Renal Rehabilitation: Present and Future Perspectives
Abstract
1. Introduction
2. CKD and Physical Inactivity
3. Chronic Effects of Exercise in CKD Animal Models
4. Chronic Effect of Exercise in Patients with CKD Undergoing Dialysis
5. Chronic Effect of Exercise in Pre-Dialysis Patients with CKD
6. Chronic Effect of Exercise in Pre-Dialysis CKD Patients with Acute Myocardial Infarction (AMI)
7. Mechanisms of Renal Protection by Chronic Exercise
8. Barriers to Exercise Participation among Patients with CKD
9. Present Status of RR
9.1. Societies and Meetings
9.2. Guidelines of RR
9.3. National Health Insurance Reimbursement
10. Future perspectives of RR: Adding Life to Years and Years to Life
11. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The Japanese Society for Dialysis Therapy. Available online: https://docs.jsdt.or.jp/overview/index.html (accessed on 23 November 2023). (In Japanese).
- Kooman, J.P.; Kotanko, P.; Schols, A.M.W.J.; Shiels, P.G.; Stenvinkel, P. Chronic kidney disease and premature ageing. Nat. Rev. Nephrol. 2014, 10, 732–742. [Google Scholar] [CrossRef] [PubMed]
- O’Hare, A.M.; Tawney, K.; Bacchetti, P.; Johansen, K.L. Decreased survival among sedentary patients undergoing dialysis: Results from the dialysis morbidity and mortality study wave 2. Am. J. Kidney Dis. 2003, 41, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Go, A.S.; Chertow, G.M.; Fan, D.; McCulloch, C.E.; Hsu, C.-y. Chronic Kidney Disease and the Risks of Death, Cardiovascular Events, and Hospitalization. N. Engl. J. Med. 2004, 351, 1296–1305. [Google Scholar] [CrossRef]
- Johansen, K.L. Exercise in the End-Stage Renal Disease Population. J. Am. Soc. Nephrol. 2007, 18, 1845–1854. [Google Scholar] [CrossRef] [PubMed]
- Kohzuki, M. Renal rehabilitation: Definition and evidence. In Renal Rehabilitation; Kohzuki, M., Ed.; Ishiyaku Publishers, Inc.: Tokyo, Japan, 2012; pp. 10–17. (In Japanese) [Google Scholar]
