Oncologic Outcomes of Patients with Early-Stage Cervical Cancer after Minimally Invasive Radical Hysterectomy and Sentinel Lymph Node Biopsy
Abstract
1. Introduction
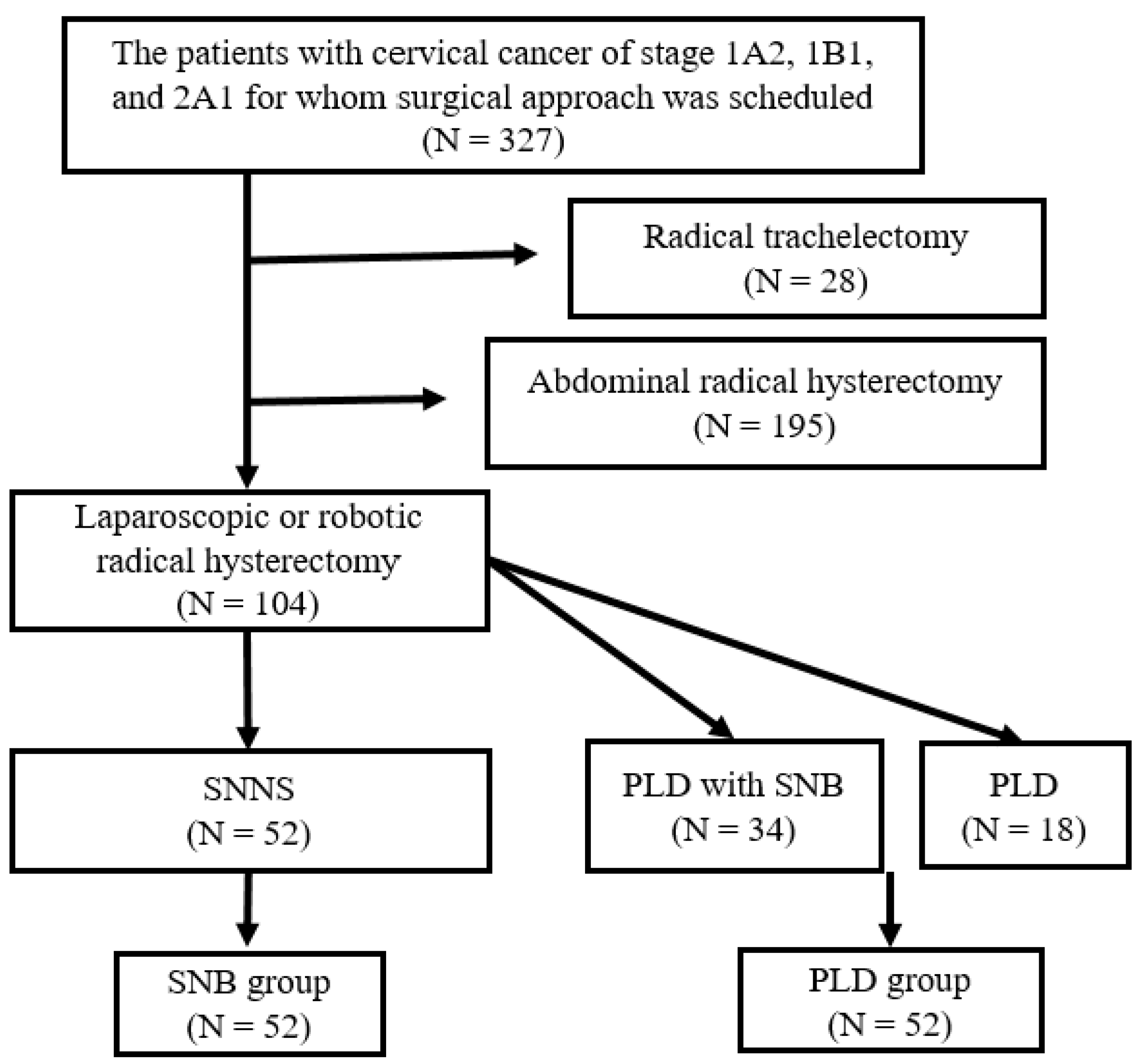
2. Materials and Methods
2.1. Participants
2.2. Diagnosis and Adjuvant Therapy
2.3. SNB Procedure
2.4. Radical Hysterectomy Procedure
2.5. Evaluation of Lymph Nodes
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Melamed, A.; Margul, D.J.; Chen, L.; Keating, N.L.; Del Carmen, M.G.; Yang, J.; Seagle, B.L.; Alexander, A.; Barber, E.L.; Rice, L.W.; et al. Survival after Minimally Invasive Radical Hysterectomy for Early-Stage Cervical Cancer. N. Engl. J. Med. 2018, 379, 1905–1914. [Google Scholar] [CrossRef]
- Zhang, X.; Bao, B.; Wang, S.; Yi, M.; Jiang, L.; Fang, X. Sentinel lymph node biopsy in early stage cervical cancer: A meta-analysis. Cancer Med. 2021, 10, 2590–2600. [Google Scholar] [CrossRef]
- Tanaka, T.; Nishie, R.; Ueda, S.; Hashida, S.; Miyamoto, S.; Terada, S.; Kogata, Y.; Taniguchi, K.; Komura, K.; Ohmichi, M. Robot-assisted modified radical hysterectomy with removal of lymphatic vessel using indocyanine green: A new method. Int. J. Med. Robot. Comput. Assist. Surg. 2022, 18, e2451. [Google Scholar] [CrossRef]
- Kadkhodayan, S.; Hasanzadeh, M.; Treglia, G.; Azad, A.; Yousefi, Z.; Zarifmahmoudi, L.; Sadeghi, R. Sentinel node biopsy for lymph nodal staging of uterine cervix cancer: A systematic review and meta-analysis of the pertinent literature. Eur. J. Surg. Oncol. 2015, 41, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Chiyoda, T.; Yoshihara, K.; Kagabu, M.; Nagase, S.; Katabuchi, H.; Mikami, M.; Tabata, T.; Hirashima, Y.; Kobayashi, Y.; Kaneuchi, M.; et al. Sentinel node navigation surgery in cervical cancer: A systematic review and metaanalysis. Int. J. Clin. Oncol. 2022, 27, 1247–1255. [Google Scholar] [CrossRef]
