Advancing Tailored Treatments: A Predictive Nomogram, Based on Ultrasound and Laboratory Data, for Assessing Nodal Involvement in Endometrial Cancer Patients
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Univariate Analysis and Multivariable Analyses
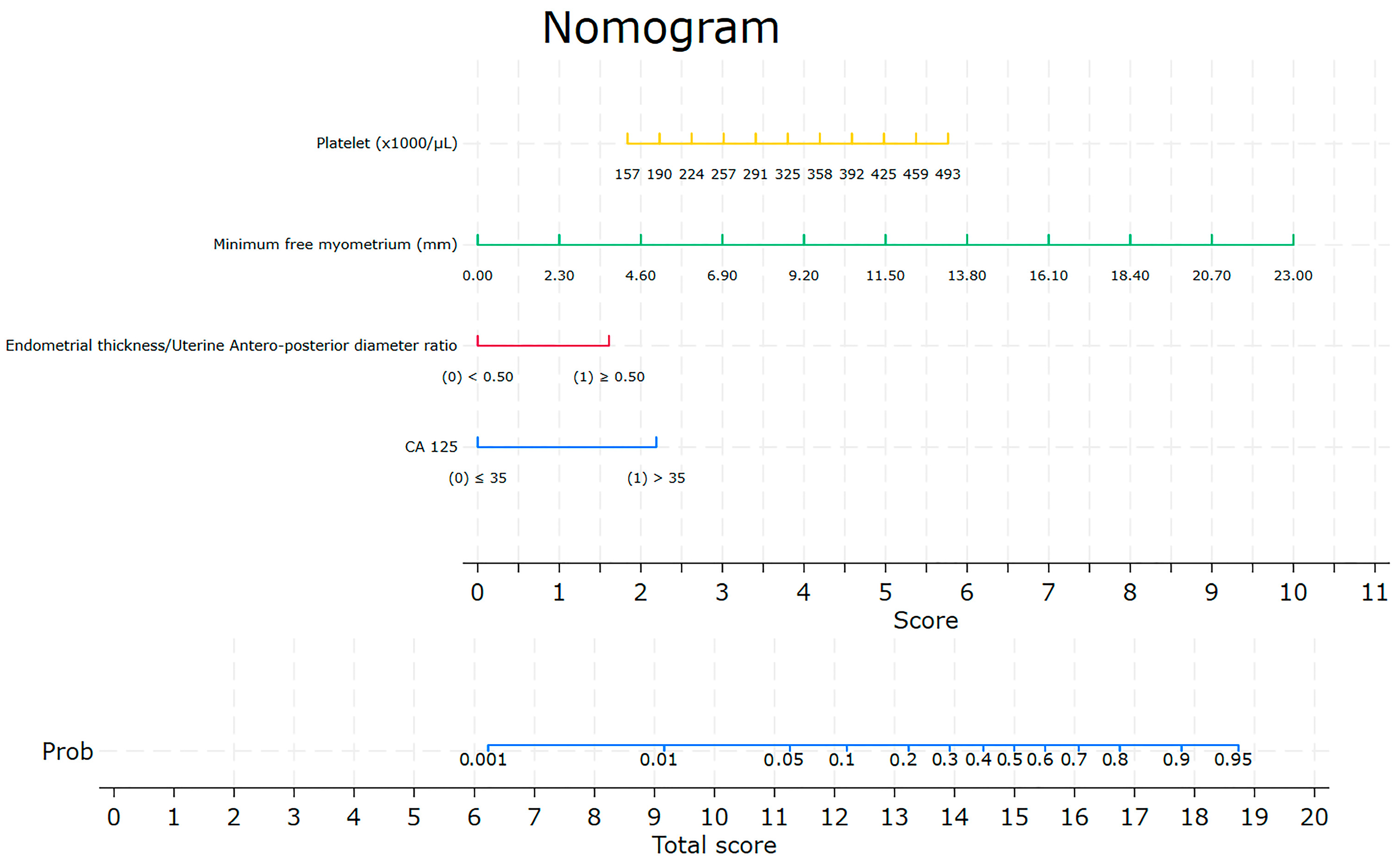
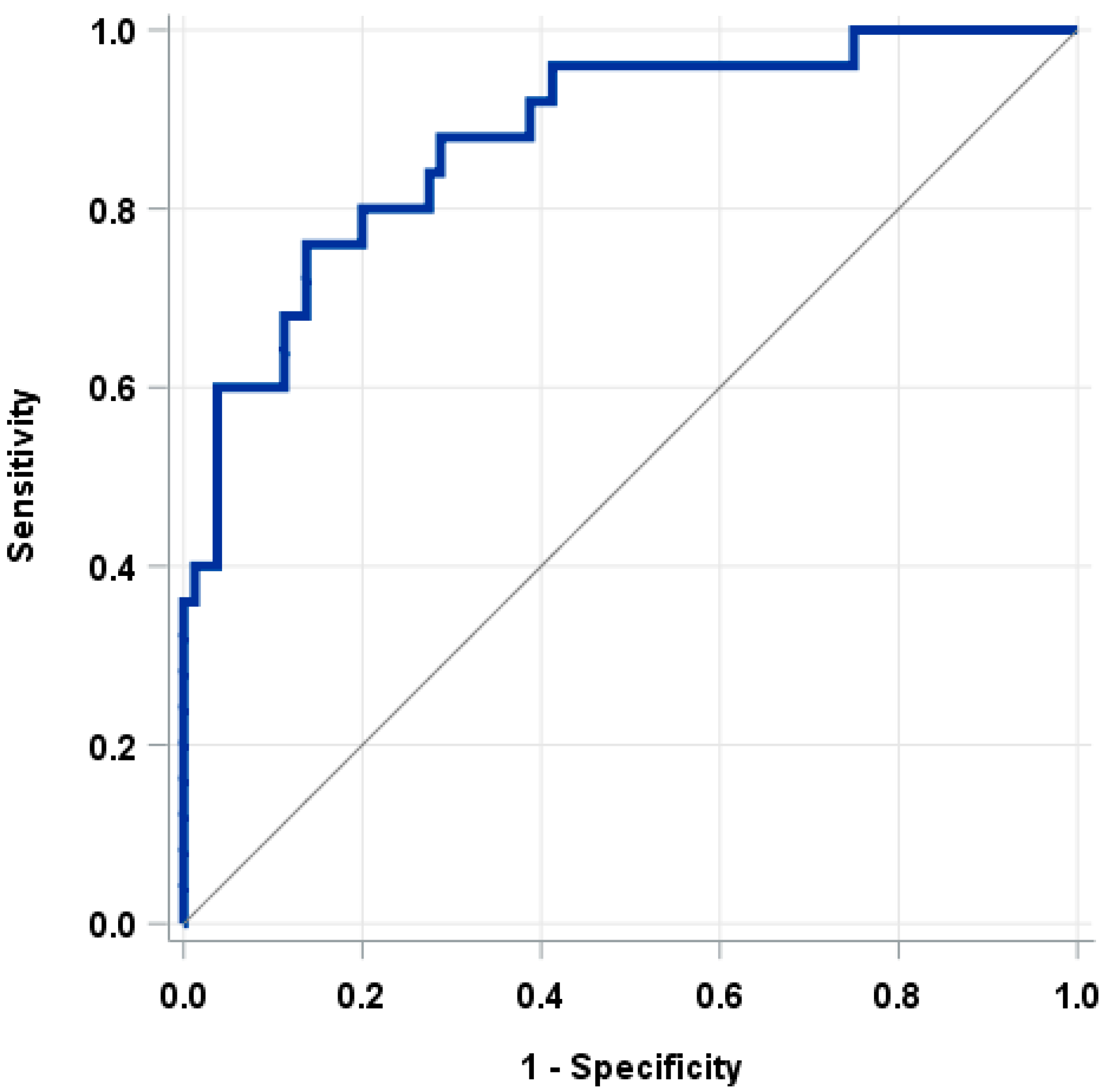
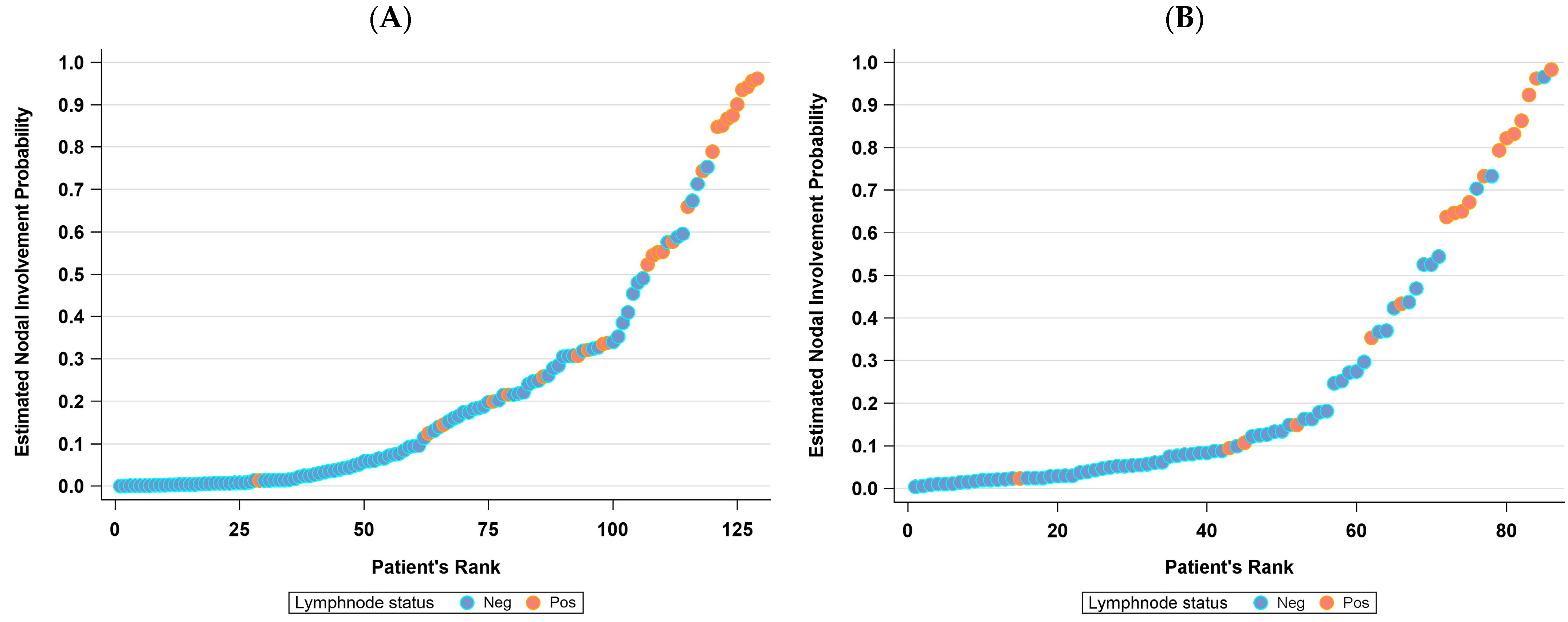
3.2. The Nomogram
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer Statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Concin, N.; Matias-Guiu, X.; Vergote, I.; Cibula, D.; Mirza, M.R.; Marnitz, S.; Ledermann, J.; Bosse, T.; Chargari, C.; Fagotti, A.; et al. ESGO/ESTRO/ESP Guidelines for the Management of Patients with Endometrial Carcinoma. Int. J. Gynecol. Cancer 2021, 31, 12–39. [Google Scholar] [CrossRef] [PubMed]
- Accorsi, G.S.; Paiva, L.L.; Schmidt, R.; Vieira, M.; Reis, R.; Andrade, C. Sentinel Lymph Node Mapping vs Systematic Lymphadenectomy for Endometrial Cancer: Surgical Morbidity and Lymphatic Complications. J. Minim. Invasive Gynecol. 2020, 27, 938–945. [Google Scholar] [CrossRef]
- ASTEC study group; Kitchener, H.; Swart, A.M.C.; Qian, Q.; Amos, C.; Parmar, M.K.B. Efficacy of Systematic Pelvic Lymphadenectomy in Endometrial Cancer (MRC ASTEC Trial): A Randomised Study. Lancet 2009, 373, 125–136. [Google Scholar] [CrossRef]
- Frost, J.A.; Webster, K.E.; Bryant, A.; Morrison, J. Lymphadenectomy for the Management of Endometrial Cancer. Cochrane Database Syst. Rev. 2017, 10, CD007585. [Google Scholar] [CrossRef] [PubMed]
- Proppe, L.; Alkatout, I.; Koch, R.; Baum, S.; Kotanidis, C.; Rody, A.; Hanker, L.C.; Gitas, G. Impact of Lymphadenectomy on Short- and Long-Term Complications in Patients with Endometrial Cancer. Arch. Gynecol. Obstet. 2022, 306, 811–819. [Google Scholar] [CrossRef] [PubMed]
- Capozzi, V.A.; Riemma, G.; Rosati, A.; Vargiu, V.; Granese, R.; Ercoli, A.; Cianci, S. Surgical Complications Occurring during Minimally Invasive Sentinel Lymph Node Detection in Endometrial Cancer Patients. A Systematic Review of the Literature and Metanalysis. Eur. J. Surg. Oncol. 2021, 47, 2142–2149. [Google Scholar] [CrossRef]
- Sozzi, G.; Fanfani, F.; Berretta, R.; Capozzi, V.A.; Uccella, S.; Buono, N.; Giallombardo, V.; Di Donna, M.C.; Monterossi, G.; Restaino, S.; et al. Laparoscopic Sentinel Node Mapping with Intracervical Indocyanine Green Injection for Endometrial Cancer: The SENTIFAIL Study—A Multicentric Analysis of Predictors of Failed Mapping. Int. J. Gynecol. Cancer 2020, 30, 1713–1718. [Google Scholar] [CrossRef]
- Polan, R.M.; Rossi, E.C.; Barber, E.L. Extent of Lymphadenectomy and Postoperative Major Complications among Women with Endometrial Cancer Treated with Minimally Invasive Surgery. Am. J. Obstet. Gynecol. 2019, 220, 263.e1–263.e8. [Google Scholar] [CrossRef]
- Imboden, S.; Mereu, L.; Siegenthaler, F.; Pellegrini, A.; Papadia, A.; Tateo, S.; Mueller, M.D. Oncological Safety and Perioperative Morbidity in Low-Risk Endometrial Cancer with Sentinel Lymph-Node Dissection. Eur. J. Surg. Oncol. 2019, 45, 1638–1643. [Google Scholar] [CrossRef]
