Immersive Virtual Reality Therapy Is Supportive for Orthopedic Rehabilitation among the Elderly: A Randomized Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
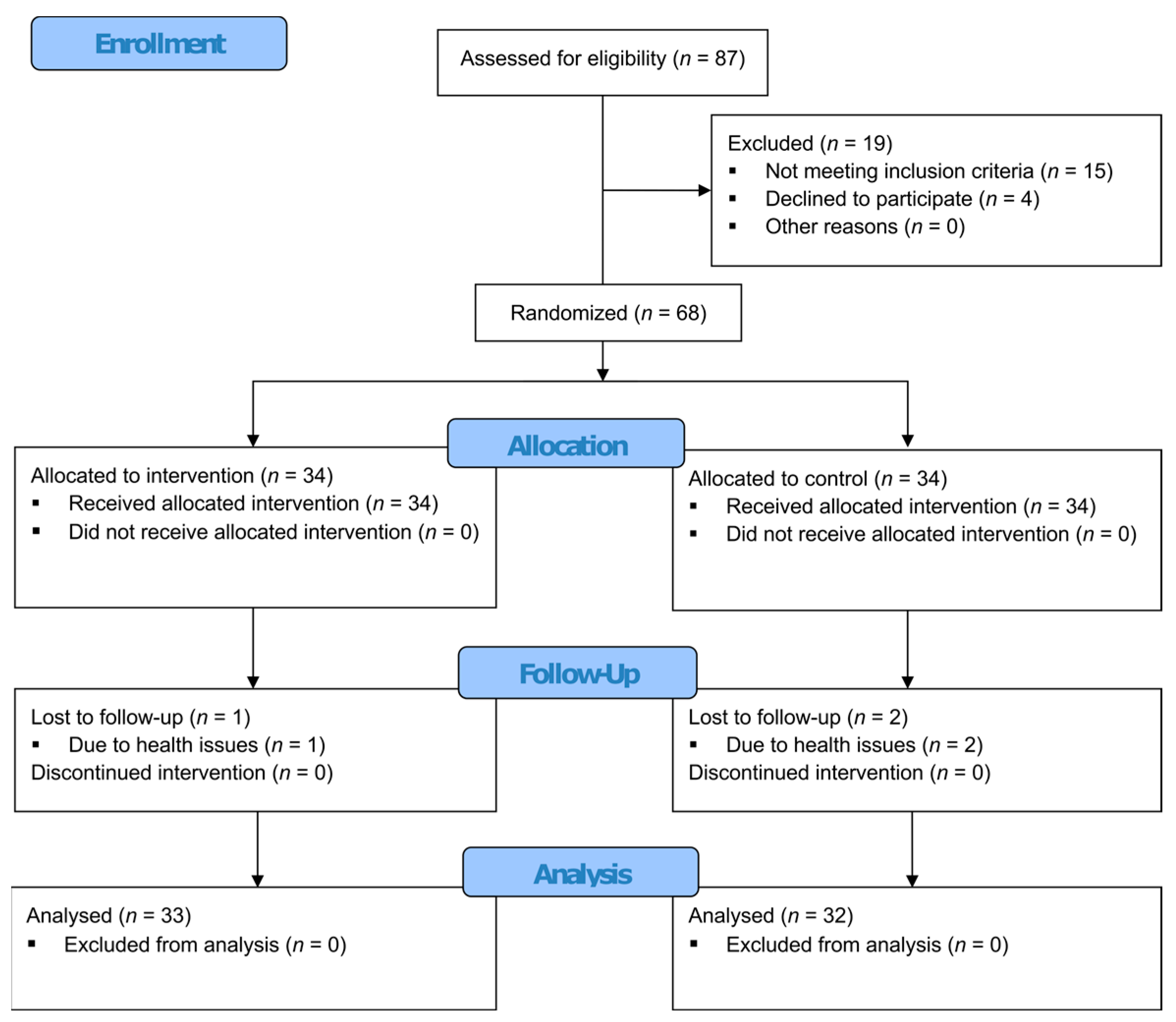
2.2. Participants
2.3. Interventions
2.4. Outcome Measures
2.5. Data Analysis
3. Results
3.1. Participant Characteristics
3.2. Effectiveness of the Interventions
3.3. Correlations and Predictors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Quicke, J.G.; Conaghan, P.G.; Corp, N.; Peat, G. Osteoarthritis Year in Review 2021: Epidemiology & Therapy. Osteoarthr. Cartil. 2022, 30, 196–206. [Google Scholar] [CrossRef]
- Ackerman, I.N.; Buchbinder, R.; March, L. Global Burden of Disease Study 2019: An Opportunity to Understand the Growing Prevalence and Impact of Hip, Knee, Hand and Other Osteoarthritis in Australia. Intern. Med. J. 2023, 53, 1875–1882. [Google Scholar] [CrossRef]
- World Health Organization Osteoarthritis. Available online: https://www.who.int/news-room/fact-sheets/detail/osteoarthritis (accessed on 26 September 2023).
