Fusiform versus Saccular Intracranial Aneurysms—Hemodynamic Evaluation of the Pre-Aneurysmal, Pathological, and Post-Interventional State
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Cohort
2.2. Virtual Stenting, Vessel Reconstruction, and Aneurysm Sac Definition
2.3. Hemodynamic Simulation
2.4. Morphological and Stent Analysis
2.5. Hemodynamic Analysis
3. Results
3.1. Morphological and Stent-Related Differences
3.2. Hemodynamic Differences
4. Discussion
4.1. Morphology and Stent
4.2. Wall-Related Parameters
4.3. Flow-Related Parameters
4.4. Energy Loss
4.5. Limitations and Future Work
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| A | Surface Area |
| AR | Aspect Ratio |
| BA | Basilar Artery |
| CFD | Computational Fluid Dynamics |
| DSA | Digital Subtraction Angiography |
| E | Energy |
| EL | Energy Loss |
| FDS | Flow Diverting Stent |
| FIA | Fusiform Intracranial Aneurysm |
| H | Healthy |
| IA | Intracranial Aneurysm |
| MCA | Middle Cerebral Artery |
| VA | Vertebral Artery |
| OVI | Oscillatory Velocity Index |
| OSI | Oscillatory Shear Index |
| P | Pathological |
| p | Pressure |
| SIA | Saccular Intracranial Aneurysm |
| T | Treated |
| TAWSS | Time Averaged Wall Shear Stress |
| Cardiac Cycle Length | |
| v | velocity |
| W | Watt |
| WSS | Wall Shear Stress |
| Fluid Density | |
| Vorticity |
References
- Boussel, L.; Rayz, V.; McCulloch, C.; Martin, A.; Acevedo-Bolton, G.; Lawton, M.; Higashida, R.; Smith, W.S.; Young, W.L.; Saloner, D. Aneurysm growth occurs at region of low wall shear stress: Patient-specific correlation of hemodynamics and growth in a longitudinal study. Stroke 2008, 39, 2997–3002. [Google Scholar] [CrossRef]
- Seibert, B.; Tummala, R.P.; Chow, R.; Faridar, A.; Mousavi, S.A.; Divani, A.A. Intracranial aneurysms: Review of current treatment options and outcomes. Front. Neurol. 2011, 2, 45. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jing, L.; Zhang, Y.; Liu, J.; Yang, X. Low wall shear stress is associated with the rupture of intracranial aneurysm with known rupture point: Case report and literature review. BMC Neurol. 2016, 16, 231. [Google Scholar] [CrossRef]