- Persson, J.; Salehi, S.; Bollino, M.; Lönnerfors, C.; Falconer, H.; Geppert, B. Pelvic Sentinel Lymph Node Detection in High-Risk Endometrial Cancer (SHREC-Trial)—The Final Step towards a Paradigm Shift in Surgical Staging. Eur. J. Cancer 2019, 116, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Cusimano, M.C.; Vicus, D.; Pulman, K.; Maganti, M.; Bernardini, M.Q.; Bouchard-Fortier, G.; Laframboise, S.; May, T.; Hogen, L.F.; Covens, A.L.; et al. Assessment of Sentinel Lymph Node Biopsy vs Lymphadenectomy for Intermediate- and High-Grade Endometrial Cancer Staging. JAMA Surg. 2021, 156, 157–164. [Google Scholar] [CrossRef]
- Persson, J.; Geppert, B.; Lönnerfors, C.; Bollino, M.; Måsbäck, A. Description of a Reproducible Anatomically Based Surgical Algorithm for Detection of Pelvic Sentinel Lymph Nodes in Endometrial Cancer. Gynecol. Oncol. 2017, 147, 120–125. [Google Scholar] [CrossRef] [PubMed]
- How, J.A.; O’Farrell, P.; Amajoud, Z.; Lau, S.; Salvador, S.; How, E.; Gotlieb, W.H. Sentinel Lymph Node Mapping in Endometrial Cancer: A Systematic Review and Meta-Analysis. Minerva Ginecol. 2018, 70, 194–214. [Google Scholar] [CrossRef] [PubMed]
- Maramai, M.; Achilarre, M.T.; Aloisi, A.; Betella, I.; Bogliolo, S.; Garbi, A.; Maruccio, M.; Quatrale, C.; Aletti, G.D.; Mariani, A.; et al. Cervical Re-Injection of Indocyanine Green to Improve Sentinel Lymph Node Detection in Endometrial Cancer. Gynecol. Oncol. 2021, 162, 38–42. [Google Scholar] [CrossRef]
- Mueller, J.J.; Pedra Nobre, S.; Braxton, K.; Alektiar, K.M.; Leitao, M.M.; Aghajanian, C.; Ellenson, L.H.; Abu-Rustum, N.R. Incidence of Pelvic Lymph Node Metastasis Using Modern FIGO Staging and Sentinel Lymph Node Mapping with Ultrastaging in Surgically Staged Patients with Endometrioid and Serous Endometrial Carcinoma. Gynecol. Oncol. 2020, 157, 619–623. [Google Scholar] [CrossRef]
- Levy, M.H.; Back, A.; Benedetti, C.; Billings, J.A.; Block, S.; Boston, B.; Bruera, E.; Dy, S.; Eberle, C.; Foley, K.M.; et al. National comprehensive cancer network NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®), Uterine Neoplasms. Version 1.2024 2023. J. Natl. Compr. Cancer Netw. 2009, 7, 436–473. [Google Scholar] [CrossRef]
- Talhouk, A.; McConechy, M.K.; Leung, S.; Li-Chang, H.H.; Kwon, J.S.; Melnyk, N.; Yang, W.; Senz, J.; Boyd, N.; Karnezis, A.N.; et al. A Clinically Applicable Molecular-Based Classification for Endometrial Cancers. Br. J. Cancer 2015, 113, 299–310. [Google Scholar] [CrossRef]
- Jamieson, A.; Thompson, E.F.; Huvila, J.; Leung, S.; Lum, A.; Morin, C.; Ennour-Idrissi, K.; Sebastianelli, A.; Renaud, M.-C.; Gregoire, J.; et al. Endometrial Carcinoma Molecular Subtype Correlates with the Presence of Lymph Node Metastases. Gynecol. Oncol. 2022, 165, 376–384. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, S.; Ma, Y.; Li, W.; Tian, J.; Liu, T. A Nomogram Prediction Model for Lymph Node Metastasis in Endometrial Cancer Patients. BMC Cancer 2021, 21, 748. [Google Scholar] [CrossRef]
- Bendifallah, S.; Genin, A.S.; Naoura, I.; Chabbert Buffet, N.; Clavel Chapelon, F.; Haddad, B.; Luton, D.; Darai, E.; Rouzier, R.; Koskas, M. A Nomogram for Predicting Lymph Node Metastasis of Presumed Stage I and II Endometrial Cancer. Am. J. Obstet. Gynecol. 2012, 207, 197.e1–197.e8. [Google Scholar] [CrossRef]