- Sambamoorthi, U.; Shah, D.; Zhao, X. Healthcare Burden of Depression in Adults with Arthritis. Expert. Rev. Pharmacoecon Outcomes Res. 2017, 17, 53–65. [Google Scholar] [CrossRef]
- Wang, S.-T.; Ni, G.-X. Depression in Osteoarthritis: Current Understanding. Neuropsychiatr. Dis. Treat. 2022, 18, 375–389. [Google Scholar] [CrossRef]
- Agarwal, P.; Sambamoorthi, U. Healthcare Expenditures Associated with Depression among Individuals with Osteoarthritis: Post-Regression Linear Decomposition Approach. J. Gen. Intern. Med. 2015, 30, 1803–1811. [Google Scholar] [CrossRef] [PubMed]
- Briggs, A.M.; Cross, M.J.; Hoy, D.G.; Sànchez-Riera, L.; Blyth, F.M.; Woolf, A.D.; March, L. Musculoskeletal Health Conditions Represent a Global Threat to Healthy Aging: A Report for the 2015 World Health Organization World Report on Ageing and Health. Gerontologist 2016, 56, S243–S255. [Google Scholar] [CrossRef] [PubMed]
- Shalhoub, M.; Anaya, M.; Deek, S.; Zaben, A.H.; Abdalla, M.A.; Jaber, M.M.; Koni, A.A.; Zyoud, S.H. The Impact of Pain on Quality of Life in Patients with Osteoarthritis: A Cross-Sectional Study from Palestine. BMC Musculoskelet. Disord. 2022, 23, 248. [Google Scholar] [CrossRef] [PubMed]
- Bonanni, R.; Cariati, I.; Tancredi, V.; Iundusi, R.; Gasbarra, E.; Tarantino, U. Chronic Pain in Musculoskeletal Diseases: Do You Know Your Enemy? J. Clin. Med. 2022, 11, 2609. [Google Scholar] [CrossRef]
- Roughan, W.H.; Campos, A.I.; García-Marín, L.M.; Cuéllar-Partida, G.; Lupton, M.K.; Hickie, I.B.; Medland, S.E.; Wray, N.R.; Byrne, E.M.; Ngo, T.T.; et al. Comorbid Chronic Pain and Depression: Shared Risk Factors and Differential Antidepressant Effectiveness. Front. Psychiatry 2021, 12, 643609. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.J.; Van Drunen, S.; Egorova-Brumley, N. Neural Correlates of Co-Occurring Pain and Depression: An Activation-Likelihood Estimation (ALE) Meta-Analysis and Systematic Review. Transl. Psychiatry 2022, 12, 196. [Google Scholar] [CrossRef] [PubMed]
- Leo, A.J.; Schuelke, M.J.; Hunt, D.M.; Metzler, J.P.; Miller, J.P.; Areán, P.A.; Armbrecht, M.A.; Cheng, A.L. A Digital Mental Health Intervention in an Orthopedic Setting for Patients with Symptoms of Depression and/or Anxiety: Feasibility Prospective Cohort Study. JMIR Form. Res. 2022, 6, e34889. [Google Scholar] [CrossRef] [PubMed]
- Hampton, M.; Riley, E.; Garneti, N.; Anderson, A.; Wembridge, K. The Orthopaedic Waiting List Crisis. Bone Jt. Open 2021, 2, 530–534. [Google Scholar] [CrossRef] [PubMed]