- Kohzuki, M. Renal rehabilitation: Present and future perspectives. In Hemodialysis; Suzuki, H., Ed.; Intech: Oakville, ON, Canada, 2013; pp. 743–751. [Google Scholar]
- Tentori, F.; Elder, S.J.; Thumma, J.; Pisoni, R.L.; Bommer, J.; Fissell, R.B.; Fukuhara, S.; Jadoul, M.; Keen, M.L.; Saran, R.; et al. Physical exercise among participants in the Dialysis Outcomes and Practice Patterns Study (DOPPS): Correlates and associated outcomes. Nephrol. Dial. Transplant. 2010, 25, 3050–3062. [Google Scholar] [CrossRef]
- Smart, N.; Steele, M. Exercise training in haemodialysis patients: A systematic review and meta-analysis. Nephrology 2011, 16, 626–632. [Google Scholar]
- Sieverdes, J.C.; Sui, X.; Lee, D.C.; Church, T.S.; McClain, A.; Hand, G.A.; Blair, S.N. Physical activity, cardiorespiratory fitness and the incidence of type 2 diabetes in a prospec-tive study of men. Br. J. Sports Med. 2010, 44, 238–244. [Google Scholar] [CrossRef]
- Blair, S.N.; Kohl, H.W., III; Paffenbarger, R.S., Jr.; Clark, D.G.; Cooper, K.H. Physical fitness and all-cause mortality: A prospective study of healthy men and women. JAMA 1989, 262, 2395–2401. [Google Scholar]
- Blair, S.N.; Sallis, R.E.; Hutber, A.; Archer, E. Exercise therapy—The public health message. Scand. J. Med. Sci. Sports 2012, 22, e24–e28. [Google Scholar] [CrossRef]
- Caspersen, C.J.; Powell, K.E.; Christenson, G.M. Physical activity, exercise, and physical fitness: Definitions and distinctions for health-related research. Public Health Rep. 1985, 100, 126–131. [Google Scholar]
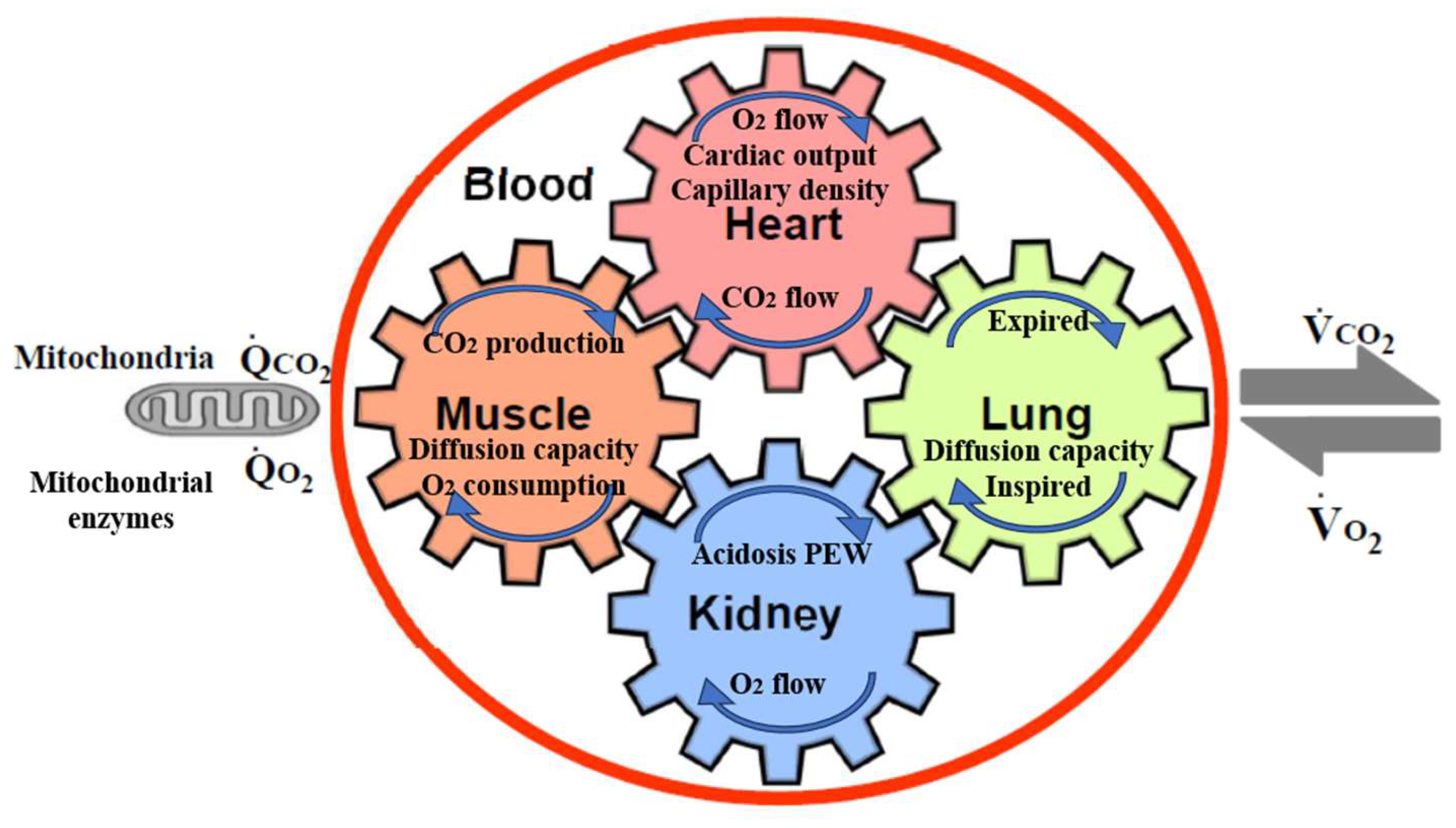
- Kohzuki, M. New Ideas on Limitations to VO2max: Five Major Determinants for VO2max. Pulm. Res. Respir. Med. Open J. 2018, 5, e1–e2. [Google Scholar] [CrossRef]