- Kang, S.; Nam, J.-H.; Bae, D.-S.; Kim, J.-W.; Kim, M.-H.; Chen, X.; No, J.-H.; Lee, J.-M.; Kim, J.-H.; Watari, H.; et al. Preoperative Assessment of Lymph Node Metastasis in Endometrial Cancer: A Korean Gynecologic Oncology Group Study. Cancer 2017, 123, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Visser, N.C.M.; Reijnen, C.; Massuger, L.F.A.G.; Nagtegaal, I.D.; Bulten, J.; Pijnenborg, J.M.A. Accuracy of Endometrial Sampling in Endometrial Carcinoma: A Systematic Review and Meta-Analysis. Obstet. Gynecol. 2017, 130, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Leitao, M.M.; Kehoe, S.; Barakat, R.R.; Alektiar, K.; Gattoc, L.P.; Rabbitt, C.; Chi, D.S.; Soslow, R.A.; Abu-Rustum, N.R. Accuracy of Preoperative Endometrial Sampling Diagnosis of FIGO Grade 1 Endometrial Adenocarcinoma. Gynecol. Oncol. 2008, 111, 244–248. [Google Scholar] [CrossRef]
- Savelli, L.; Ceccarini, M.; Ludovisi, M.; Fruscella, E.; De Iaco, P.A.; Salizzoni, E.; Mabrouk, M.; Manfredi, R.; Testa, A.C.; Ferrandina, G. Preoperative Local Staging of Endometrial Cancer: Transvaginal Sonography vs. Magnetic Resonance Imaging. Ultrasound Obstet. Gynecol. 2008, 31, 560–566. [Google Scholar] [CrossRef]
- Epstein, E.; Blomqvist, L. Imaging in Endometrial Cancer. Best Pract. Res. Clin. Obstet. Gynaecol. 2014, 28, 721–739. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, L.S.E.; Epstein, E.; Testa, A.C.; Fischerova, D.; Valentin, L.; Sladkevicius, P.; Franchi, D.; Frühauf, F.; Fruscio, R.; Haak, L.A.; et al. Ultrasound-Based Risk Model for Preoperative Prediction of Lymph-Node Metastases in Women with Endometrial Cancer: Model-Development Study. Ultrasound Obstet. Gynecol. 2020, 56, 443–452. [Google Scholar] [CrossRef]
- Fares, R.; Kehoe, S.; Shams, N. Preoperative Prediction of Lymph Nodal Metastases in Endometrial Carcinoma: Is It Possible? Int. J. Gynecol. Cancer 2018, 28, 394–400. [Google Scholar] [CrossRef]
- Ünsal, M.; Comert, G.K.; Karalok, A.; Basaran, D.; Turkmen, O.; Boyraz, G.; Tasci, T.; Koc, S.; Boran, N.; Tulunay, G.; et al. The Preoperative Serum CA 125 Can Predict the Lymph Node Metastasis in Endometrioid-Type Endometrial Cancer. Ginekol. Pol. 2018, 89, 599–606. [Google Scholar] [CrossRef]
- Son, J.-H.; Kong, T.-W.; Kim, S.H.; Paek, J.; Chang, S.-J.; Lee, E.J.; Ryu, H.-S. Prediction of Lymph Node Metastasis in Patients with Apparent Early Endometrial Cancer. Obstet. Gynecol. Sci. 2015, 58, 385–390. [Google Scholar] [CrossRef][Green Version]
- Lee, J.; Kong, T.-W.; Paek, J.; Chang, S.-J.; Ryu, H.-S. Predicting Model of Lymph Node Metastasis Using Preoperative Tumor Grade, Transvaginal Ultrasound, and Serum CA-125 Level in Patients With Endometrial Cancer. Int. J. Gynecol. Cancer 2016, 26, 1630–1635. [Google Scholar] [CrossRef] [PubMed]
- Capozzi, V.A.; Sozzi, G.; Uccella, S.; Ceni, V.; Cianciolo, A.; Gambino, G.; Armano, G.; Pugliese, M.; Scambia, G.; Chiantera, V.; et al. Novel Preoperative Predictive Score to Evaluate Lymphovascular Space Involvement in Endometrial Cancer: An Aid to the Sentinel Lymph Node Algorithm. Int. J. Gynecol. Cancer 2020, 30, 806–812. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Chen, X.; Ni, J.; Li, Z.; Su, T.; Li, S.; Wan, X. A Model to Identify Candidates for Lymph Node Dissection Among Patients With High-Risk Endometrial Endometrioid Carcinoma According to Mayo Criteria. Front. Oncol. 2022, 12, 895834. [Google Scholar] [CrossRef]
- Capozzi, V.A.; Sozzi, G.; Rosati, A.; Restaino, S.; Gambino, G.; Cianciolo, A.; Ceccaroni, M.; Uccella, S.; Franchi, M.; Chiantera, V.; et al. Predictive Score of Nodal Involvement in Endometrial Cancer Patients: A Large Multicentre Series. Ann. Surg. Oncol. 2022, 29, 2594–2599. [Google Scholar] [CrossRef]