- Patten, R.K.; Asilioglu, A.; Levinger, I.; Tacey, A.; Pascoe, M.; Tran, P.; McKenna, M.J.; Said, C.M.; Coric, N.; De Gori, M.; et al. Prevalence of Diagnosable Depression in Patients Awaiting Orthopaedic Specialist Consultation: A Cross-Sectional Analysis. BMC Musculoskelet. Disord. 2023, 24, 599. [Google Scholar] [CrossRef] [PubMed]
- National Guideline Centre (UK). Evidence Review for Inpatient Hip and Knee Postoperative Rehabilitation: Joint Replacement (Primary): Hip, Knee and Shoulder: Evidence Review P; NICE Evidence Reviews Collection; National Institute for Health and Care Excellence (NICE): London, UK, 2020; ISBN 978-1-4731-3722-6. [Google Scholar]
- Gazendam, A.; Zhu, M.; Chang, Y.; Phillips, S.; Bhandari, M. Virtual Reality Rehabilitation Following Total Knee Arthroplasty: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Knee Surg. Sports Traumatol. Arthrosc. 2022, 30, 2548–2555. [Google Scholar] [CrossRef] [PubMed]
- Szczepańska-Gieracha, J.; Jóźwik, S.; Cieślik, B.; Mazurek, J.; Gajda, R. Immersive Virtual Reality Therapy as a Support for Cardiac Rehabilitation: A Pilot Randomized-Controlled Trial. Cyberpsychol Behav. Soc. Netw. 2021, 24, 543–549. [Google Scholar] [CrossRef]
- Peng, L.; Zeng, Y.; Wu, Y.; Si, H.; Shen, B. Virtual Reality-Based Rehabilitation in Patients Following Total Knee Arthroplasty: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Chin. Med. J. 2021, 135, 153–163. [Google Scholar] [CrossRef]
- Gumaa, M.; Rehan Youssef, A. Is Virtual Reality Effective in Orthopedic Rehabilitation? A Systematic Review and Meta-Analysis. Phys. Ther. 2019, 99, 1304–1325. [Google Scholar] [CrossRef]
- García-Sánchez, M.; García-Robles, P.; Osuna-Pérez, M.C.; Lomas-Vega, R.; Obrero-Gaitán, E.; Cortés-Pérez, I. Effectiveness of Virtual Reality-Based Early Postoperative Rehabilitation after Total Knee Arthroplasty: A Systematic Review with Meta-Analysis of Randomized Controlled Trials. Appl. Sci. 2023, 13, 4597. [Google Scholar] [CrossRef]
- Birckhead, B.; Khalil, C.; Liu, X.; Conovitz, S.; Rizzo, A.; Danovitch, I.; Bullock, K.; Spiegel, B. Recommendations for Methodology of Virtual Reality Clinical Trials in Health Care by an International Working Group: Iterative Study. JMIR Ment. Health 2019, 6, e11973. [Google Scholar] [CrossRef]
- Matthews, W.J. Ericksonian Approaches to Hypnosis and Therapy: Where Are We Now? Int. J. Clin. Exp. Hypn. 2000, 48, 418–426, discussion 433–437. [Google Scholar] [CrossRef]
- Cieślik, B.; Juszko, K.; Kiper, P.; Szczepańska-Gieracha, J. Immersive Virtual Reality as Support for the Mental Health of Elderly Women: A Randomized Controlled Trial. Virtual Real. 2023, 27, 2227–2235. [Google Scholar] [CrossRef] [PubMed]