- Xiang, J.; Natarajan, S.K.; Tremmel, M.; Ma, D.; Mocco, J.; Hopkins, L.N.; Siddiqui, A.H.; Levy, E.I.; Meng, H. Hemodynamic-morphologic discriminants for intracranial aneurysm rupture. Stroke 2011, 42, 144–152. [Google Scholar] [CrossRef]
- Al-Mufti, F.; Cohen, E.R.; Amuluru, K.; Patel, V.; El-Ghanem, M.; Nuoman, R.; Majmundar, N.; Dangayach, N.S.; Meyers, P.M. Bailout Strategies and Complications Associated with the Use of Flow-Diverting Stents for Treating Intracranial Aneurysms. Interv. Neurol. 2020, 8, 38–54. [Google Scholar] [CrossRef]
- Salem, M.M.; Elfil, M.; Aboutaleb, P.E.; Dmytriw, A.A.; Thomas, A.J.; Hassan, A.E.; Mascitelli, J.R.; Kan, P.; Jankowitz, B.T.; Burkhardt, J.K. National Survey on Flow-Diverting Stents for Intracranial Aneurysms in the United States. World Neurosurg. 2022, 166, e958–e967. [Google Scholar] [CrossRef]
- Diestro, J.D.B.; Adeeb, N.; Dibas, M.; Boisseau, W.; Harker, P.; Brinjikji, W.; Xiang, S.; Joyce, E.; Shapiro, M.; Raz, E.; et al. Flow Diversion for Middle Cerebral Artery Aneurysms: An International Cohort Study. Neurosurgery 2021, 89, 1112–1121. [Google Scholar] [CrossRef]
- Pierot, L.; Wakhloo, A.K. Endovascular treatment of intracranial aneurysms: Current status. Stroke 2013, 44, 2046–2054. [Google Scholar] [CrossRef]
- Briganti, F.; Leone, G.; Marseglia, M.; Mariniello, G.; Caranci, F.; Brunetti, A.; Maiuri, F. Endovascular treatment of cerebral aneurysms using flow-diverter devices: A systematic review. Neuroradiol. J. 2015, 28, 365–375. [Google Scholar] [CrossRef]
- Echiverri, H.C.; Rubino, F.A.; Gupta, S.R.; Gujrati, M. Fusiform aneurysm of the vertebrobasilar arterial system. Stroke 1989, 20, 1741–1747. [Google Scholar] [CrossRef] [PubMed]