- Sponholtz, S.E.; Ezendam, N.P.M.; de Rooij, B.H.; Parner, E.; Mogensen, O.; Hildebrandt, M.G.; Schledermann, D.; Markauskas, A.; Frøding, L.P.; Fuglsang, K.; et al. SENTIREC—The sentinel node mapping in women with cervical cancer study—Patient-reported early lymphedema and its impact on quality of life. Gynecol. Oncol. 2022, 164, 463–472. [Google Scholar] [CrossRef]
- Ronsini, C.; De Franciscis, P.; Carotenuto, R.M.; Pasanisi, F.; Cobellis, L.; Colacurci, N. The Oncological Implication of Sentinel Lymph Node in Early Cervical Cancer: A Meta-Analysis of Oncological Outcomes and Type of Recurrences. Medicina 2022, 58, 1539. [Google Scholar] [CrossRef]
- Favre, G.; Guani, B.; Balaya, V.; Magaud, L.; Lecuru, F.; Mathevet, P. Sentinel Lymph-Node Biopsy in Early-Stage Cervical Cancer: The 4-Year Follow-Up Results of the Senticol 2 Trial. Front. Oncol. 2020, 10, 621518. [Google Scholar] [CrossRef]
- Niikura, H.; Okamoto, S.; Otsuki, T.; Yoshinaga, K.; Utsunomiya, H.; Nagase, S.; Takano, T.; Ito, K.; Watanabe, M.; Yaegashi, N. Prospective study of sentinel lymph node biopsy without further pelvic lymphadenectomy in patients with sentinel lymph node-negative cervical cancer. Int. J. Gynecol. Cancer 2012, 22, 1244–1250. [Google Scholar] [CrossRef]
- Yahata, H.; Kobayashi, H.; Sonoda, K.; Kodama, K.; Yagi, H.; Yasunaga, M.; Ohgami, T.; Onoyama, I.; Kaneki, E.; Okugawa, K.; et al. Prognostic outcome and complications of sentinel lymph node navigation surgery for early-stage cervical cancer. Int. J. Clin. Oncol. 2018, 23, 1167–1172. [Google Scholar] [CrossRef] [PubMed]
- Devaja, O.; Papadopoulos, A.J.; Bharathan, R.; Montalto, S.A.; Coutts, M.; Tan, A.; Corrigan, A.; Perovic, M.; Lalami, S.Z.R. Sentinel lymph node biopsy alone in the management of early cervical carcinoma. Int. J. Gynecol. Cancer 2022, 32, 15–20. [Google Scholar] [CrossRef]
