The Kynurenine Pathway in Attention-Deficit/Hyperactivity Disorder: A Systematic Review and Meta-Analysis of Blood Concentrations of Tryptophan and Its Catabolites
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Search Strategy and Inclusion Criteria
2.3. Data Extraction
2.4. Data Analysis
3. Results
3.1. Study Selection and Characteristics
3.2. TRYCAT Concentrations in People with ADHD (Overall Analyses)
3.3. TRYCAT Concentrations in Drug-Free Children with ADHD
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Posner, J.; Polanczyk, G.V.; Sonuga-Barke, E.J. Attention-deficit hyperactivity disorder. Lancet 2020, 395, 450–462. [Google Scholar] [CrossRef] [PubMed]
- Faraone, S.V.; Asherson, P.; Banaschewski, T.; Biederman, J.; Buitelaar, J.K.; Ramos-Quiroga, J.A.; Rohde, L.A.; Sonuga-Barke, E.J.; Tannock, R.; Franke, B. Attention-deficit/hyperactivity disorder. Nat. Rev. Dis. Primers 2015, 1, 15020. [Google Scholar] [CrossRef] [PubMed]
- Kessler, R.C.; Adler, L.; Barkley, R.; Biederman, J.; Conners, C.K.; Demler, O.; Faraone, S.V.; Greenhill, L.L.; Howes, M.J.; Secnik, K.; et al. The prevalence and correlates of adult ADHD in the United States: Results from the National Comorbidity Survey Replication. Am. J. Psychiatry 2006, 163, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Wilens, T.E.; Faraone, S.V.; Biederman, J. Attention-deficit/hyperactivity disorder in adults. JAMA 2004, 292, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Bartoli, F.; Callovini, T.; Cavaleri, D.; Cioni, R.M.; Bachi, B.; Calabrese, A.; Moretti, F.; Canestro, A.; Morreale, M.; Nasti, C.; et al. Clinical correlates of comorbid attention deficit hyperactivity disorder in adults suffering from bipolar disorder: A meta-analysis. Austr. N. Z. J. Psychiatry 2023, 57, 34–48. [Google Scholar] [CrossRef]
- Bitter, I.; Mohr, P.; Balogh, L.; Látalová, K.; Kakuszi, B.; Stopková, P.; Zmeškalová-Jelenová, D.; Pulay, A.; Czobor, P. ADHD: A hidden comorbidity in adult psychiatric patients. Atten. Defic. Hyperact. Disord. 2019, 11, 83–89. [Google Scholar] [CrossRef]
- Bonvicini, C.; Faraone, S.V.; Scassellati, C. Attention-deficit hyperactivity disorder in adults: A systematic review and meta-analysis of genetic, pharmacogenetic and biochemical studies. Mol. Psychiatry 2016, 21, 872–884. [Google Scholar] [CrossRef]
- Thapar, A.; Cooper, M.; Eyre, O.; Langley, K. What have we learnt about the causes of ADHD? J. Child. Psychol. Psychiatry 2013, 54, 3–16. [Google Scholar] [CrossRef]
- Takahashi, N.; Ishizuka, K.; Inada, T. Peripheral biomarkers of attention-deficit hyperactivity disorder: Current status and future perspective. J. Psychiatr. Res. 2021, 137, 465–470. [Google Scholar] [CrossRef]
- Misiak, B.; Wójta-Kempa, M.; Samochowiec, J.; Schiweck, C.; Aichholzer, M.; Reif, A.; Samochowiec, A.; Stańczykiewicz, B. Peripheral blood inflammatory markers in patients with attention deficit/hyperactivity disorder (ADHD): A systematic review and meta-analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry 2022, 118, 110581. [Google Scholar] [CrossRef]
- Park, J.H. Potential Inflammatory Biomarker in Patients with Attention Deficit Hyperactivity Disorder. Int. J. Mol. Sci. 2022, 23, 13054. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yang, Y.; Odisho, D.; Wu, S.; Yi, C.; Oliver, B.G. Can biomarkers be used to diagnose attention deficit hyperactivity disorder? Front. Psychiatry 2023, 14, 1026616. [Google Scholar] [CrossRef]
- Mehta, T.; Mannem, N.; Yarasi, N.K.; Bollu, P.C. Biomarkers for ADHD: The present and future directions. Curr. Dev. Disord. Rep. 2020, 7, 85–92. [Google Scholar] [CrossRef]
- Savitz, J. The kynurenine pathway: A finger in every pie. Mol. Psychiatry 2020, 25, 131–147. [Google Scholar] [CrossRef] [PubMed]