- Peeters, S.M.; Colby, G.P.; Kim, W.J.; Bae, W.I.; Sparks, H.; Reitz, K.; Tateshima, S.; Jahan, R.; Szeder, V.; Nour, M.; et al. Proximal Internal Carotid Artery Occlusion and Extracranial-Intracranial Bypass for Treatment of Fusiform and Giant Internal Carotid Artery Aneurysms. World Neurosurg. 2023, 180, e494–e505. [Google Scholar] [CrossRef]
- Serrone, J.C.; Gozal, Y.M.; Grossman, A.W.; Andaluz, N.; Abruzzo, T.; Zuccarello, M.; Ringer, A. Vertebrobasilar Fusiform Aneurysms. Neurosurg. Clin. N. Am. 2014, 25, 471–484. [Google Scholar] [CrossRef]
- Day, A.L.; Gaposchkin, C.G.; Yu, C.J.; Rivet, D.J.; Dacey, R.G. Spontaneous fusiform middle cerebral artery aneurysms: Characteristics and a proposed mechanism of formation. J. Neurosurg. 2003, 99, 228–240. [Google Scholar] [CrossRef]
- al Yamany, M.; Ross, I.B. Giant fusiform aneurysm of the middle cerebral artery: Successful Hunterian ligation without distal bypass. Br. J. Neurosurg. 1998, 12, 572–575. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Yim, M.B.; Lee, C.Y.; Kim, E.; Son, E.I. Intracranial Fusiform Aneurysms: It’s Pathogenesis, Clinical Characteristics and Managements. J. Korean Neurosurg. Soc. 2008, 44, 116–123. [Google Scholar] [CrossRef]
- Städt, M.; Holtmannspötter, M.; Eff, F.; Voit-Höhne, H. Non-visualizable stent-occlusion after treatment of a fusiform PCA-aneurysm–a case report. Radiol. Case Rep. 2021, 16, 2573–2578. [Google Scholar] [CrossRef]
- Elbaroody, M.; Fiki, A.E.; Eldabaa, K.A.; Ghaneim, M.E.; Gabr, M. Multiple clips reconstruction for giant fusiform Middle Cerebral Artery aneurysm. Interdiscip. Neurosurg. 2022, 29, 101538. [Google Scholar] [CrossRef]
- Telles, J.P.M.; Solla, D.J.F.; Yamaki, V.N.; Rabelo, N.N.; da Silva, S.A.; Caldas, J.G.P.; Teixeira, M.J.; Junior, J.R.; Figueiredo, E.G. Comparison of surgical and endovascular treatments for fusiform intracranial aneurysms: Systematic review and individual patient data meta-analysis. Neurosurg. Rev. 2020, 44, 2405–2414. [Google Scholar] [CrossRef]