- Caso, G.; Garlick, P.J. Control of muscle protein kinetics by acid-base balance. Curr. Opin. Clin. Nutr. Metab. Care 2005, 8, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.L.; Wang, X.; England, B.K.; Price, S.R.; Ding, X.; Mitch, W.E. The acidosis of chronic renal failure activates muscle proteolysis in rats by augmenting transcription of genes encoding proteins of the ATP-dependent ubiquitin-proteasome pathway. J. Clin. Investig. 1996, 97, 1447–1453. [Google Scholar] [CrossRef]
- Mitch, W.E. Influence of metabolic acidosis on nutrition. Am. J. Kidney Dis. 1997, 29, XLVI–XLVIII. [Google Scholar] [CrossRef] [PubMed]
- Fahal, I.H. Uraemic sarcopenia: Aetiology and implications. Nephrol. Dial. Transplant. 2014, 29, 1655–1665. [Google Scholar] [CrossRef]
- Kolb, E.M.; Kelly, S.A.; Middleton, K.M.; Sermsakdi, L.S.; Chappell, M.A.; Garland, T., Jr. Erythropoietin elevates VO2, max but not voluntary wheel running in mice. J. Exp. Biol. 2010, 213, 510–519. [Google Scholar] [CrossRef]
- Bach, T.M.; Clement, D.B. Exercise induced acute renal failure in an athlete. Can. Fam. Physician 1980, 26, 591–595. [Google Scholar]
- Jackson, C.R. Exercise-induced renal failure and muscle damage. Proc. R. Soc. Med. 1970, 63, 566–570. [Google Scholar]
- Kohzuki, M.; Kamimoto, M.; Wu, X.M.; Xu, H.L.; Kawamura, T.; Mori, N.; Nagasaka, M.; Kurosawa, H.; Minami, N.; Kanazawa, M.; et al. Renal protective effects of chronic exercise and antihypertensive therapy in hyper-tensive rats with chronic renal failure. J. Hypertens. 2001, 19, 1877–1882. [Google Scholar] [CrossRef]
- Kanazawa, M.; Kawamura, T.; Li, L.; Sasaki, Y.; Matsumoto, K.; Kataoka, H.; Ito, O.; Minami, N.; Sato, T.; Ootaka, T.; et al. Combination of Exercise and Enalapril Enhances Renoprotective and Peripheral Effects in Rats With Renal Ablation. Am. J. Hypertens. 2006, 19, 80–86. [Google Scholar] [CrossRef]