- Bjelland, I.; Dahl, A.A.; Haug, T.T.; Neckelmann, D. The Validity of the Hospital Anxiety and Depression Scale. An Updated Literature Review. J. Psychosom. Res. 2002, 52, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S. Perceived Stress in a Probability Sample of the United States. In The Social Psychology of Health; The Claremont Symposium on Applied Social Psychology; Sage Publications, Inc.: Thousand Oaks, CA, USA, 1988; pp. 31–67. ISBN 978-0-8039-3162-6. [Google Scholar]
- Luszczynska, A.; Scholz, U.; Schwarzer, R. The General Self-Efficacy Scale: Multicultural Validation Studies. J. Psychol. 2005, 139, 439–457. [Google Scholar] [CrossRef] [PubMed]
- Collin, C.; Wade, D.T.; Davies, S.; Horne, V. The Barthel ADL Index: A Reliability Study. Int. Disabil. Stud. 1988, 10, 61–63. [Google Scholar] [CrossRef] [PubMed]
- Collen, F.M.; Wade, D.T.; Robb, G.F.; Bradshaw, C.M. The Rivermead Mobility Index: A Further Development of the Rivermead Motor Assessment. Int. Disabil. Stud. 1991, 13, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Lauretani, F.; Ticinesi, A.; Gionti, L.; Prati, B.; Nouvenne, A.; Tana, C.; Meschi, T.; Maggio, M. Short-Physical Performance Battery (SPPB) Score Is Associated with Falls in Older Outpatients. Aging Clin. Exp. Res. 2019, 31, 1435–1442. [Google Scholar] [CrossRef]
- Plopa, M.; Makarowski, R. The Perception of Stress Questionnaire. Manual; Vizja Press & IT: Warsaw, Poland, 2010; ISBN 978-83-61086-79-6. [Google Scholar]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A Flexible Statistical Power Analysis Program for the Social, Behavioral, and Biomedical Sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Vincent, H.K.; Hagen, J.E.; Zdziarski-Horodyski, L.A.; Patrick, M.; Sadasivan, K.K.; Guenther, R.; Vasilopoulos, T.; Sharififar, S.; Horodyski, M. Patient-Reported Outcomes Measurement Information System Outcome Measures and Mental Health in Orthopaedic Trauma Patients During Early Recovery. J. Orthop. Trauma. 2018, 32, 467–473. [Google Scholar] [CrossRef]
- Flanigan, D.C.; Everhart, J.S.; Glassman, A.H. Psychological Factors Affecting Rehabilitation and Outcomes Following Elective Orthopaedic Surgery. J. Am. Acad. Orthop. Surg. 2015, 23, 563–570. [Google Scholar] [CrossRef]
- Kiper, P.; Przysiężna, E.; Cieślik, B.; Broniec-Siekaniec, K.; Kucińska, A.; Szczygieł, J.; Turek, K.; Gajda, R.; Szczepańska-Gieracha, J. Effects of Immersive Virtual Therapy as a Method Supporting Recovery of Depressive Symptoms in Post-Stroke Rehabilitation: Randomized Controlled Trial. Clin. Interv. Aging 2022, 17, 1673–1685. [Google Scholar] [CrossRef]
- Peters, M.L. Emotional and Cognitive Influences on Pain Experience. Mod. Trends Pharmacopsychiatry 2015, 30, 138–152. [Google Scholar] [CrossRef]