- Kim, J.; Hwang, G.; Kim, B.T.; Park, S.Q.; Oh, J.S.; Ban, S.P.; Kwon, O.K.; Chung, J. Safety and Efficacy of Flow Diverter Therapy for Unruptured Intracranial Aneurysm Compared to Traditional Endovascular Strategy: A Multi-Center, Randomized, Open-Label Trial. J. Korean Neurosurg. Soc. 2022, 65, 772–778. [Google Scholar] [CrossRef] [PubMed]
- Saalfeld, S.; Stahl, J.; Korte, J.; Miller Marsh, L.M.; Preim, B.; Beuing, O.; Cherednychenko, Y.; Behme, D.; Berg, P. Can Endovascular Treatment of Fusiform Intracranial Aneurysms Restore the Healthy Hemodynamic Environment?–A Virtual Pilot Study. Front. Neurol. 2022, 12, 771694. [Google Scholar] [CrossRef]
- Barletta, E.A.; Ricci, R.L.; Di Silva, R.G.; Gaspar, R.H.M.L.; Araújo, J.F.M.; Neves, M.W.F.; de Aquino, J.L.B.; Barba Belsuzarri, T.A. Fusiform aneurysms: A review from its pathogenesis to treatment options. Surg. Neurol. Int. 2018, 9, 189. [Google Scholar] [CrossRef]
- Reorowicz, P.; Tyfa, Z.; Obidowski, D.; Wiśniewski, K.; Stefańczyk, L.; Jóźwik, K.; Levy, M.L. Blood flow through the fusiform aneurysm treated with the Flow Diverter stent—Numerical investigations. Biocybern. Biomed. Eng. 2022, 42, 375–390. [Google Scholar] [CrossRef]
- Sindeev, S.; Kirschke, J.S.; Prothmann, S.; Frolov, S.; Liepsch, D.; Berg, P.; Zimmer, C.; Friedrich, B. Evaluation of flow changes after telescopic stenting of a giant fusiform aneurysm of the vertebrobasilar junction. Biomed. Eng. Online 2019, 18, 82. [Google Scholar] [CrossRef] [PubMed]
- Lv, N.; Cao, W.; Larrabide, I.; Karmonik, C.; Zhu, D.; Liu, J.; Huang, Q.; Fang, Y. Hemodynamic Changes Caused by Multiple Stenting in Vertebral Artery Fusiform Aneurysms: A Patient-Specific Computational Fluid Dynamics Study. AJNR Am. J. Neuroradiol. 2018, 39, 118–122. [Google Scholar] [CrossRef]