- Tufescu, A.; Kanazawa, M.; Ishida, A.; Lu, H.; Sasaki, Y.; Ootaka, T.; Sato, T.; Kohzuki, M. Combination of exercise and losartan enhances renoprotective and peripheral effects in spontaneously type 2 diabetes mellitus rats with nephropathy. J. Hypertens. 2008, 26, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Ito, D.; Cao, P.; Kakihana, T.; Sato, E.; Suda, C.; Muroya, Y.; Ogawa, Y.; Hu, G.; Ishii, T.; Ito, O.; et al. Chronic Running Exercise Alleviates Early Progression of Nephropathy with Upregulation of Nitric Oxide Synthases and Suppression of Glycation in Zucker Diabetic Rats. PLoS ONE 2015, 10, e0138037. [Google Scholar] [CrossRef] [PubMed]
- Bogataj, S.; Pajek, M.; Pajek, J.; Ponikvar, J.B.; Paravlic, A.H. Exercise-based interventions in hemodialysis patients: A systematic review with a me-ta-analysis of randomized controlled trials. J. Clin. Med. 2019, 9, 43. [Google Scholar] [PubMed]
- Hu, H.; Liu, X.; Chau, P.H.; Choi, E.P.H. Effects of intradialytic exercise on health-related quality of life in patients undergoing mainte-nance haemodialysis: A systematic review and meta-analysis. Qual. Life Res. 2022, 31, 1915–1932. [Google Scholar] [CrossRef]
- Scapini, K.B.; Bohlke, M.; Moraes, O.A.; Rodrigues, C.G.; Inácio, H.F.; Sbruzzi, G.; Leguisamo, C.P.; Sanches, I.C.; Filho, H.T.; Irigoyen, M.C. Combined training is the most effective training modality to improve aerobic capaci-ty and blood pressure control in people requiring haemodialysis for endstage renal disease: Systematic review and network meta-analysis. J. Physiother. 2019, 65, 4–15. [Google Scholar] [CrossRef]
- Sheng, K.; Zhang, P.; Chen, L.; Wu, C.; Chen, J. Intradialytic exercise in hemodialysis patients: A systematic review and meta-analysis. Am. J. Nephrol. 2014, 40, 478–490. [Google Scholar] [CrossRef]
