021, 42, 563–645. [Google Scholar] [CrossRef] [PubMed]
- Mueller, G.C.; Becker, O.; Mir, T.; Arndt, F.; Kozlik-Feldmann, R.; Dodge-Khatami, A. Functional Outcomes after the Ross Procedure. Thorac. Cardiovasc. Surg. 2015, 63. [Google Scholar] [CrossRef]
- Marino, B.S.; Pasquali, S.K.; Wernovsky, G.; Bockoven, J.R.; McBride, M.; Cho, C.J.; Spray, T.L.; Paridon, S.M. Exercise performance in children and adolescents after the Ross procedure. Cardiol. Young 2006, 16, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Doty, D.B.; Flores, J.H.; Yanowitz, F.G.; Oury, J.H. Maximum Exercise after Aortic Valve Replacement with Pulmonary Autograft. Asian Cardiovasc. Thorac. Ann. 1999, 7, 37–39. [Google Scholar] [CrossRef]
- Alsoufi, B.; Manlhiot, C.; Fadel, B.; Al-Ahmadi, M.; Tamim, M.; McCrindle, B.W.; Canver, C.C.; Al-Halees, Z. The Ross procedure in children: Preoperative haemodynamic manifestation has significant effect on late autograft re-operation. Eur. J. Cardio-Thorac. Surg. 2010, 38, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Al-Halees, Z.; Pieters, F.; Qadoura, F.; Shahid, M.; Al-Amri, M.; Al-Fadley, F. The Ross procedure is the procedure of choice for congenital aortic valve disease. J. Thorac. Cardiovasc. Surg. 2002, 123, 437–442. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fadel, B.M.; Manlhiot, C.; Al-Halees, Z.; Di Salvo, G.; Al-Ahmadi, M.; McCrindle, B.; Alsoufi, B. The fate of the neoaortic valve and root after the modified Ross–Konno procedure. J. Thorac. Cardiovasc. Surg. 2013, 145, 430–437.e1. [Google Scholar] [CrossRef] [PubMed]
- Al Halees, Z. The Mini-Ross–Konno procedure. Eur. J. Cardio-Thorac. Surg. 2011, 39, 1067–1069. [Google Scholar] [CrossRef][Green Version]
- Alsoufi, B.; Manlhiot, C.; Fadel, B.; Al-Fayyadh, M.; McCrindle, B.W.; Alwadai, A.; Al-Halees, Z. Is the ross procedure a suitable choice for aortic valve replacement in children with rheumatic aortic valve disease? World J. Pediatr. Congenit. Heart Surg. 2012, 3, 8–15. [Google Scholar] [CrossRef]
- Le Guillou, V.; Bouchart, F.; Gay, A.; Nafeh-Bizet, C.; Hubscher, C.; Tabley, A.; Bessou, J.P.; Doguet, F. The Ross procedure in endocarditis: A report of 28 cases. Eur. J. Cardio-Thoracic Surg. 2014, 45, 153–158. [Google Scholar] [CrossRef][Green Version]
- Ratschiller, T.; Sames-Dolzer, E.; Paulus, P.; Schimetta, W.; Müller, H.; Zierer, A.F.; Mair, R. Long-term Evaluation of the Ross Procedure in Acute Infective Endocarditis. In Seminars in Thoracic and Cardiovascular Surgery; WB Saunders: Philadelphia, PA, USA, 2017. [Google Scholar] [CrossRef]
- Loobuyck, V.; Soquet, J.; Moussa, M.D.; Coisne, A.; Pinçon, C.; Richardson, M.; Rousse, N.; Mugnier, A.; Juthier, F.; Marechaux, S.; et al. Active Aortic Endocarditis in Young Adults: Long-term Results of the Ross Procedure. Ann. Thorac. Surg. 2020, 110, 856–861. [Google Scholar] [CrossRef] [PubMed]
- Lamelas, J. Minimally invasive aortic valve replacement: The “Miami Method”. Ann. Cardiothorac. Surg. 2015, 4, 71–77. [Google Scholar] [PubMed]
- Huygens, S.A.; Rutten-van Mölken, M.P.M.H.; Noruzi, A.; Etnel, J.R.G.; Corro Ramos, I.; Bouten, C.V.C.; Kluin, J.; Takkenberg, J.J.M. What Is the Potential of Tissue-Engineered Pulmonary Valves in Children? Ann. Thorac. Surg. 2019, 107, 1845–1853. [Google Scholar] [CrossRef] [PubMed]