- Temur, I.; Kucukgoz Gulec, U.; Paydas, S.; Guzel, A.B.; Sucu, M.; Vardar, M.A. Prognostic Value of Pre-Operative Neutrophil/Lymphocyte Ratio, Monocyte Count, Mean Platelet Volume, and Platelet/Lymphocyte Ratio in Endometrial Cancer. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 226, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Tal, O.; Eitan, R.; Gemer, O.; Helpman, L.; Vaknin, Z.; Leytes, S.; Lavie, O.; Ben-Arie, A.; Amit, A.; Namazov, A.; et al. Prognostic Significance of Pretreatment Thrombocytosis in Endometrial Cancer: An Israeli Gynecologic Oncology Group Study. Int. J. Gynecol. Cancer 2021, 31, 1437–1442. [Google Scholar] [CrossRef]
- Epstein, E.; Fischerova, D.; Valentin, L.; Testa, A.C.; Franchi, D.; Sladkevicius, P.; Frühauf, F.; Lindqvist, P.G.; Mascilini, F.; Fruscio, R.; et al. Ultrasound Characteristics of Endometrial Cancer as Defined by International Endometrial Tumor Analysis (IETA) Consensus Nomenclature: Prospective Multicenter Study. Ultrasound Obstet. Gynecol. 2018, 51, 818–828. [Google Scholar] [CrossRef]
- Leone, F.P.G.; Timmerman, D.; Bourne, T.; Valentin, L.; Epstein, E.; Goldstein, S.R.; Marret, H.; Parsons, A.K.; Gull, B.; Istre, O.; et al. Terms, Definitions and Measurements to Describe the Sonographic Features of the Endometrium and Intrauterine Lesions: A Consensus Opinion from the International Endometrial Tumor Analysis (IETA) Group. Ultrasound Obstet. Gynecol. 2010, 35, 103–112. [Google Scholar] [CrossRef]
- Multinu, F.; Ducie, J.A.; Eriksson, A.G.Z.; Schlappe, B.A.; Cliby, W.A.; Glaser, G.E.; Grassi, T.; Keeney, G.L.; Weaver, A.L.; Abu-Rustum, N.R.; et al. Role of Lymphadenectomy in Endometrial Cancer with Nonbulky Lymph Node Metastasis: Comparison of Comprehensive Surgical Staging and Sentinel Lymph Node Algorithm. Gynecol. Oncol. 2019, 155, 177–185. [Google Scholar] [CrossRef]
- Schwartz, L.H.; Bogaerts, J.; Ford, R.; Shankar, L.; Therasse, P.; Gwyther, S.; Eisenhauer, E.A. Evaluation of Lymph Nodes with RECIST 1.1. Eur. J. Cancer 2009, 45, 261–267. [Google Scholar] [CrossRef]
- Barlin, J.N.; Khoury-Collado, F.; Kim, C.H.; Leitao, M.M.; Chi, D.S.; Sonoda, Y.; Alektiar, K.; DeLair, D.F.; Barakat, R.R.; Abu-Rustum, N.R. The Importance of Applying a Sentinel Lymph Node Mapping Algorithm in Endometrial Cancer Staging: Beyond Removal of Blue Nodes. Gynecol. Oncol. 2012, 125, 531–535. [Google Scholar] [CrossRef]
- Kim, C.H.; Soslow, R.A.; Park, K.J.; Barber, E.L.; Khoury-Collado, F.; Barlin, J.N.; Sonoda, Y.; Hensley, M.L.; Barakat, R.R.; Abu-Rustum, N.R. Pathologic Ultrastaging Improves Micrometastasis Detection in Sentinel Lymph Nodes during Endometrial Cancer Staging. Int. J. Gynecol. Cancer 2013, 23, 964–970. [Google Scholar] [CrossRef] [PubMed]
- Brierley, J.D.; Gospodarowicz, M.K. Christian Wittekind. In TNM Classification of Malignant Tumours, 8th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2016; ISBN 978-1-4443-5896-4. [Google Scholar]
- Oaknin, A.; Bosse, T.J.; Creutzberg, C.L.; Giornelli, G.; Harter, P.; Joly, F.; Lorusso, D.; Marth, C.; Makker, V.; Mirza, M.R.; et al. Endometrial Cancer: ESMO Clinical Practice Guideline for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2022, 33, 860–877. [Google Scholar] [CrossRef] [PubMed]
- Rafaniello-Raviele, P.; Betella, I.; Rappa, A.; Vacirca, D.; Tolva, G.; Guerrieri-Gonzaga, A.; Bertario, L.; Barberis, M.; Bonanni, B.; Marabelli, M. Microsatellite Instability Evaluation: Which Test to Use for Endometrial Cancer? J. Clin. Pathol. 2023, 76, 29–33. [Google Scholar] [CrossRef]