- Cervenka, I.; Agudelo, L.Z.; Ruas, J.L. Kynurenines: Tryptophan’s metabolites in exercise, inflammation, and mental health. Science 2017, 357, eaaf9794. [Google Scholar] [CrossRef]
- Schwarcz, R.; Stone, T.W. The kynurenine pathway and the brain: Challenges, controversies and promises. Neuropharmacology 2017, 112 Pt B, 237–247. [Google Scholar] [CrossRef]
- Schwarcz, R.; Bruno, J.P.; Muchowski, P.J.; Wu, H.Q. Kynurenines in the mammalian brain: When physiology meets pathology. Nat. Rev. Neurosci. 2012, 13, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Fujigaki, H.; Kato, K.; Yamazaki, K.; Fujigaki, S.; Kunisawa, K.; Yamamoto, Y.; Mouri, A.; Oda, A.; Nabeshima, T.; et al. Selective and competitive inhibition of kynurenine aminotransferase 2 by glycyrrhizic acid and its analogues. Sci. Rep. 2019, 9, 10243. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ding, L.; Zhang, H.; Mellor, D.; Wu, H.; Zhao, D.; Wu, C.; Lin, Z.; Yuan, J.; Peng, D. The Metabolic Factor Kynurenic Acid of Kynurenine Pathway Predicts Major Depressive Disorder. Front. Psychiatry 2018, 9, 552. [Google Scholar] [CrossRef] [PubMed]
- Myint, A.M. Kynurenines: From the perspective of major psychiatric disorders. FEBS J. 2012, 279, 1375–1385. [Google Scholar] [CrossRef] [PubMed]
- Fazio, F.; Lionetto, L.; Curto, M.; Iacovelli, L.; Cavallari, M.; Zappulla, C.; Ulivieri, M.; Napoletano, F.; Capi, M.; Corigliano, V.; et al. Xanthurenic Acid Activates mGlu2/3 Metabotropic Glutamate Receptors and is a Potential Trait Marker for Schizophrenia. Sci. Rep. 2015, 5, 17799. [Google Scholar] [CrossRef]
- Bohár, Z.; Toldi, J.; Fülöp, F.; Vécsei, L. Changing the face of kynurenines and neurotoxicity: Therapeutic considerations. Int. J. Mol. Sci. 2015, 16, 9772–9793. [Google Scholar] [CrossRef] [PubMed]
- Guillemin, G.J. Quinolinic acid, the inescapable neurotoxin. FEBS J. 2012, 279, 1356–1365. [Google Scholar] [CrossRef] [PubMed]
- Murakami, Y.; Imamura, Y.; Saito, K.; Sakai, D.; Motyama, J. Altered kynurenine pathway metabolites in a mouse model of human attention-deficit hyperactivity/ autism spectrum disorders: A potential new biological diagnostic marker. Sci. Rep. 2019, 9, 13182. [Google Scholar] [CrossRef] [PubMed]
- Puurunen, J.; Sulkama, S.; Tiira, K.; Araujo, C.; Lehtonen, M.; Hanhineva, K.; Lohi, H. A non-targeted metabolite profiling pilot study suggests that tryptophan and lipid metabolisms are linked with ADHD-like behaviours in dogs. Behav. Brain Funct. 2016, 12, 27. [Google Scholar] [CrossRef] [PubMed]
- Evangelisti, M.; De Rossi, P.; Rabasco, J.; Donfrancesco, R.; Lionetto, L.; Capi, M.; Sani, G.; Simmaco, M.; Nicoletti, F.; Villa, M.P. Changes in serum levels of kynurenine metabolites in paediatric patients affected by ADHD. Eur. Child. Adolesc. Psychiatry 2017, 26, 1433–1441. [Google Scholar] [CrossRef]
- Aarsland, T.I.M.; Landaas, E.T.; Hegvik, T.A.; Ulvik, A.; Halmøy, A.; Ueland, P.M.; Haavik, J. Serum concentrations of kynurenines in adult patients with attention-deficit hyperactivity disorder (ADHD): A case-control study. Behav. Brain. Funct. 2015, 11, 36. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Wolraich, M.L.; Hannah, J.N.; Pinnock, T.Y.; Baumgaertel, A.; Brown, J. Comparison of diagnostic criteria for attention-deficit hyperactivity disorder in a county-wide sample. J. Am. Acad. Child Adolesc. Psychiatry 1996, 35, 319–324. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Li, T.; Deeks, J.J. Chapter 6: Choosing Effect Measures and Computing Estimates of Effect. Cochrane Handbook for Systematic Reviews of Interventions Version 6.4; (Updated August 2023). Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; Cochrane: London, UK, 2023. Available online: www.training.cochrane.org/handbook (accessed on 1 December 2023).