- Song, Y.-Y.; Hu, R.-J.; Diao, Y.-S.; Chen, L.; Jiang, X.-L. Effects of Exercise Training on Restless Legs Syndrome, Depression, Sleep Quality, and Fatigue Among Hemodialysis Patients: A Systematic Review and Meta-analysis. J. Pain Symptom Manag. 2018, 55, 1184–1195. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, X.; Deng, S.; Chen, J.; Zhang, L.; Huang, Y.; Hu, H. Combined aerobic and resistance exercise in maintenance hemodialysis patients: A meta-analysis. Semin. Dial. 2023, 36, 278–293. [Google Scholar] [CrossRef]
- Baria, F.; Kamimura, M.A.; Aoike, D.T.; Ammirati, A.; Rocha, M.L.; de Mello, M.T.; Cuppari, L. Randomized controlled trial to evaluate the impact of aerobic exercise on visceral fat in overweight chronic kidney disease patients. Nephrol. Dial. Transplant. 2014, 29, 857–864. [Google Scholar] [CrossRef]
- Greenwood, S.A.; Koufaki, P.; Mercer, T.H.; MacLaughlin, H.; Rush, R.; Lindup, H.; O’Connor, E.M.; Jones, C.; Hendry, B.; Macdougall, I.; et al. Effect of exercise training on estimated GFR, vascular health, and cardiorespira-tory fitness in patients with CKD: A pilot randomized controlled trial. Am. J. Kidney Dis. 2015, 65, 425–434. [Google Scholar] [CrossRef]
- Chen, I.-R.; Wang, S.-M.; Liang, C.-C.; Kuo, H.-L.; Chang, C.-T.; Liu, J.-H.; Lin, H.-H.; Wang, I.-K.; Yang, Y.-F.; Chou, C.-Y.; et al. Association of Walking with Survival and RRT Among Patients with CKD Stages 3–5. Clin. J. Am. Soc. Nephrol. 2014, 9, 1183–1189. [Google Scholar] [CrossRef] [PubMed]
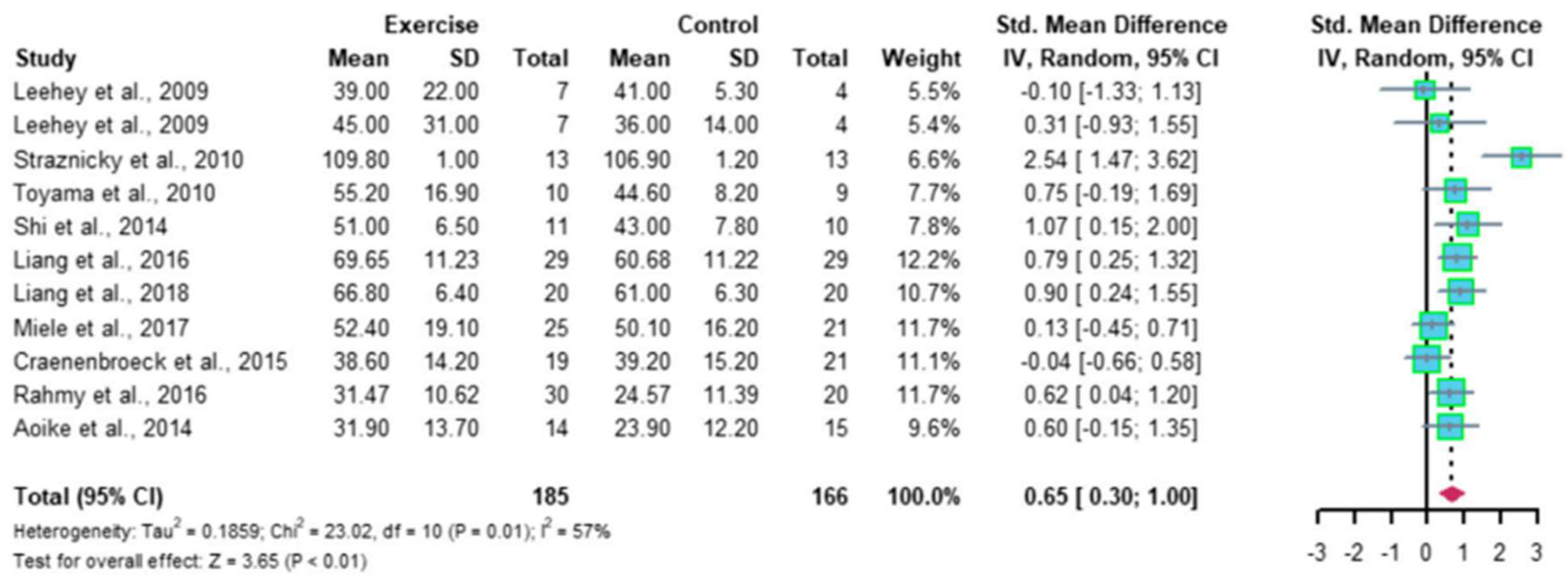
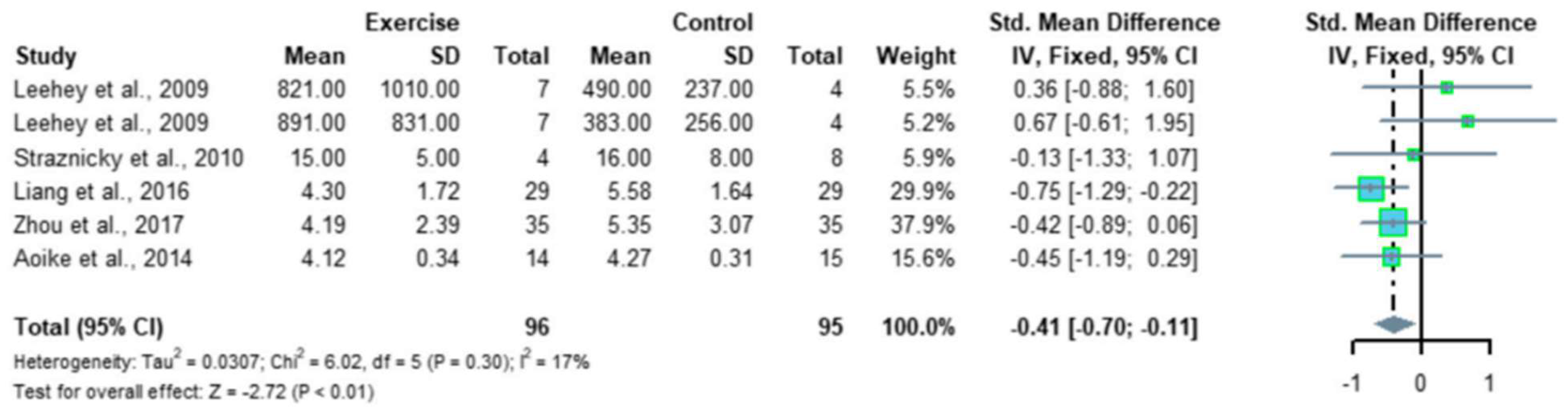
- Ma, Q.; Gao, Y.; Lu, J.; Liu, X.; Wang, R.; Shi, Y.; Liu, J.; Su, H. The effect of regular aerobic exercise on renal function in patients with CKD: A systematic review and meta-analysis. Front. Physiol. 2022, 13, 901164. [Google Scholar] [CrossRef]