- Talhouk, A.; McConechy, M.K.; Leung, S.; Yang, W.; Lum, A.; Senz, J.; Boyd, N.; Pike, J.; Anglesio, M.; Kwon, J.S.; et al. Confirmation of ProMisE: A Simple, Genomics-Based Clinical Classifier for Endometrial Cancer. Cancer 2017, 123, 802–813. [Google Scholar] [CrossRef]
- Liro, M.; Śniadecki, M.; Wycinka, E.; Wojtylak, S.; Brzeziński, M.; Jastrzębska, J.; Wydra, D. Incorporation of Tumor-Free Distance and Other Alternative Ultrasound Biomarkers into a Myometrial Invasion-Based Model Better Predicts Lymph Node Metastasis in Endometrial Cancer: Evidence and Future Prospects. Diagnostics 2022, 12, 2604. [Google Scholar] [CrossRef]
- Celik, C.; Ozdemir, S.; Kiresi, D.; Emlik, D.; Tazegül, A.; Esen, H. Evaluation of Cervical Involvement in Endometrial Cancer by Transvaginal Sonography, Magnetic Resonance Imaging and Frozen Section. J. Obstet. Gynaecol. J. Inst. Obstet. Gynaecol. 2010, 30, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Pergialiotis, V.; Zachariou, E.; Vlachos, D.E.; Vlachos, A.; Goula, K.; Thomakos, N.; Rodolakis, A.; Haidopoulos, D. Tumor Free Distance from Serosa and Survival Rates of Endometrial Cancer Patients: A Meta-Analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2023, 286, 16–22. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Galaal, K.; Patel, A.; Fisher, A.; Nayar, A.; Cross, P.; Naik, R. Tumour-Free Distance from Serosa Is a Better Prognostic Indicator than Depth of Invasion and Percentage Myometrial Invasion in Endometrioid Endometrial Cancer. BJOG Int. J. Obstet. Gynaecol. 2012, 119, 1162–1170. [Google Scholar] [CrossRef]
- Liro, M.; Śniadecki, M.; Wycinka, E.; Wojtylak, S.; Brzeziński, M.; Stańczak, A.; Wydra, D. Ultrasound Measurement of Tumor-Free Distance from the Serosal Surface as the Alternative to Measuring the Depth of Myometrial Invasion in Predicting Lymph Node Metastases in Endometrial Cancer. Diagnostics 2021, 11, 1472. [Google Scholar] [CrossRef]
- Eriksson, L.S.E.; Lindqvist, P.G.; Flöter Rådestad, A.; Dueholm, M.; Fischerova, D.; Franchi, D.; Jokubkiene, L.; Leone, F.P.; Savelli, L.; Sladkevicius, P.; et al. Transvaginal Ultrasound Assessment of Myometrial and Cervical Stromal Invasion in Women with Endometrial Cancer: Interobserver Reproducibility among Ultrasound Experts and Gynecologists. Ultrasound Obstet. Gynecol. 2015, 45, 476–482. [Google Scholar] [CrossRef]
- Mariani, A.; Webb, M.J.; Keeney, G.L.; Haddock, M.G.; Calori, G.; Podratz, K.C. Low-Risk Corpus Cancer: Is Lymphadenectomy or Radiotherapy Necessary? Am. J. Obstet. Gynecol. 2000, 182, 1506–1519. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhang, Z.; Tian, Y.; Li, Z.; Liu, Z.; Zhu, S. The Critical Role of Platelet in Cancer Progression and Metastasis. Eur. J. Med. Res. 2023, 28, 385. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, M. Role of Platelets and Platelet Receptors in Cancer Metastasis. J. Hematol. Oncol.J Hematol Oncol 2018, 11, 125. [Google Scholar] [CrossRef] [PubMed]
- Qu, C.; Li, T.; Tang, Z.; Zhu, X.-R.; Han, J.; Tian, H. Platelet Count Is Associated with the Rate of Lymph Node Metastasis in Lung Adenocarcinoma. Cancer Manag. Res. 2020, 12, 9765–9774. [Google Scholar] [CrossRef]
- Çatal, O.; Özer, B.; Şit, M. Prediction of Lymph Node Metastasis in Colon Cancer via Platelet to Lymphocyte Ratio and Platelet Count. Jcpsp-J. Coll. Physicians Surg. Pak. 2020, 30, 250–253. [Google Scholar] [CrossRef]