- Luo, D.; Wan, X.; Liu, J.; Tong, T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat. Methods Med. Res. 2018, 27, 1785–1805. [Google Scholar] [CrossRef]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef] [PubMed]
- Andrade, C. Mean Difference, Standardized Mean Difference (SMD), and Their Use in Meta-Analysis: As Simple as It Gets. J. Clin. Psychiatry 2020, 81, 20f13681. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Higgins, J.P.T.; Sterne, J.A.C. Chapter 13: Assessing Risk of Bias Due to Missing Results in a Synthesis. Cochrane Handbook for Systematic Reviews of Interventions Version 6.4; (Updated August 2023). Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; Cochrane: London, UK, 2023. Available online: www.training.cochrane.org/handbook (accessed on 1 December 2023).
- StataCorp. Stata Statistical Software: Release 18; StataCorp LLC: College Station, TX, USA, 2023. [Google Scholar]
- Wallace, B.C.; Dahabreh, I.J.; Trikalinos, T.A.; Lau, J.; Trow, P.; Schmid, C.H. Closing the Gap between Methodologists and End-Users: R as a Computational Back-End. J. Stat. Softw. 2012, 49, 1–15. [Google Scholar] [CrossRef]
- Kilany, A.; Nashaat, N.H.; Zeidan, H.M.; Hashish, A.F.; El-Saied, M.M.; Abdelraouf, E.R. Kynurenine and oxidative stress in children having learning disorder with and without attention deficit hyperactivity disorder: Possible role and involvement. BMC Neurol. 2022, 22, 356. [Google Scholar] [CrossRef] [PubMed]
- Molina-Carballo, A.; Cubero-Millán, I.; Fernández-López, L.; Checa-Ros, A.; Machado-Casas, I.; Jerez-Calero, A.; Blanca-Jover, E.; Cantarero-Malagón, A.M.; Uberos, J.; Muñoz-Hoyos, A. Methylphenidate ameliorates the homeostatic balance between levels of kynurenines in ADHD children. Psychiatry Res. 2021, 303, 114060. [Google Scholar] [CrossRef]
- Sağlam, E.; Bilgiç, A.; Abuşoğlu, S.; Ünlü, A.; Sivrikaya, A. The role of tryptophan metabolic pathway in children with attention deficit hyperactivity disorder with and without comorbid oppositional defiant disorder and conduct disorder. Psychiatry Res. 2021, 298, 113770. [Google Scholar] [CrossRef]
- Skalny, A.V.; Mazaletskaya, A.L.; Zaitseva, I.P.; Skalny, A.A.; Spandidos, D.A.; Tsatsakis, A.; Lobanova, Y.N.; Skalnaya, M.G.; Aschner, M.; Tinkov, A.A. Alterations in serum amino acid profiles in children with attention deficit/hyperactivity disorder. Biomed. Rep. 2021, 14, 47. [Google Scholar] [CrossRef]