- Swamy, S.; Noor, S.M.; Mathew, R.O. Cardiovascular Disease in Diabetes and Chronic Kidney Disease. J. Clin. Med. 2023, 12, 6984. [Google Scholar] [CrossRef] [PubMed]
- Xanthopoulos, A.; Papamichail, A.; Briasoulis, A.; Loritis, K.; Bourazana, A.; Magouliotis, D.E.; Sarafidis, P.; Stefanidis, I.; Skoularigis, J.; Triposkiadis, F. Heart Failure in Patients with Chronic Kidney Disease. J. Clin. Med. 2023, 12, 6105. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Kohzuki, M.; Ono, M.; Muto, M.; Osugi, T.; Kawamura, K.; Naganuma, W.; Sato, M.; Shishito, N. Association between physical activity and change in renal function in patients after acute myocardial infarction. PLoS ONE 2019, 14, e0212100. [Google Scholar] [CrossRef]
- Séronie-Vivien, S.; Delanaye, P.; Piéroni, L.; Mariat, C.; Froissart, M.; Cristol, J.-P. Cystatin C: Current position and future prospects. Clin. Chem. Lab. Med. 2008, 46, 1664–1686. [Google Scholar] [CrossRef] [PubMed]
- Poortmans, J.R.; Gulbis, B.; De Bruyn, E.; Baudry, S.; Carpentier, A. Limitations of serum values to estimate glomerular filtration rate during exercise. Br. J. Sports Med. 2012, 47, 1166–1170. [Google Scholar]
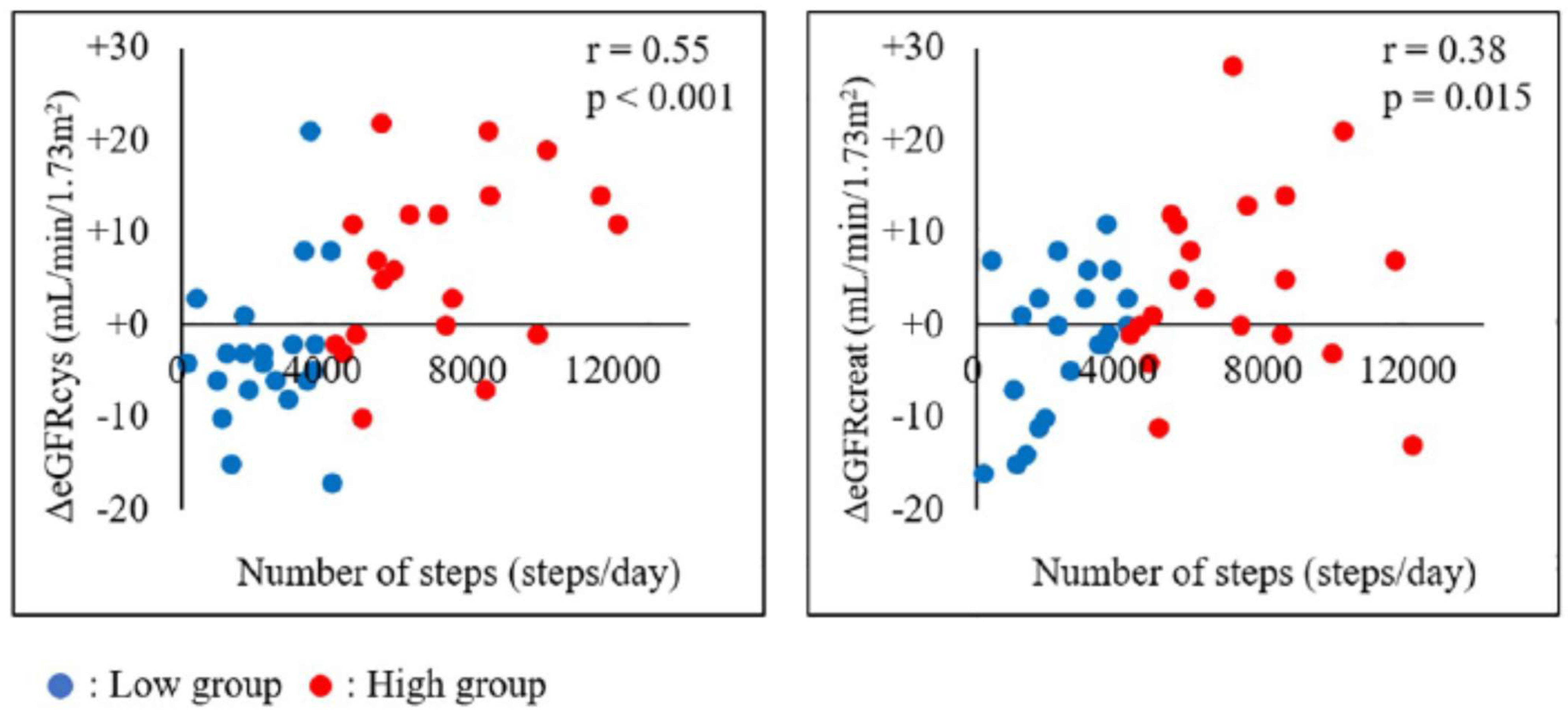
- Robinson-Cohen, C.; Littman, A.J.; Duncan, G.E.; Weiss, N.S.; Sachs, M.C.; Ruzinski, J.; Kundzins, J.; Rock, D.; de Boer, I.H.; Ikizler, T.A.; et al. Physical Activity and Change in Estimated GFR among Persons with CKD. J. Am. Soc. Nephrol. 2014, 25, 399–406. [Google Scholar] [CrossRef]
- Burlacu, A.; Covic, A. Special Issue: “Cardiovascular Complications in Renal Diseases”. J. Clin. Med. 2023, 12, 5307. [Google Scholar]
- Bishop, N.C.; Burton, J.O.; Graham-Brown, M.P.M.; Stensel, D.J.; Viana, J.L.; Watson, E.L. Exercise and chronic kidney disease: Potential mechanisms underlying the physiological benefits. Nat. Rev. Nephrol. 2023, 19, 244–256. [Google Scholar] [CrossRef]