- Lin, I.; Wiles, L.; Waller, R.; Goucke, R.; Nagree, Y.; Gibberd, M.; Straker, L.; Maher, C.G.; O’Sullivan, P.P.B. What Does Best Practice Care for Musculoskeletal Pain Look like? Eleven Consistent Recommendations from High-Quality Clinical Practice Guidelines: Systematic Review. Br. J. Sports Med. 2020, 54, 79–86. [Google Scholar] [CrossRef]
- Moseley, G.L.; Vlaeyen, J.W.S. Beyond Nociception: The Imprecision Hypothesis of Chronic Pain. Pain 2015, 156, 35–38. [Google Scholar] [CrossRef]
- Vincent, H.K.; Horodyski, M.; Vincent, K.R.; Brisbane, S.T.; Sadasivan, K.K. Psychological Distress After Orthopedic Trauma: Prevalence in Patients and Implications for Rehabilitation. PM&R 2015, 7, 978–989. [Google Scholar] [CrossRef]
- Shamim, Q.; Fatima, L.; Albab, H. The Impact of Psychological Factors on Rehabilitation Outcomes in Patients with Chronic Pain. J. Health Rehabil. Res. 2023, 3, 48–52. [Google Scholar]
- Cherkin, D.C.; Sherman, K.J.; Balderson, B.H.; Cook, A.J.; Anderson, M.L.; Hawkes, R.J.; Hansen, K.E.; Turner, J.A. Effect of Mindfulness-Based Stress Reduction vs Cognitive Behavioral Therapy or Usual Care on Back Pain and Functional Limitations in Adults with Chronic Low Back Pain: A Randomized Clinical Trial. JAMA 2016, 315, 1240–1249. [Google Scholar] [CrossRef] [PubMed]
- Gil, J.A.; Goodman, A.D.; Mulcahey, M.K. Psychological Factors Affecting Outcomes after Elective Shoulder Surgery. J. Am. Acad. Orthop. Surg. 2018, 26, e98–e104. [Google Scholar] [CrossRef] [PubMed]
- Sheikhzadeh, A.; Wertli, M.M.; Weiner, S.S.; Rasmussen-Barr, E.; Weiser, S. Do Psychological Factors Affect Outcomes in Musculoskeletal Shoulder Disorders? A Systematic Review. BMC Musculoskelet. Disord. 2021, 22, 560. [Google Scholar] [CrossRef] [PubMed]
- Szczepańska-Gieracha, J.; Mazurek, J. The Role of Self-Efficacy in the Recovery Process of Stroke Survivors. Psychol. Res. Behav. Manag. 2020, 13, 897–906. [Google Scholar] [CrossRef]
- Marconcin, P.; Espanha, M.; Teles, J.; Bento, P.; Campos, P.; André, R.; Yázigi, F. A Randomized Controlled Trial of a Combined Self-Management and Exercise Intervention for Elderly People with Osteoarthritis of the Knee: The PLE2NO Program. Clin. Rehabil. 2018, 32, 223–232. [Google Scholar] [CrossRef]
- Khachian, A.; Seyedoshohadaei, M.; Haghani, H.; Amiri, F. Effect of Self-Management Program on Outcome of Adult Knee Osteoarthritis. Int. J. Orthop. Trauma. Nurs. 2020, 39, 100797. [Google Scholar] [CrossRef] [PubMed]


| Variable | Overall | VR Therapy | Control | p Value | |
|---|---|---|---|---|---|
| N | 68 | 34 | 34 | - | |
| n (%) of women | 42 (61.76) | 21 (61.76) | 21 (61.76) | 1.00 b | |
| Age, years | 69.59 (6.16) | 69.71 (6.82) | 69.47 (5.52) | 0.88 a | |