- Parpinel, G.; Laas-Faron, E.; Balaya, V.; Guani, B.; Zola, P.; Mathevet, P.; Paoletti, X.; Lecuru, F.R. Survival after sentinel lymph node biopsy for early cervical cancers: A systematic review and meta-analysis. Int. J. Gynecol. Cancer 2023, 33, 1853–1860. [Google Scholar] [CrossRef] [PubMed]
- Togami, S.; Kubo, R.; Kawamura, T.; Yanazume, S.; Kamio, M.; Kobayashi, H. Comparison of lymphatic complications between sentinel node navigation surgery and pelvic lymphadenectomy in patients with cervical cancer. Jpn. J. Clin. Oncol. 2020, 50, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Cibula, D.; Pötter, R.; Planchamp, F.; Avall-Lundqvist, E.; Fischerova, D.; Haie Meder, C.; Köhler, C.; Landoni, F.; Lax, S.; Lindegaard, J.C.; et al. The European Society of Gynaecological Oncology/European Society for Radiotherapy and Oncology/European Society of Pathology guidelines for the management of patients with cervical cancer. Radiother. Oncol. 2018, 127, 404–416. [Google Scholar] [CrossRef] [PubMed]
| SNB | PLD | p Value | |
|---|---|---|---|
| Number of patients | 52 | 52 | |
| Age *, years old | 46 (41–70) | 45 (36–51) | 0.3 |
| FIGO staging | 0.03 | ||
| 1A2 | 8 | 3 | |
| 1B1 | 43 | 43 | |
| 2A1 | 1 | 6 | |
| Pathological analysis | |||
| Histological type | 0.5 | ||
| Squamous cell carcinoma | 24 | 27 | |
| Adenocarcinoma | 28 | 25 | |
| Tumor size, mm * | 12 (7–20) | 20 (13–25) | 0.001 |
| Large tumor size (>4 cm) | 0 | 1 | 0.5 |
| Lymph node metastasis | 1 | 9 | 0.001 |
| Parametrial invasion | 0 | 2 | 0.5 |
| Deep stromal invasion | 7 | 17 | 0.02 |
| Lymphovascular space invasion | 5 | 13 | 0.03 |
| Positive vaginal cut end | 0 | 1 | 0.5 |
| No adjuvant therapy | 45 | 28 | <0.001 |