- Bergwerff, C.E.; Luman, M.; Blom, H.J.; Oosterlaan, J. No Tryptophan, Tyrosine and Phenylalanine Abnormalities in Children with Attention-Deficit/Hyperactivity Disorder. PLoS ONE 2016, 11, e0151100. [Google Scholar] [CrossRef]
- Oades, R.D.; Dauvermann, M.R.; Schimmelmann, B.G.; Schwarz, M.J.; Myint, A.M. Attention-deficit hyperactivity disorder (ADHD) and glial integrity: S100B, cytokines and kynurenine metabolism—Effects of medication. Behav. Brain Funct. 2010, 6, 29. [Google Scholar] [CrossRef]
- Ramtekkar, U.P.; Reiersen, A.M.; Todorov, A.A.; Todd, R.D. Sex and age differences in attention-deficit/hyperactivity disorder symptoms and diagnoses: Implications for DSM-V and ICD-11. J. Am. Acad. Child Adolesc. Psychiatry 2010, 49, 217–228.e283. [Google Scholar] [CrossRef]
- Friedman, M. Analysis, Nutrition, and Health Benefits of Tryptophan. Int. J. Tryptophan Res. 2018, 11, 1178646918802282. [Google Scholar] [CrossRef]
- Sorgdrager, F.J.H.; Naudé, P.J.W.; Kema, I.P.; Nollen, E.A.; Deyn, P.P. Tryptophan Metabolism in Inflammaging: From Biomarker to Therapeutic Target. Front. Immunol. 2019, 10, 2565. [Google Scholar] [CrossRef]
- Theofylaktopoulou, D.; Midttun, Ø.; Ulvik, A.; Ueland, P.M.; Tell, G.S.; Vollset, S.E.; Nygård, O.; Eussen, S.J. A community-based study on determinants of circulating markers of cellular immune activation and kynurenines: The Hordaland Health Study. Clin. Exp. Immunol. 2013, 173, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Dolina, S.; Margalit, D.; Malitsky, S.; Rabinkov, A. Attention-deficit hyperactivity disorder (ADHD) as a pyridoxine-dependent condition: Urinary diagnostic biomarkers. Med. Hypot. 2014, 82, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Cavaleri, D.; Bartoli, F.; Capogrosso, C.A.; Guzzi, P.; Moretti, F.; Riboldi, I.; Misiak, B.; Kishi, T.; Rubin, R.T.; Fuchs, D.; et al. Blood concentrations of neopterin and biopterin in subjects with depression: A systematic review and meta-analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry 2023, 120, 110633. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Miller, B.J.; Stefanek, M.E.; Miller, A.H. Inflammation-induced activation of the indoleamine 2,3-dioxygenase pathway: Relevance to cancer-related fatigue. Cancer 2015, 121, 2129–2136. [Google Scholar] [CrossRef] [PubMed]
- Saad, A.; Yekutieli, D.; Lev-Ran, S.; Gross, R.; Guyatt, G. Getting more out of meta-analyses: A new approach to meta-analysis in light of unexplained heterogeneity. J. Clin. Epidemiol. 2019, 107, 101–106. [Google Scholar] [CrossRef]