- Farag, C.M.; Antar, R.; Akosman, S.; Ng, M.; Whalen, M.J. What Is Hemoglobin, Albumin, Lymphocyte, Platelet (HALP) Score? A Comprehensive Literature Review of HALP’s Prognostic Ability in Different Cancer Types. Oncotarget 2023, 14, 153–172. [Google Scholar] [CrossRef]
- Chen, H.; Wu, Q.; Zhang, Y.; Li, Q.; Ma, J.; Kong, F.; Ma, X. Nomograms Based on the Novel Platelet Index Score Predict Postoperative Prognosis in Endometrial Cancer. Gynecol. Oncol. 2020, 158, 689–697. [Google Scholar] [CrossRef]
- Bosse, T.; Peters, E.E.M.; Creutzberg, C.L.; Jürgenliemk-Schulz, I.M.; Jobsen, J.J.; Mens, J.W.M.; Lutgens, L.C.H.W.; van der Steen-Banasik, E.M.; Smit, V.T.H.B.M.; Nout, R.A. Substantial Lymph-Vascular Space Invasion (LVSI) Is a Significant Risk Factor for Recurrence in Endometrial Cancer—A Pooled Analysis of PORTEC 1 and 2 Trials. Eur. J. Cancer 2015, 51, 1742–1750. [Google Scholar] [CrossRef]
- Capozzi, V.A.; Monfardini, L.; Sozzi, G.; Butera, D.; Armano, G.; Riccò, M.; Giovanna, G.; Berretta, R. Obesity, an Independent Predictor of Pre and Postoperative Tumor Grading Disagreement in Endometrial Cancer. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 262, 160–165. [Google Scholar] [CrossRef]
- Kommoss, S.; McConechy, M.K.; Kommoss, F.; Leung, S.; Bunz, A.; Magrill, J.; Britton, H.; Kommoss, F.; Grevenkamp, F.; Karnezis, A.; et al. Final Validation of the ProMisE Molecular Classifier for Endometrial Carcinoma in a Large Population-Based Case Series. Ann. Oncol. 2018, 29, 1180–1188. [Google Scholar] [CrossRef] [PubMed]
- Creasman, W.T.; Morrow, C.P.; Bundy, B.N.; Homesley, H.D.; Graham, J.E.; Heller, P.B. Surgical Pathologic Spread Patterns of Endometrial Cancer: A Gynecologic Oncology Group Study. Cancer 1987, 60, 2035–2041. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | All Patients N = 210 | Nodal Involvement | p-Value a | |
|---|---|---|---|---|
| No N = 167 | Yes N = 43 | |||
| Age at Surgery, years | 60.2 (53.6, 69.4) | 60.4 (53.6, 69.4) | 58.2 (52.1, 70.4) | 0.73 |
| BMI (kg/m2) | 26.5 (23.0, 31.3) | 27.0 (23.0, 31.5) | 25.7 (22.7, 29.6) | 0.66 |
| CA 125 | 18.7 (11.9, 30.6) | 16.3 (11.1, 23.4) | 41.3 (23.4, 101) | <0.001 |
| Platelet count (103/μL) | 249 (212, 299) | 243 (211, 283) | 270 (213, 335) | 0.01 |
| Lymphocytes (103/μL) | 1.73 (1.40, 2.15) | 1.74 (1.43, 2.26) | 1.63 (1.32, 2.13) | 0.32 |
| Monocytes (103/μL) | 0.45 (0.36, 0.58) | 0.45 (0.36, 0.56) | 0.52 (0.35, 0.63) | 0.20 |
| Neutrophils (103/μL) | 4.09 (3.30, 5.22) | 3.92 (3.19, 5.04) | 4.98 (3.87, 5.68) | 0.002 |
| NLR (Neutrophil/Lymphocyte ratio) | 2.31 (1.70, 3.15) | 2.24 (1.69, 2.81) | 3.13 (1.72, 3.72) | 0.01 |
| PLR (Platelet/Lymphocyte ratio) | 142 (113, 188) | 138 (112, 178) | 169 (117, 231) | 0.05 |
| MLR (Monocyte/Lymphocyte ratio) | 0.26 (0.20, 0.34) | 0.25 (0.19, 0.34) | 0.29 (0.22, 0.36) | 0.08 |
| Uterine thickness (mm) | 40.0 (33.0, 49.0) | 39.0 (33.0, 49.0) | 45.0 (36.0, 56.0) | 0.03 |
| Endometrial thickness (mm) | 12.8 (7.0, 23.0) | 11.0 (6.2, 19.4) | 24.3 (14.3, 35.5) | <0.001 |
| Minimum free myometrium (mm) | 4.5 (2.4, 9.0) | 5.0 (3.0, 10.4) | 1.9 (0.0, 3.3) | <0.001 |
| Lesion diameter (mm) | ||||
| Sagittal | 29.0 (18.0, 41.0) | 25.0 (15.0, 37.0) | 42.0 (32.0, 59.0) | <0.001 |
| Antero-posterior | 18.0 (10.0, 27.0) | 16.0 (9.0, 24.0) | 26.5 (22.0, 41.0) | <0.001 |
| Transverse | 25.0 (16.0, 34.0) | 24.0 (14.0, 32.0) | 32.5 (25.0, 41.5) | <0.001 |