- Griffin, A.; Lerner, E.; Zuchowski, A.; Zomorodi, A.; Gonzalez, L.F.; Hauck, E.F. Flow diversion of fusiform intracranial aneurysms. Neurosurg. Rev. 2020, 44, 1471–1478. [Google Scholar] [CrossRef]
- Lyu, M.; Ventikos, Y.; Peach, T.W.; Makalanda, L.; Bhogal, P. Virtual Flow-T Stenting for Two Patient-Specific Bifurcation Aneurysms. Front. Neurol. 2021, 12, 726980. [Google Scholar] [CrossRef]
- Zhong, L.; Zhang, J.M.; Su, B.; Tan, R.S.; Allen, J.C.; Kassab, G.S. Application of Patient-Specific Computational Fluid Dynamics in Coronary and Intra-Cardiac Flow Simulations: Challenges and Opportunities. Front. Physiol. 2018, 9, 742. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Lu, G.; Ge, L.; Zou, R.; Li, G.; Wan, H.; Leng, X.; Xiang, J.; Zhang, X. Hemodynamic Comparison of Treatment Strategies for Intracranial Vertebral Artery Fusiform Aneurysms. Front. Neurol. 2022, 13, 927135. [Google Scholar] [CrossRef]
- Findlay, J.; Hao, C.; Emery, D. Non-Atherosclerotic Fusiform Cerebral Aneurysms. Can. J. Neurol. Sci. Le J. Can. Des Sci. Neurol. 2002, 29, 41–48. [Google Scholar] [CrossRef]
- Janiga, G.; Daróczy, L.; Berg, P.; Thévenin, D.; Skalej, M.; Beuing, O. An automatic CFD-based flow diverter optimization principle for patient-specific intracranial aneurysms. J. Biomech. 2015, 48, 3846–3852. [Google Scholar] [CrossRef]
- Janiga, G.; Berg, P.; Beuing, O.; Neugebauer, M.; Gasteiger, R.; Preim, B.; Rose, G.; Skalej, M.; Thévenin, D. Recommendations for accurate numerical blood flow simulations of stented intracranial aneurysms. Biomed. Tech. Eng. 2013, 58, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Berg, P.; Stucht, D.; Janiga, G.; Beuing, O.; Speck, O.; Thévenin, D. Cerebral blood flow in a healthy Circle of Willis and two intracranial aneurysms: Computational fluid dynamics versus four-dimensional phase-contrast magnetic resonance imaging. J. Biomech. Eng. 2014, 136, 041003. [Google Scholar] [CrossRef]