- Dinu, L.M.; Phattharakulnij, N.; Dommett, E.J. Tryptophan modulation in individuals with attention deficit hyperactivity disorder: A systematic review. J. Neural. Transm. 2022, 129, 361–377. [Google Scholar] [CrossRef] [PubMed]
- Bartoli, F.; Cioni, R.M.; Callovini, T.; Cavaleri, D.; Crocamo, C.; Carrà, G. The kynurenine pathway in schizophrenia and other mental disorders: Insight from meta-analyses on the peripheral blood levels of tryptophan and related metabolites. Schizophr. Res. 2021, 232, 61–62. [Google Scholar] [CrossRef] [PubMed]
- Pu, J.; Liu, Y.; Zhang, H.; Tian, L.; Gui, S.; Yu, Y.; Chen, X.; Chen, Y.; Yang, L.; Ran, Y.; et al. An integrated meta-analysis of peripheral blood metabolites and biological functions in major depressive disorder. Mol. Psychiatry 2021, 26, 4265–4276. [Google Scholar] [CrossRef]
- Bartoli, F.; Cioni, R.M.; Cavaleri, D.; Callovini, T.; Crocamo, C.; Misiak, B.; Savitz, J.B.; Carrà, G. The association of kynurenine pathway metabolites with symptom severity and clinical features of bipolar disorder: An overview. Eur. Psychiatry 2022, 65, e82. [Google Scholar] [CrossRef]
- Bartoli, F.; Misiak, B.; Callovini, T.; Cavaleri, D.; Cioni, R.M.; Crocamo, C.; Savitz, J.B.; Carrà, G. The kynurenine pathway in bipolar disorder: A meta-analysis on the peripheral blood levels of tryptophan and related metabolites. Mol. Psychiatry 2021, 26, 3419–3429. [Google Scholar] [CrossRef] [PubMed]
- Meinzer, M.C.; Pettit, J.W.; Viswesvaran, C. The co-occurrence of attention-deficit/hyperactivity disorder and unipolar depression in children and adolescents: A meta-analytic review. Clin. Psychol. Rev. 2014, 34, 595–607. [Google Scholar] [CrossRef] [PubMed]
- Erhardt, S.; Olsson, S.K.; Engberg, G. Pharmacological Manipulation of Kynurenic Acid. CNS Drugs 2009, 23, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Leo, D.; Sorrentino, E.; Volpicelli, F.; Eyman, M.; Greco, D.; Viggiano, D.; di Porzio, U.; Perrone-Capano, C. Altered midbrain dopaminergic neurotransmission during development in an animal model of ADHD. Neurosci. Biobehav. Rev. 2003, 27, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Sonnenschein, S.F.; Gomes, F.V.; Grace, A.A. Dysregulation of Midbrain Dopamine System and the Pathophysiology of Schizophrenia. Front. Psychiatry 2020, 11, 613. [Google Scholar] [CrossRef] [PubMed]