- Kirkman, D.L.; Sequeira-Lopez, M.L.S. Call for Papers: Exercise and the kidneys in health and disease. Am. J. Physiol. Ren. Physiol. 2023, 324, F461–F463. [Google Scholar]
- K/DOQI Workshop. K/DOQI clinical practice guidelines dor cardiovascular disease in dialysis patients. Am J Kidney Dis. 2005, 45, S16–S153. [Google Scholar] [CrossRef]
- Delgado, C.; Johansen, K.L. Deficient Counseling on Physical Activity among Nephrologists. Nephron Clin. Pract. 2010, 116, c330–c336. [Google Scholar] [CrossRef] [PubMed]
- Delgado, C.; Johansen, K.L. Barriers to exercise participation among dialysis patients. Nephrol. Dial. Transplant. 2012, 27, 1152–1157. [Google Scholar] [CrossRef] [PubMed]
- Konstantinidou, E.; Koukouvou, G.; Kouidi, E.; Deligiannis, A.; Tourkantonis, A. Exercise training in patients with end-stage renal disease on hemodialysis: Comparison of three rehabilitation programs. J. Rehabil. Med. 2002, 34, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Wilund, K.R.; Painter, P. Formation of an Exercise in CKD Working Group. Am. J. Kidney Dis. 2016, 67, 812. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Krause, R.; WGRR-European Working Group on Renal Rehabilitation and Exercise Physiology (affiliated to the ERA-EDTA); KfH-Kuratorium für Dialyse und Nieren transplantation e.V.; Nephrological Centre Berlin-Moabit. Nephrologists’ view on exercise training in chronic kidney disease (results of the questionnaire at the WCN 2003). Clin. Nephrol. 2004, 61, S2–S4. [Google Scholar]
- Schrag, W.F.; Campbell, M.; Ewert, J.; Hartley, S.; Niemann, J.; Ross, D. Multidisciplinary Team Renal Rehabilitation: Interventions and Outcomes. Adv. Ren. Replace. Ther. 1999, 6, 282–288. [Google Scholar] [CrossRef]
- Japanese Society of Renal Rehabilitation. Available online: https://jsrr.smoosy.atlas.jp/ja/ (accessed on 23 November 2023). (In Japanese).