- Valen-Sendstad, K.; Piccinelli, M.; KrishnankuttyRema, R.; Steinman, D.A. Estimation of inlet flow rates for image-based aneurysm CFD models: Where and how to begin? Ann. Biomed. Eng. 2015, 43, 1422–1431. [Google Scholar] [CrossRef]
- Hodis, S.; Kargar, S.; Kallmes, D.; Dragomir-Daescu, D. Artery Length Sensitivity in Patient-Specific Cerebral Aneurysm Simulations. Am. J. Neuroradiol. 2014, 36, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Saalfeld, S.; Voß, S.; Beuing, O.; Preim, B.; Berg, P. Flow-splitting-based computation of outlet boundary conditions for improved cerebrovascular simulation in multiple intracranial aneurysms. Int. J. Comput. Assist. Radiol. Surg. 2019, 14, 1805–1813. [Google Scholar] [CrossRef]
- Korte, J.; Voß, S.; Janiga, G.; Beuing, O.; Behme, D.; Saalfeld, S.; Berg, P. Is Accurate Lumen Segmentation More Important than Outlet Boundary Condition in Image-Based Blood Flow Simulations for Intracranial Aneurysms? Cardiovasc. Eng. Technol. 2023, 14, 617–630. [Google Scholar] [CrossRef] [PubMed]
- Cebral, J.R.; Mut, F.; Weir, J.; Putman, C.M. Association of hemodynamic characteristics and cerebral aneurysm rupture. AJNR Am. J. Neuroradiol. 2011, 32, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Tutino, V.M.; Snyder, K.V.; Meng, H. CFD: Computational fluid dynamics or confounding factor dissemination? The role of hemodynamics in intracranial aneurysm rupture risk assessment. AJNR Am. J. Neuroradiol. 2014, 35, 1849–1857. [Google Scholar] [CrossRef]
- Tanioka, S.; Ishida, F.; Kishimoto, T.; Tsuji, M.; Tanaka, K.; Shimosaka, S.; Toyoda, M.; Kashiwagi, N.; Sano, T.; Suzuki, H. Quantification of hemodynamic irregularity using oscillatory velocity index in the associations with the rupture status of cerebral aneurysms. J. Neurointerv. Surg. 2019, 11, 614–617. [Google Scholar] [CrossRef]