- Ashok, A.H.; Marques, T.R.; Jauhar, S.; Nour, M.M.; Goodwin, G.M.; Young, A.H.; Howes, O.D. The dopamine hypothesis of bipolar affective disorder: The state of the art and implications for treatment. Mol. Psychiatry 2017, 22, 666–679. [Google Scholar] [CrossRef]
- Chaudhury, D.; Walsh, J.J.; Friedman, A.K.; Juarez, B.; Ku, S.M.; Koo, J.W.; Ferguson, D.; Tsai, H.C.; Pomeranz, L.; Christoffel, D.J.; et al. Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature 2013, 493, 532–536. [Google Scholar] [CrossRef]
- Fujigaki, H.; Yamamoto, Y.; Saito, K. L-Tryptophan-kynurenine pathway enzymes are therapeutic target for neuropsychiatric diseases: Focus on cell type differences. Neuropharmacology 2017, 112, 264–274. [Google Scholar] [CrossRef]
- Cheng, J.; Liu, A.; Shi, M.Y.; Yan, Z. Disrupted Glutamatergic Transmission in Prefrontal Cortex Contributes to Behavioral Abnormality in an Animal Model of ADHD. Neuropsychopharmacology 2017, 42, 2096–2104. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.P.; Lane, H.Y.; Tsai, G.E. Attention deficit hyperactivity disorder and N-methyl-D-aspartate (NMDA) dysregulation. Curr. Pharm. Des. 2014, 20, 5180–5185. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.M.; Biederman, J.; Wozniak, J.; Mick, E.; Aleardi, M.; Wardrop, M.; Dougherty, M.; Harpold, T.; Hammerness, P.; Randall, E.; et al. Differences in brain chemistry in children and adolescents with attention deficit hyperactivity disorder with and without comorbid bipolar disorder: A proton magnetic resonance spectroscopy study. Am. J. Psychiatry 2006, 163, 316–318. [Google Scholar] [CrossRef] [PubMed]
- Mithaiwala, M.N.; Santana-Coelho, D.; Porter, G.A.; O’Connor, J.C. Neuroinflammation and the Kynurenine Pathway in CNS Disease: Molecular Mechanisms and Therapeutic Implications. Cells 2021, 10, 1548. [Google Scholar] [CrossRef] [PubMed]
- Ryan, K.M.; Allers, K.A.; McLoughlin, D.M.; Harkin, A. Tryptophan metabolite concentrations in depressed patients before and after electroconvulsive therapy. Brain. Behav. Immun. 2020, 83, 153–162. [Google Scholar] [CrossRef]
- Huang, X.; Wang, M.; Zhang, Q.; Chen, X.; Wu, J. The role of glutamate receptors in attention-deficit/hyperactivity disorder: From physiology to disease. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2019, 180, 272–286. [Google Scholar] [CrossRef] [PubMed]
- Stone, T.W.; Darlington, L.G. The kynurenine pathway as a therapeutic target in cognitive and neurodegenerative disorders. Br. J. Pharmacol. 2013, 169, 1211–1227. [Google Scholar] [CrossRef]