| Body mass, kg | 80.25 (16.08) | 79.26 (16.60) | 81.24 (15.72) | 0.62 a | |
| Body height, cm | 167.69 (10.02) | 166.76 (8.07) | 168.62 (11.79) | 0.45 a | |
| Body mass index, kg/m2 | 28.54 (5.12) | 28.46 (5.25) | 28.62 (5.05) | 0.89 a | |
| Normal (BMI 18.5–24.9), n (%) | 13 (19.12) | 6 (17.65) | 7 (20.59) | 0.76 b | |
| Overweight (BMI 25–29.9), n (%) | 33 (48.53) | 18 (52.94) | 15 (44.12) | 0.47 b | |
| Obese (BMI > 30), n (%) | 22 (32.35) | 10 (29.41) | 12 (35.29) | 0.60 b | |
| Arthroplasty area, n (%) | |||||
| Hip | 45 (66.18) | 22 (64.70) | 23 (67.65) | 0.80 b | |
| Knee | 23 (33.83) | 12 (35.30) | 11 (32.35) | ||
| Marital status, n (%) | |||||
| Married | 46 (67.65) | 21 (61.76) | 25 (73.53) | 0.30 b | |
| Single | 1 (1.47) | 1 (2.94) | 0 (0.00) | - | |
| Widowed | 21 (30.88) | 12 (35.29) | 9 (26.47) | 0.43 b | |
| Education, n (%) | |||||
| Primary/vocational | 27 (39.71) | 13 (38.24) | 14 (41.18) | 0.80 b | |
| Secondary | 29 (42.65) | 16 (47.06) | 13 (38.24) | 0.46 b | |
| Higher | 12 (17.65) | 5 (14.71) | 7 (20.59) | 0.52 b | |
| Outcome | Mean Square | F | ηp2 | p Value |
|---|---|---|---|---|
| Psychological outcomes | ||||
| HADS | 605.87 | 25.48 | 0.29 | <0.001 |
| HADS-A | 156.29 | 21.23 | 0.25 | <0.001 |
| HADS-D | 146.72 | 14.79 | 0.19 | <0.001 |
| VAS | 53.95 | 30.88 | 0.32 | <0.001 |
| PSS-10 | 94.67 | 17.77 | 0.22 | <0.001 |
| GSES | 837.89 | 47.85 | 0.43 | <0.001 |
| PSQ | 4563.31 | 41.26 | 0.40 | <0.001 |
| ES | 345.40 | 23.88 | 0.28 | <0.001 |
| IS | 452.43 | 30.83 | 0.33 | <0.001 |
| ET | 767.11 | 41.03 | 0.39 | <0.001 |
| Functional outcomes | ||||
| Tinetti | 92.01 | 44.79 | 0.42 | <0.001 |
| BI | 3520.84 | 47.48 | 0.43 | <0.001 |
| RMA-GF | 35.19 | 23.74 | 0.27 | <0.001 |
| SPPB | 111.54 | 41.03 | 0.39 | <0.001 |
| Outcome | VR Therapy (n = 34) | Control (n = 34) | Between-Group Comparison | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Post-Treatment | p Value | Baseline | Post-Treatment | p Value | Mean Difference | p Value | |
| Psychological outcomes | ||||||||
| HADS | 13.15 (5.94) | 5.55 (4.64) | <0.001 | 13.88 (7.68) | 14.94 (7.00) | 0.02 | −8.66 | <0.001 |
| HADS-A | 7.35 (3.33) | 2.76 (2.50) | <0.001 | 7.56 (3.92) | 7.34 (4.04) | 0.72 | −4.37 | <0.001 |
| HADS-D | 5.79 (2.59) | 2.79 (3.13) | 0.004 | 6.32 (4.35) | 7.59 (4.14) | 0.06 | −4.27 | <0.001 |
| VAS | 5.27 (1.97) | 0.88 (1.02) | <0.001 | 4.35 (2.07) | 2.59 (1.94) | <0.001 | −2.63 | <0.001 |
| PSS-10 | 24.94 (3.59) | 22.15 (1.77) | <0.001 | 25.18 (3.77) | 25.69 (4.27) | 0.14 | −3.30 | <0.001 |
| GSES | 28.59 (6.62) | 38.70 (1.29) | <0.001 | 30.74 (5.71) | 30.41 (6.36) | 0.88 | 10.44 | <0.001 |