- Bennett, P.N.; Kohzuki, M.; Bohm, C.; Roshanravan, B.; Bakker, S.J.; Viana, J.L.; MacRae, J.M.; Wilkinson, T.J.; Wilund, K.R.; Van Craenenbroeck, A.H.; et al. Global Policy Barriers and Enablers to Exercise and Physical Activity in Kidney Care. J. Ren. Nutr. 2022, 32, 441–449. [Google Scholar] [CrossRef]
- Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int. 2012, 2, 337–414. [Google Scholar]
- American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription, 11th ed.; Liguori, G., Feito, Y., Fountaine, C., Roy, B.A., Eds.; Wolters Kluwer: Philadelphia, PA, USA, 2022; pp. 336–341. [Google Scholar]
- Japanese Society of Renal Rehabilitation. Guideline for Renal Rehabilitation; Nankodo, Inc.: Tokyo, Japan, 2018; pp. 1–87. (In Japanese) [Google Scholar]
- Yamagata, K.; Hoshino, J.; Sugiyama, H.; Hanafusa, N.; Shibagaki, Y.; Komatsu, Y.; Konta, T.; Fujii, N.; Kanda, E.; Sofue, T.; et al. Clinical practice guideline for renal rehabilitation: Systematic reviews and recommendations of exercise therapies in patients with kidney diseases. Ren. Replace. Ther. 2019, 5, 4. [Google Scholar] [CrossRef]
- Williams, A.D.; Fassett, R.G.; Coombes, J.S. Exercise in CKD: Why is it important and how should it be delivered? Am J Kidney Dis. 2014, 64, 329–331. [Google Scholar] [CrossRef] [PubMed]
- Zelle, D.M.; Klaassen, G.; van Adrichem, E.; Bakker, S.J.; Corpeleijn, E.; Navis, G. Physical inactivity: A risk factor and target for intervention in renal care. Nat. Rev. Nephrol. 2017, 13, 152–168. [Google Scholar] [CrossRef] [PubMed]
- Kohzuki, M. Paradigm shift in rehabilitation medicine in the era of multimorbidity and multiple disabilities (MMD). Phys. Med. Rehabil. Int. 2014, 1, 4. [Google Scholar]
- Kohzuki, M.; Sakata, Y.; Kawamura, T. A paradigm shift in rehabilitation medicine: From “adding life to years” to “adding life to years and years to life”. Asian J. Hum. Serv. 2012, 2, 1–8. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kohzuki, M. Renal Rehabilitation: Present and Future Perspectives. J. Clin. Med. 2024, 13, 552. https://doi.org/10.3390/jcm13020552
Kohzuki M. Renal Rehabilitation: Present and Future Perspectives. Journal of Clinical Medicine. 2024; 13(2):552. https://doi.org/10.3390/jcm13020552
Chicago/Turabian StyleKohzuki, Masahiro. 2024. "Renal Rehabilitation: Present and Future Perspectives" Journal of Clinical Medicine 13, no. 2: 552. https://doi.org/10.3390/jcm13020552
APA StyleKohzuki, M. (2024). Renal Rehabilitation: Present and Future Perspectives. Journal of Clinical Medicine, 13(2), 552. https://doi.org/10.3390/jcm13020552