- Dohmen, P.M.; Lembcke, A.; Holinski, S.; Kivelitz, D.; Braun, J.P.; Pruss, A.; Konertz, W. Mid-Term Clinical Results Using a Tissue-Engineered Pulmonary Valve to Re-construct the Right Ventricular Outflow Tract During the Ross Procedure. Ann. Thorac. Surg. 2007, 84, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Karamlou, T.; Pettersson, G.; Nigro, J.J. Commentary: A pediatric perspective on the Ozaki procedure. J. Thorac. Cardiovasc. Surg. 2021, 161, 1582–1583. [Google Scholar] [CrossRef] [PubMed]
- Drullinsky, D.; Mehta, C.K.; Scott, M.B.; Crawford, E.; Markl, M.; Bonow, R.O.; Mendelson, M.A.; El-Hamamsy, I.; Malaisrie, S.C. Four-Dimensional Magnetic Resonance after Ross Procedure for Unicuspid Aortic Valve. Circ. Cardiovasc. Imaging 2021, 14, e011500. [Google Scholar] [CrossRef] [PubMed]
- Knott-Craig, C.J.; Goldberg, S.P.; Pastuszko, P.; Peyton, M.D.; Kirklin, J.K. The Ross operation for aortic valve disease: Previous sternotomy results in improved long-term outcome. J. Heart Valve Dis. 2007, 16, 394–397. [Google Scholar]
- El-Hamamsy, I.; Bouhout, I. The Ross procedure: Time for a hard look at current practices and a reexamination of the guidelines. Ann. Transl. Med. 2017, 5, 142. [Google Scholar] [CrossRef]
- Misfeld, M.; Borger, M.A. The Ross procedure: Time to reevaluate the guidelines. J. Thorac. Cardiovasc. Surg. 2019, 157, 211–212. [Google Scholar] [CrossRef]

| Relevant Study | Ross Procedure Outcomes |
|---|---|
| El-Hamamsy et al., RCT n(228) 2010 [9] | 10 Year Survival 97% |
| Ryan et al., 2021 n(225) [26] | 20 Year Survival 81.3% (74.8–88.3%) |
| Mortality (In-hospital) 0.9% (30 day) 2.2% | |
| Pergola et al., n(536) 2020 [18] | 15 Year Freedom from all Re-operation 83% |
| Freedom from Autograft reoperation 81% | |
| David et al., n(212) 2019 [23] | 20 Year Mortality 10.8% |
| Stelzer et al., n(702) 2021 [25] | Perioperative Mortality 1% |
| Aboud et al., n(2444) 2021 [28] | 25 Year Survival 75.8% |
| Early mortality 1% |
| Candidate Selection | |
|---|---|
| Better | Worse |
| Congenital etiology | Rheumatic |
| Aortic stenosis | Pure Aortic regurgitation |
| Aortic root diameter < 15 mm/m2 | Older age (homograft re-intervention) |
| Features | Benefit |
|---|---|
| Silent | ++++ |
| Non-thrombogenic | ++++ |
| Normal Hemodynamic | ++++ |
| Readily Available–Low cost | ++++ |
| Has Potential for growth | +++ |
| Infection Resistant | +++ |
| Easy to Implant | ++ |
| Durable-Non-Rheumatics -Rheumatics | ++++ ++ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galzerano, D.; Kholaif, N.; Al Amro, B.; Al Admawi, M.; Eltayeb, A.; Alshammari, A.; Di Salvo, G.; Al-Halees, Z.Y. The Ross Procedure: Imaging, Outcomes and Future Directions in Aortic Valve Replacement. J. Clin. Med. 2024, 13, 630. https://doi.org/10.3390/jcm13020630
Galzerano D, Kholaif N, Al Amro B, Al Admawi M, Eltayeb A, Alshammari A, Di Salvo G, Al-Halees ZY. The Ross Procedure: Imaging, Outcomes and Future Directions in Aortic Valve Replacement. Journal of Clinical Medicine. 2024; 13(2):630. https://doi.org/10.3390/jcm13020630
Chicago/Turabian StyleGalzerano, Domenico, Naji Kholaif, Bandar Al Amro, Mohammed Al Admawi, Abdalla Eltayeb, Amal Alshammari, Giovanni Di Salvo, and Zohair Y. Al-Halees. 2024. "The Ross Procedure: Imaging, Outcomes and Future Directions in Aortic Valve Replacement" Journal of Clinical Medicine 13, no. 2: 630. https://doi.org/10.3390/jcm13020630
APA StyleGalzerano, D., Kholaif, N., Al Amro, B., Al Admawi, M., Eltayeb, A., Alshammari, A., Di Salvo, G., & Al-Halees, Z. Y. (2024). The Ross Procedure: Imaging, Outcomes and Future Directions in Aortic Valve Replacement. Journal of Clinical Medicine, 13(2), 630. https://doi.org/10.3390/jcm13020630