| PSQ | 59.32 (18.47) | 35.73 (7.71) | <0.001 | 54.59 (18.64) | 54.91 (15.08) | 0.94 | −23.91 | <0.001 |
| ES | 18.06 (6.33) | 12.73 (3.38) | <0.001 | 16.47 (6.03) | 17.69 (4.97) | 0.09 | −6.55 | <0.001 |
| IS | 19.56 (6.65) | 11.30 (2.84) | <0.001 | 18.94 (7.09) | 18.38 (6.01) | 0.42 | −7.70 | <0.001 |
| ET | 21.71 (7.20) | 11.70 (3.85) | <0.001 | 19.18 (6.52) | 18.84 (5.41) | 0.69 | −9.67 | <0.001 |
| Functional outcomes | ||||||||
| Tinetti | 3.29 (2.42) | 9.64 (0.96) | <0.001 | 3.59 (2.50) | 6.53 (2.27) | <0.001 | −3.41 | <0.001 |
| BI | 54.56 (16.49) | 93.94 (6.47) | <0.001 | 56.18 (17.41) | 74.38 (16.84) | <0.001 | −21.18 | <0.001 |
| RMA-GF | 4.56 (2.35) | 10.00 (1.00) | <0.001 | 4.41 (2.55) | 7.81 (2.47) | <0.001 | −2.04 | <0.001 |
| SPPB | 2.62 (2.34) | 9.12 (2.41) | <0.001 | 2.88 (2.43) | 5.59 (2.98) | <0.001 | −3.79 | <0.001 |
| Variable | B | Beta | t | p Value | F | R2 | |
|---|---|---|---|---|---|---|---|
| ΔBI | <0.001 | 18.39 | 0.37 | ||||
| ΔHADS-A | 1.53 | 0.42 | 4.06 | ||||
| ΔPSS-10 | 1.53 | 0.35 | 3.38 | ||||
| ΔTinetti | <0.001 | 13.25 | 0.30 | ||||
| ΔGSES | 0.12 | 0.36 | 2.96 | ||||
| ΔHADS-A | 0.17 | 0.28 | 2.33 | ||||
| ΔRMA-GF | <0.001 | 10.67 | 0.26 | ||||
| ΔGSES | 0.09 | 0.34 | 2.88 | ||||
| ΔPSS-10 | 0.15 | 0.28 | 2.42 | ||||
| ΔSPPB | <0.001 | 13.46 | 0.40 | ||||
| ΔGSES | 0.11 | 0.29 | 2.47 | ||||
| ΔPSS-10 | 0.23 | 0.29 | 2.69 | ||||
| ΔHADS-A | 0.18 | 0.26 | 2.32 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazurek, J.; Cieślik, B.; Wrzeciono, A.; Gajda, R.; Szczepańska-Gieracha, J. Immersive Virtual Reality Therapy Is Supportive for Orthopedic Rehabilitation among the Elderly: A Randomized Controlled Trial. J. Clin. Med. 2023, 12, 7681. https://doi.org/10.3390/jcm12247681
Mazurek J, Cieślik B, Wrzeciono A, Gajda R, Szczepańska-Gieracha J. Immersive Virtual Reality Therapy Is Supportive for Orthopedic Rehabilitation among the Elderly: A Randomized Controlled Trial. Journal of Clinical Medicine. 2023; 12(24):7681. https://doi.org/10.3390/jcm12247681
Chicago/Turabian StyleMazurek, Justyna, Błażej Cieślik, Adam Wrzeciono, Robert Gajda, and Joanna Szczepańska-Gieracha. 2023. "Immersive Virtual Reality Therapy Is Supportive for Orthopedic Rehabilitation among the Elderly: A Randomized Controlled Trial" Journal of Clinical Medicine 12, no. 24: 7681. https://doi.org/10.3390/jcm12247681
APA StyleMazurek, J., Cieślik, B., Wrzeciono, A., Gajda, R., & Szczepańska-Gieracha, J. (2023). Immersive Virtual Reality Therapy Is Supportive for Orthopedic Rehabilitation among the Elderly: A Randomized Controlled Trial. Journal of Clinical Medicine, 12(24), 7681. https://doi.org/10.3390/jcm12247681