- Hughes, T.D.; Güner, O.F.; Iradukunda, E.C.; Phillips, R.S.; Bowen, J.P. The Kynurenine Pathway and Kynurenine 3-Monooxygenase Inhibitors. Molecules 2022, 27, 273. [Google Scholar] [CrossRef]
- Ciapała, K.; Mika, J.; Rojewska, E. The Kynurenine Pathway as a Potential Target for Neuropathic Pain Therapy Design: From Basic Research to Clinical Perspectives. Int. J. Mol. Sci. 2021, 22, 11055. [Google Scholar] [CrossRef]
- Deeks, J.J.; Higgins, J.P.T.; Altman, D.G. Chapter 10: Analysing Data and Undertaking Meta-Analyses. Cochrane Handbook for Systematic Reviews of Interventions Version 6.4 (Updated August 2023); Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; Cochrane: London, UK, 2023. Available online: www.training.cochrane.org/handbook (accessed on 8 January 2024).

| Study | Country | Sample | Participants with ADHD | Healthy Controls | Compounds | Ratios | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample Size | Age (Years) Mean ± SD | Sex Male % (M/F) | Drug-Free % | Sample Size | Age (Years) Mean ± SD | Sex Male % (M/F) | |||||
| Aarsland et al., 2015 [27] | Norway | serum | 133 | 28.0 ± 9.7 | 46.6% | 31.6% | 131 | 22.5 ± 4.2 | 42.1% | TRP, KYN, KYNA, 3HK, XA, AA, 3HAA, QA | KYN/TRP |
| (62 M/71 F) | (56 M/75 F) | ||||||||||
| Bergwerff et al., 2016 [42] | The Netherlands | dried blood spots | 83 | 9.7 ± 1.7 | 74.7% | 39.8% | 72 | 9.9 ± 1.7 | 51.4% | TRP | – |
| (62 M/21 F) | (37 M/35 F) | ||||||||||
| Evangelisti et al., 2017 [26] | Italy | serum | 102 | 9.3 ± 2.7 | 73.5% | 100% § | 62 | 9.6 ± 1.7 | 77.4% | TRP, KYN, KYNA, XA, AA, 3HAA, QA | KYN/TRP |
| (75 M/27 F) | (48 M/14 F) | ||||||||||
| Kilany et al., 2022 [38] | Egypt | plasma | 31 | 8.2 ± 1.4 | 64.5% | 100% | 54 | 8.5 ± 1.8 | 64.8% | KYN | – |
| (20 M/11 F) | (35 M/19 F) | ||||||||||
| Molina-Caraballo et al., 2021 [39] | Spain | serum | 130 | 9.5 ± 2.5 | 78.5% | 100% § | 49 | 10.4 ± 2.6 | 67.3% | AA | – |
| (102 M/28 F) | (33 M/16 F) | ||||||||||
| Oades et al., 2010a [43] | Germany | serum | 35 | 10.4 ± 2.5 | 74.3% | 60.0% § | 21 | 11.0 ± 1.5 | 95.2% | TRP, KYN, KYNA, 3HK | KYN/TRP, KYNA/KYN, KYNA/3HK |
| (26 M/9 F) | (20 M/1 F) | ||||||||||
| Sağlam et al., 2021 [40] | Turkey | serum | 122 | 11.1 ± 2.5 | 77.0% | 100% | 50 | 11.3 ± 2.7 | 70.0% | TRP, KYN, KYNA, 3HK, 3HAA | KYN/TRP, KYNA/KYN, KYNA/3HK, 3HK/KYN |
| (94 M/28 F) | (35 M/15 F) | ||||||||||