| d/D (mm) | 0.43 (0.23, 0.64) | 0.38 (0.20, 0.60) | 0.67 (0.47, 0.78) | <0.001 |
| Distance between tumor and external os (mm) | 24.0 (17.0, 32.0) | 26.0 (20.0, 35.0) | 17.5 (0.1, 23.5) | <0.001 |
| PCR | 0.20 (0.10, 0.50) | 0.20 (0.10, 0.55) | 0.25 (0.10, 0.50) | 0.84 |
| Characteristics | Level | Nodal Involvement N (%) a | p-Value b | |
|---|---|---|---|---|
| No | Yes | |||
| Parity (N = 171) | 107 (78.1) | 24 (70.6) | 0.37 | |
| Menopausal status | Post | 126 (75.5) | 33 (76.7) | 1.00 |
| Pre | 41 (24.6) | 10 (23.4) | ||
| Abnormal Uterine Bleeding | None | 59 (35.5) | 17 (39.5) | 0.97 |
| Post-menopausal | 86 (51.5) | 22 (51.2) | ||
| Heavy menstrual | 5 (3.0) | 1 (2.3) | ||
| Inter menstrual | 9 (5.4) | 1 (2.3) | ||
| Unknown | 8 (4.8) | 2 (4.7) | ||
| Hormone Replacement Therapy | No HRT | 166 (99.4) | 43 (100) | 1.00 |
| Continuous combined estro-progesterone scheme | 1 (0.6) | 0 | ||
| Endometrial echo pattern | Non-uniform | 88 (52.7) | 14 (32.6) | 0.02 |
| Uniform | 68 (40.7) | 22 (51.2) | ||
| Unspecified | 11 (6.6) | 7 (16.3) | ||
| Uniform endometrium pattern (N = 81) | Hyper echogenic | 46 (76.7) | 15 (71.4) | 0.10 |
| Hypo echogenic | 2 (3.3) | 3 (14.3) | ||
| Iso echogenic | 3 (5.0) | 3 (14.3) | ||
| Three-layer pattern | 5 (8.3) | 0 | ||
| Unspecified | 4 (6.7) | 0 | ||
| Endometrial midline | Trilaminar | 84 (50.3) | 27 (62.8) | 0.005 |
| Irregular | 53 (31.7) | 6 (14.0) | ||
| Linear | 10 (6.0) | 4 (9.3) | ||
| Not linear | 13 (7.8) | 0 | ||
| Unspecified | 7 (4.2) | 6 (14.0) | ||
| Endometrial junction (N = 209) | Interrupted | 73 (44.0) | 29 (67.4) | 0.008 |
| Regular | 42 (25.3) | 4 (9.3) | ||
| Irregular | 28 (16.9) | 3 (7.0) | ||
| Undefined | 15 (9.0) | 2 (4.7) | ||
| Unspecified | 8 (4.8) | 5 (11.6) | ||
| Myometrial invasion (N = 189) | No invasion | 51 (34.2) | 4 (10.0) | <0.001 |
| <50% | 63 (42.3) | 8 (20.0) | ||
| >50% | 25 (16.8) | 22 (55.0) | ||
| Unspecified | 10 (6.7) | 6 (15.0) | ||
| SLN performed c | 165 (99.4) | 20 (47.6) | <0.001 | |
| Left SLN Pathology (N = 167) | Negative | 145 (98.0) | 2 (10.5) | <0.001 |
| Isolated Tumor Cells | 2 (1.4) | 5 (26.3) | ||
| Micrometastasis | 0 | 7 (36.8) | ||
| Macrometastasis | 0 | 4 (21.1) | ||
| SLND not found in the specimen | 1 (0.7) | 0 | ||
| Positive, of unknown type | 0 | 1 (5.3) | ||
| Right SLN Pathology (N = 185) | Negative | 154 (93.9) | 4 (19.1) | <0.001 |
| Isolated Tumor Cells | 8 (4.9) | 3 (14.3) | ||
| Micrometastasis | 0 | 7 (33.3) | ||
| Macrometastasis | 0 | 6 (28.6) | ||
| SLND not found in the specimen | 2 (1.2) | 0 | ||
| Positive, of unknown type | 0 | 1 (4.8) | ||
| Measurable endometrium thickness | 154 (92.2) | 41 (95.4) | 0.74 | |
| Indentifiable lesion | 131 (78.4) | 38 (88.4) | 0.20 | |
| Cervical invasion | 10 (6.0) | 15 (34.9) | <0.001 | |
| Synchronous ovarian cancer | 0 | 0 | - | |
| LVSI (N = 209) | No | 147 (88.6) | 21 (48.8) | <0.001 |
| Yes | 19 (11.5) | 22 (51.2) | ||
| LVSI Type (N = 41) | Diffuse | 8 (42.1) | 13 (59.1) | 0.35 |
| Local | 11 (57.9) | 9 (40.9) | ||
| CA 125 | ≤35 | 149 (89.2) | 21 (48.8) | <0.001 |
| >35 | 18 (10.8) | 22 (51.2) | ||
| Risk Factor | Level | OR (95% CI) | p-Value | AUC (95% CI) |
|---|---|---|---|---|
| Age at Surgery, years | 0.94 a (0.83, 1.06) | 0.30 | 0.54 (0.46, 0.63) | |
| BMI (kg/m2) | 0.81 a (0.65, 1.02) | 0.07 | 0.56 (0.49, 0.64) | |