- Hu, P.; Qian, Y.; Lee, C.J.; Zhang, H.Q.; Ling, F. The energy loss may predict rupture risks of anterior communicating aneurysms: A preliminary result. Int. J. Clin. Exp. Med. 2015, 8, 4128–4133. [Google Scholar]
- Guo, H.; Liu, J.F.; Li, C.H.; Wang, J.W.; Li, H.; Gao, B.L. Effects of stent-assisted coiling in comparison with flow diversion on intracranial aneurysms. Front. Neurol. 2022, 13, 937536. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Tian, Z.; Liu, J.; Jing, L.; Paliwal, N.; Wang, S.; Zhang, Y.; Xiang, J.; Siddiqui, A.H.; Meng, H.; et al. Hemodynamic alterations after stent implantation in 15 cases of intracranial aneurysm. Acta Neurochir. 2016, 158, 811–819. [Google Scholar] [CrossRef] [PubMed]
- Rhee, K.; Han, M.H.; Cha, S.H. Changes of flow characteristics by stenting in aneurysm models: Influence of aneurysm geometry and stent porosity. Ann. Biomed. Eng. 2002, 30, 894–904. [Google Scholar] [CrossRef] [PubMed]
- Brunozzi, D.; Theiss, P.; Andrews, A.; Amin-Hanjani, S.; Charbel, F.T.; Alaraj, A. Correlation Between Laminar Wall Shear Stress and Growth of Unruptured Cerebral Aneurysms: In Vivo Assessment. World Neurosurg. 2019, 131, e599–e605. [Google Scholar] [CrossRef]
- Takehara, Y. Clinical Application of 4D Flow MR Imaging for the Abdominal Aorta. Magn. Reson. Med. Sci. MRMS Off. J. Jpn. Soc. Magn. Reson. Med. 2022, 21, 354–364. [Google Scholar] [CrossRef]
- Chong, W.; Zhang, Y.; Qian, Y.; Lai, L.; Parker, G.; Mitchell, K. Computational hemodynamics analysis of intracranial aneurysms treated with flow diverters: Correlation with clinical outcomes. AJNR Am. J. Neuroradiol. 2014, 35, 136–142. [Google Scholar] [CrossRef]
- Hayes, W.T.; Bernhardt, H.; Young, J.M. Fusiform arteriosclerotic aneurysm of the basilar artery. Five cases including two ruptures. Vasc. Surg. 1967, 1, 171–178. [Google Scholar] [CrossRef]
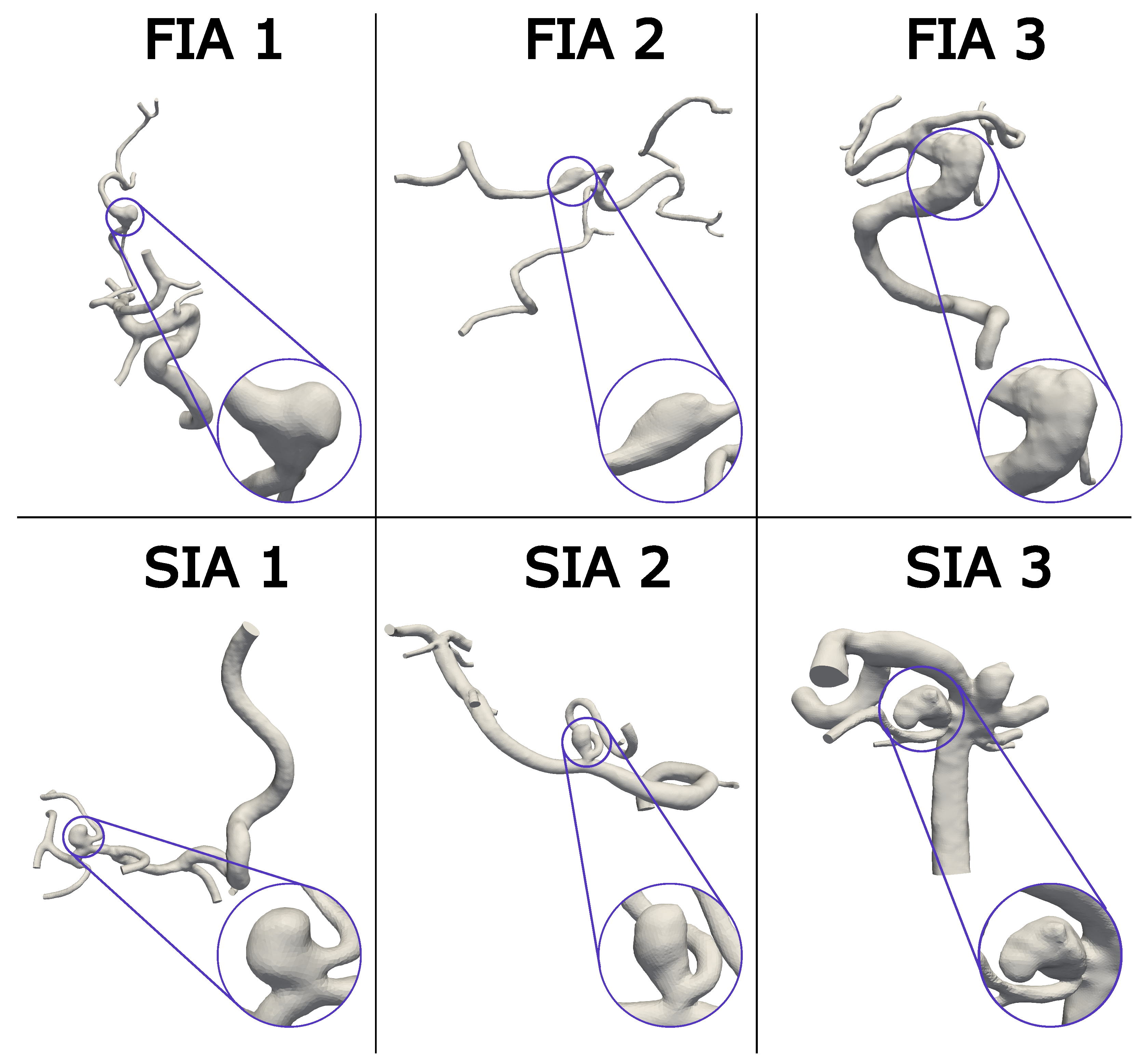
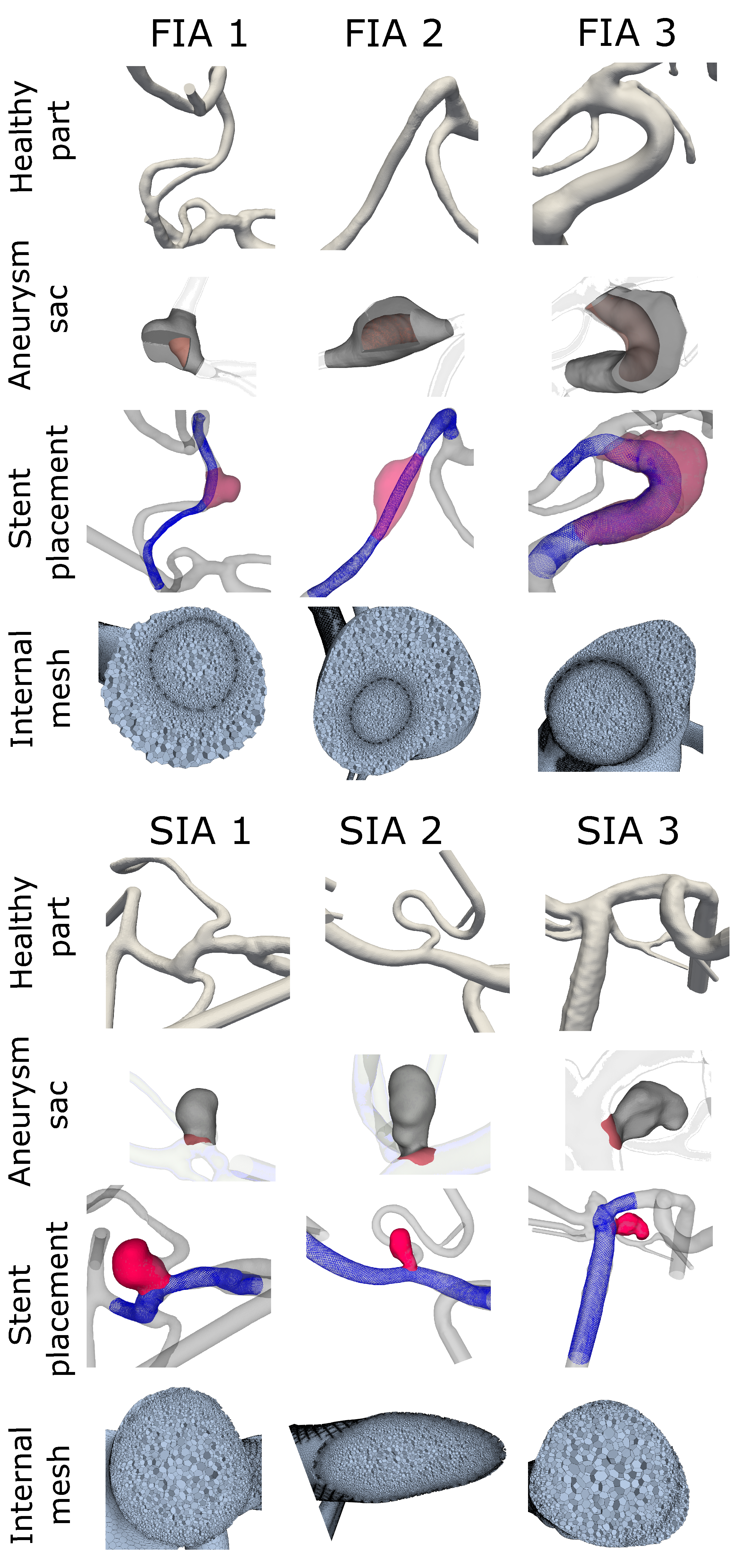

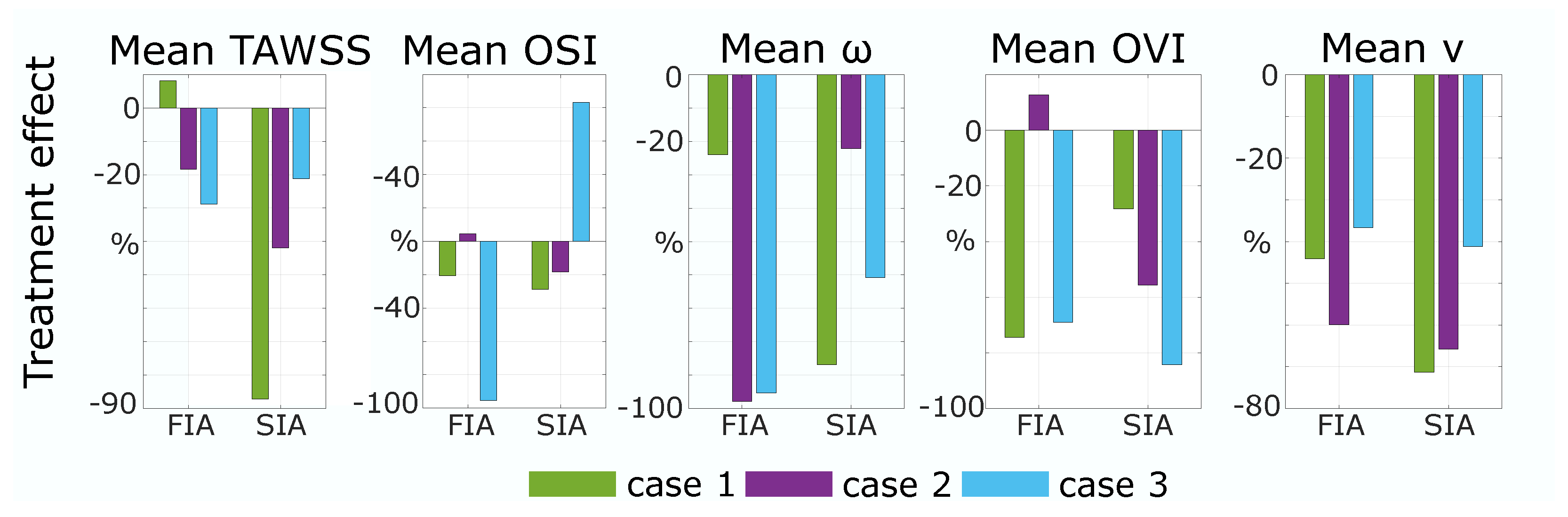