| Skalny et al., 2021 [41] | Russia | serum | 71 | 8.4 ± 2.6 | 76.1% | 100% | 31 | 8.0 ± 2.9 | 77.4% | TRP | – |
| (54 M/17 F) | (24 M/7 F) | ||||||||||
| k | n | n ADHD | n HCs | SMD | 95%CI | p-Value | I2 | |
|---|---|---|---|---|---|---|---|---|
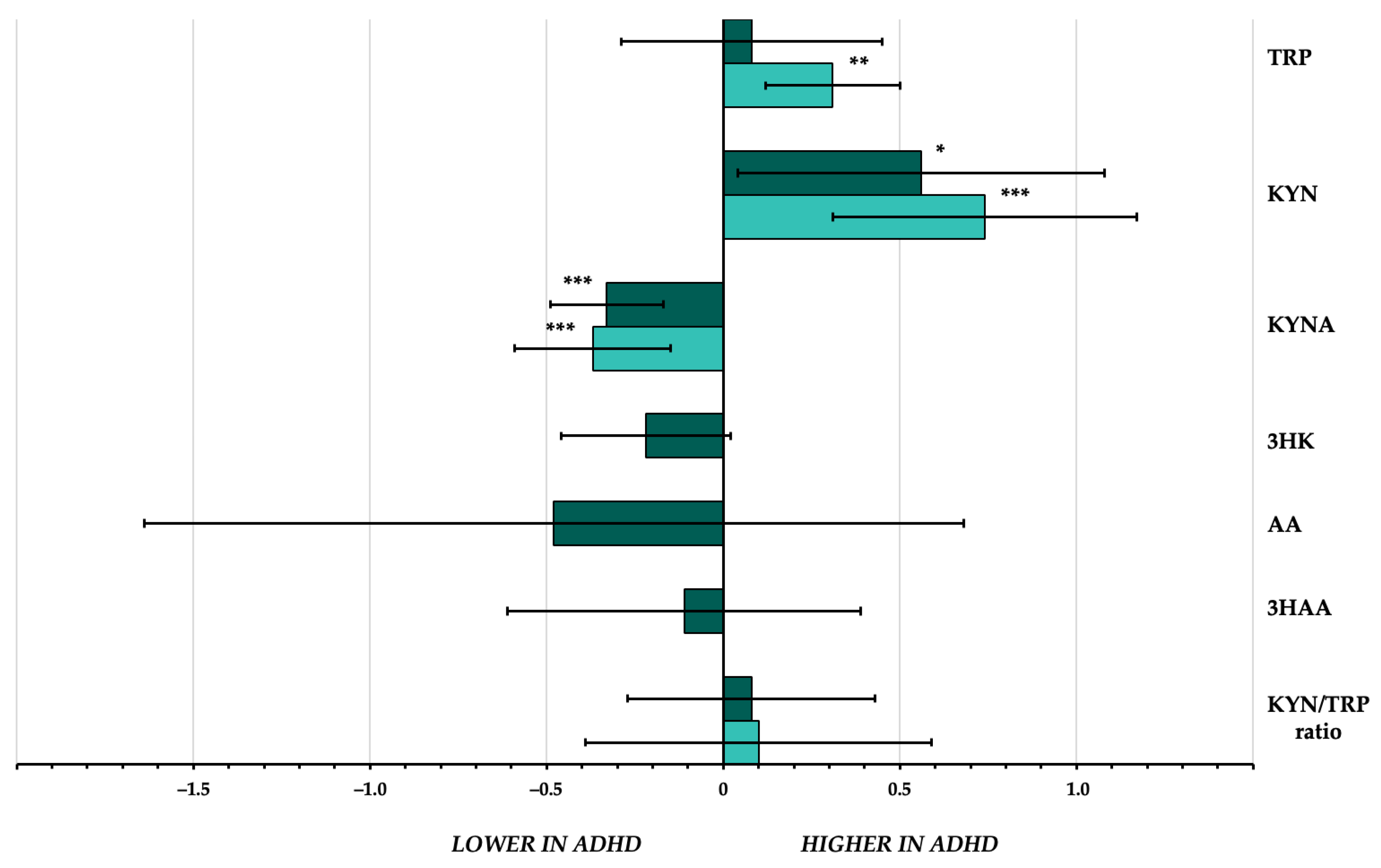
| TRP | 6 | 909 | 545 | 364 | 0.08 | −0.29 to 0.45 | 0.68 | 85.7% |
| KYN | 5 | 737 | 422 | 315 | 0.56 | 0.04 to 1.08 | 0.033 | 90.3% |
| KYNA | 4 | 650 | 389 | 261 | −0.33 | −0.49 to −0.17 | <0.001 | 0% |
| 3HK | 3 | 486 | 287 | 199 | −0.22 | −0.45 to 0.02 | 0.08 | 30.4% |
| AA | 3 | 604 | 363 | 241 | −0.48 | −1.64 to 0.68 | 0.41 | 97.6% |
| 3HAA | 3 | 597 | 355 | 242 | −0.11 | −0.62 to 0.39 | 0.66 | 88.5% |
| KYN/TRP ratio | 4 | 652 | 391 | 261 | 0.08 | −0.28 to 0.43 | 0.68 | 77.4% |
| k | n | n ADHD | n HCs | SMD | 95%CI | p-Value | I2 | |
|---|---|---|---|---|---|---|---|---|
| TRP | 4 | 477 | 315 | 162 | 0.31 | 0.11 to 0.50 | 0.002 | 0% |
| KYN | 4 | 460 | 275 | 185 | 0.74 | 0.30 to 1.17 | <0.001 | 76.5% |
| KYNA | 3 | 375 | 244 | 131 | −0.37 | −0.59 to −0.15 | <0.001 | 0% |
| KYN/TRP ratio | 3 | 375 | 244 | 131 | 0.10 | −0.39 to 0.59 | 0.68 | 77.1% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavaleri, D.; Crocamo, C.; Morello, P.; Bartoli, F.; Carrà, G. The Kynurenine Pathway in Attention-Deficit/Hyperactivity Disorder: A Systematic Review and Meta-Analysis of Blood Concentrations of Tryptophan and Its Catabolites. J. Clin. Med. 2024, 13, 583. https://doi.org/10.3390/jcm13020583
Cavaleri D, Crocamo C, Morello P, Bartoli F, Carrà G. The Kynurenine Pathway in Attention-Deficit/Hyperactivity Disorder: A Systematic Review and Meta-Analysis of Blood Concentrations of Tryptophan and Its Catabolites. Journal of Clinical Medicine. 2024; 13(2):583. https://doi.org/10.3390/jcm13020583
Chicago/Turabian StyleCavaleri, Daniele, Cristina Crocamo, Pietro Morello, Francesco Bartoli, and Giuseppe Carrà. 2024. "The Kynurenine Pathway in Attention-Deficit/Hyperactivity Disorder: A Systematic Review and Meta-Analysis of Blood Concentrations of Tryptophan and Its Catabolites" Journal of Clinical Medicine 13, no. 2: 583. https://doi.org/10.3390/jcm13020583
APA StyleCavaleri, D., Crocamo, C., Morello, P., Bartoli, F., & Carrà, G. (2024). The Kynurenine Pathway in Attention-Deficit/Hyperactivity Disorder: A Systematic Review and Meta-Analysis of Blood Concentrations of Tryptophan and Its Catabolites. Journal of Clinical Medicine, 13(2), 583. https://doi.org/10.3390/jcm13020583