| CA 125 | 1.84 b (1.42, 2.39) | <0.001 | 0.80 (0.73, 0.86) | |
| Platelet count (103/μL) | 1.27 c (1.06, 1.54) | 0.01 | 0.60 (0.52, 0.69) | |
| Lymphocytes (103/μL) | 4.08 c (0.37, 44.8) | 0.25 | 0.45 (0.37, 0.53) | |
| Monocytes (103/μL) | NE | 0.16 | 0.56 (0.47, 0.64) | |
| Neutrophils (103/μL) | NE | 0.01 | 0.60 (0.52, 0.69) | |
| NLR (Neutrophil/Lymphocyte ratio) | 1.22 d (0.98, 1.51) | 0.07 | 0.60 (0.51, 0.69) | |
| PLR (Platelet/Lymphocyte ratio) | 1.00 d (0.99, 1.01) | 0.09 | 0.59 (0.50, 0.67) | |
| MLR (Monocyte/Lymphocyte ratio) | 1.70 c (0.35, 8.26) | 0.51 | 0.58 (0.33, 0.47) | |
| Uterine thickness (mm) | 1.16 a (1.06, 1.27) | 0.002 | 0.63 (0.56, 0.71) | |
| Endometrial thickness (mm) | 1.26 a (1.14, 1.40) | <0.001 | 0.68 (0.59, 0.77) | |
| Minimum free myometrium (mm) | 0.18 a (0.08, 0.40) | <0.001 | 0.78 (0.70, 0.86) | |
| Lesion diameter (mm) | Sagittal | 1.25 a (1.15, 1.36) | <0.001 | 0.74 (0.66, 0.81) |
| Antero-posterior | 1.36 a (1.21, 1.53) | <0.001 | 0.75 (0.68, 0.82) | |
| Transverse | 1.26 a (1.13, 1.40) | <0.001 | 0.71 (0.63, 0.78) | |
| d/D (mm) | 31.9 d (8.04, 127) | <0.001 | 0.72 (0.64, 0.80) | |
| Distance between tumor and external os (mm) | 0.65 a (0.55, 0.77) | <0.001 | 0.75 (0.66, 0.84) | |
| PCR | 1.22 c (0.91, 1.65) | 0.18 | 0.46 (0.37, 0.56) |
| Risk Factor Entered | Level | OR (95% CI) | p-Value |
|---|---|---|---|
| CA 125 | ≤35 | ref | <0.001 |
| >35 | 7.74 (1.86, 32.2) | ||
| d/D | <0.50 | ref | <0.001 |
| ≥0.50 | 3.44 (0.61, 19.3) | ||
| Minimum free myometrium | 0.73 (0.53, 1.01) | 0.05 | |
| Platelets | 1.01 (1.00, 1.02) | 0.13 |
| Cohort | Observed N (%) a | ||
|---|---|---|---|
| Predicted | No | Yes | |
| Training b | No | 60 (58.8) | 1 (3.7) |
| Yes | 42 (41.2) | 26 (96.3) | |
| Validation c | No | 25 (64.1) | 0 |
| Yes | 14 (35.9) | 10 (100) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pino, I.; Gozzini, E.; Radice, D.; Boveri, S.; Iacobone, A.D.; Vidal Urbinati, A.M.; Multinu, F.; Gullo, G.; Cucinella, G.; Franchi, D. Advancing Tailored Treatments: A Predictive Nomogram, Based on Ultrasound and Laboratory Data, for Assessing Nodal Involvement in Endometrial Cancer Patients. J. Clin. Med. 2024, 13, 496. https://doi.org/10.3390/jcm13020496
Pino I, Gozzini E, Radice D, Boveri S, Iacobone AD, Vidal Urbinati AM, Multinu F, Gullo G, Cucinella G, Franchi D. Advancing Tailored Treatments: A Predictive Nomogram, Based on Ultrasound and Laboratory Data, for Assessing Nodal Involvement in Endometrial Cancer Patients. Journal of Clinical Medicine. 2024; 13(2):496. https://doi.org/10.3390/jcm13020496
Chicago/Turabian StylePino, Ida, Elisa Gozzini, Davide Radice, Sara Boveri, Anna Daniela Iacobone, Ailyn Mariela Vidal Urbinati, Francesco Multinu, Giuseppe Gullo, Gaspare Cucinella, and Dorella Franchi. 2024. "Advancing Tailored Treatments: A Predictive Nomogram, Based on Ultrasound and Laboratory Data, for Assessing Nodal Involvement in Endometrial Cancer Patients" Journal of Clinical Medicine 13, no. 2: 496. https://doi.org/10.3390/jcm13020496
APA StylePino, I., Gozzini, E., Radice, D., Boveri, S., Iacobone, A. D., Vidal Urbinati, A. M., Multinu, F., Gullo, G., Cucinella, G., & Franchi, D. (2024). Advancing Tailored Treatments: A Predictive Nomogram, Based on Ultrasound and Laboratory Data, for Assessing Nodal Involvement in Endometrial Cancer Patients. Journal of Clinical Medicine, 13(2), 496. https://doi.org/10.3390/jcm13020496