| Case | Aneurysm Volume (mm3) | Stent Area (mm2) | Area/ Volume (1/mm) | Nominal Stent Diameter (mm) | Max Stent Expansion (%) | Mean Stent Expansion (%) |
|---|---|---|---|---|---|---|
| FIA 1 | 30.4 | 38.6 | 1.3 | 2.3 | 104 | 91 |
| SIA 1 | 33.2 | 7.84 | 0.2 | 2.3 | 91 | 82 |
| FIA 2 | 159 | 168 | 1.1 | 3.0 | 122 | 87 |
| SIA 2 | 52.7 | 6.35 | 0.1 | 4.5 | 103 | 58 |
| FIA 3 | 920 | 656 | 0.7 | 7.0 | 103 | 80 |
| SIA 3 | 34.1 | 4.4 | 0.1 | 4.0 | 83 | 72 |
| Case | EL (W) | ||
|---|---|---|---|
| P | T | H | |
| FIA 1 | −8.17 | −9.57 | 4.60 |
| SIA 1 | 5.02 | 8.16 | 3.79 |
| FIA 2 | 2.69 | 4.96 | 3.22 |
| SIA 2 | 1.79 | 2.65 | 1.76 |
| FIA 3 | 9.50 | −5.17 | 9.33 |
| SIA 3 | 2.48 | 2.91 | 1.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korte, J.; Marsh, L.M.M.; Saalfeld, S.; Behme, D.; Aliseda, A.; Berg, P. Fusiform versus Saccular Intracranial Aneurysms—Hemodynamic Evaluation of the Pre-Aneurysmal, Pathological, and Post-Interventional State. J. Clin. Med. 2024, 13, 551. https://doi.org/10.3390/jcm13020551
Korte J, Marsh LMM, Saalfeld S, Behme D, Aliseda A, Berg P. Fusiform versus Saccular Intracranial Aneurysms—Hemodynamic Evaluation of the Pre-Aneurysmal, Pathological, and Post-Interventional State. Journal of Clinical Medicine. 2024; 13(2):551. https://doi.org/10.3390/jcm13020551
Chicago/Turabian StyleKorte, Jana, Laurel M. M. Marsh, Sylvia Saalfeld, Daniel Behme, Alberto Aliseda, and Philipp Berg. 2024. "Fusiform versus Saccular Intracranial Aneurysms—Hemodynamic Evaluation of the Pre-Aneurysmal, Pathological, and Post-Interventional State" Journal of Clinical Medicine 13, no. 2: 551. https://doi.org/10.3390/jcm13020551
APA StyleKorte, J., Marsh, L. M. M., Saalfeld, S., Behme, D., Aliseda, A., & Berg, P. (2024). Fusiform versus Saccular Intracranial Aneurysms—Hemodynamic Evaluation of the Pre-Aneurysmal, Pathological, and Post-Interventional State. Journal of Clinical Medicine, 13(2), 551. https://doi.org/10.3390/jcm13020551