| Adjuvant RT or CCRT | 6 | 16 | |
| Adjuvant chemotherapy | 1 | 12 | |
| Follow-up, months * | 42 (24–60) | 82 (19–101) | <0.001 |
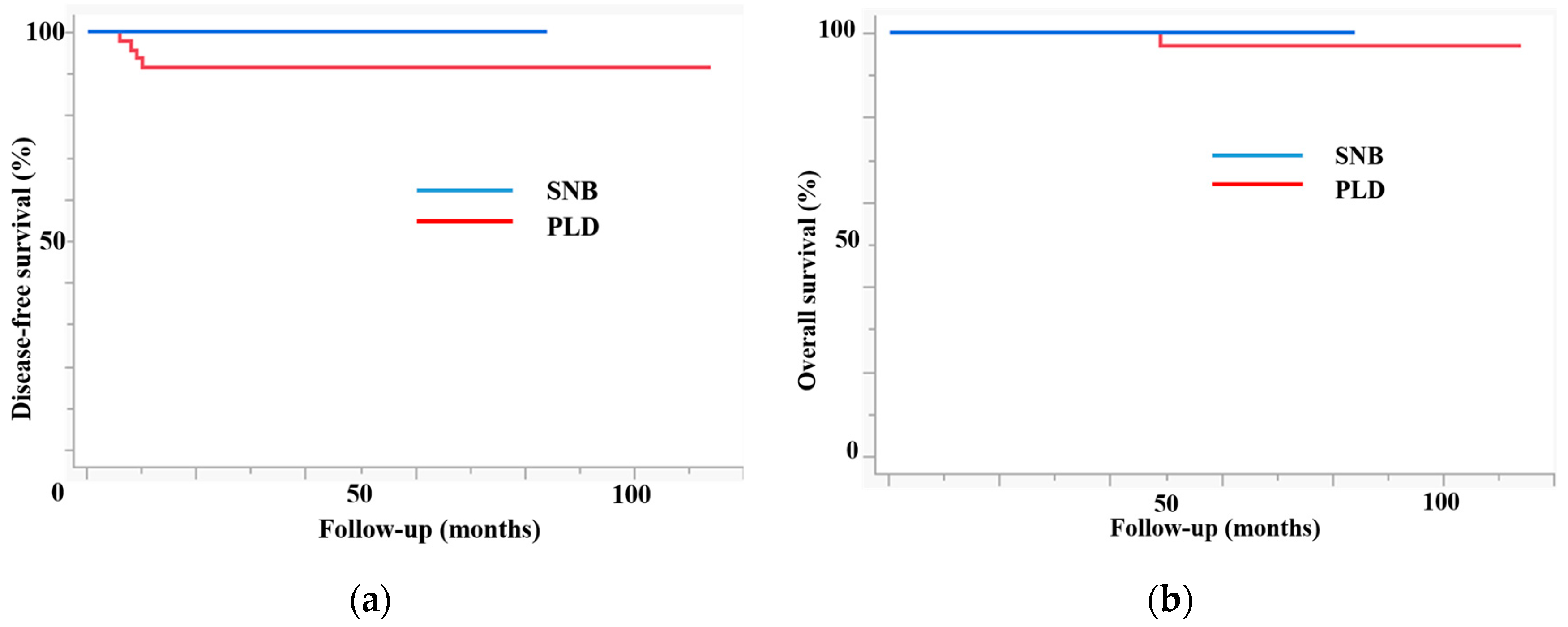
| Recurrence | 0 | 4 | 0.04 |
| 3-year DFS | 100 | 91.5 | 0.04 |
| 3-year OS | 100 | 100 | 0.5 |
| Case 1 | Case 2 | Case 3 | Case 4 | |
|---|---|---|---|---|
| Age, years old | 68 | 45 | 46 | 52 |
| FIGO staging | 1B1 | 1B1 | 1B1 | 1B1 |
| Pathological analysis | ||||
| Histological type | SCC | AD | SCC | AD |
| Tumor size, mm | 18 | 14 | 20 | 11 |
| Large tumor size (>4 cm) | - | - | - | - |
| Lymph node metastasis | - | - | - | - |
| Parametrial invasion | - | - | - | - |
| Deep stromal invasion | + | - | - | - |
| Lymphovascular space invasion | + | - | - | - |
| Positive vaginal cut end | - | - | - | - |
| Adjuvant therapy | CCRT | - | - | - |
| DFS, months | 9 | 6 | 8 | 10 |
| OS, months | 84 | 49 | 104 | 22 |
| Recurrent site | Lung | Lung | Pelvis | Vagina |
| Outcome | Alive | Died | Alive | Alive |
| SNB | PLD | p Value | |
|---|---|---|---|
| Number of patients | 44 | 30 | |
| Age *, years old | 47 (42–55) | 45 (35–50) | 0.2 |
| FIGO staging | 0.2 | ||
| 1A2 | 8 | 2 | |
| 1B1 | 35 | 26 | |
| 2A1 | 1 | 2 | |
| Pathological analysis | |||
| Histological type | 0.7 | ||
| Squamous cell carcinoma | 20 | 15 | |
| Adenocarcinoma | 24 | 15 | |
| Tumor size, mm * | 10 (7–17) | 15 (10–18) | 0.08 |
| Large tumor size (>4 cm) | 0 | 0 | |
| Lymph node metastasis | 0 | 4 | 0.01 |
| Parametrial invasion | 0 | 2 | 0.5 |
| Deep stromal invasion | 4 | 7 | 0.1 |
| Lymphovascular space invasion | 4 | 6 | 0.2 |
| Positive vaginal cut end | 0 | 1 | 0.2 |
| No adjuvant therapy | 41 | 20 | 0.02 |
| Adjuvant RT or CCRT | 2 | 4 | |
| Adjuvant chemotherapy | 1 | 6 | |
| Follow-up, months * | 43 (24–60) | 84 (62–105) | <0.001 |
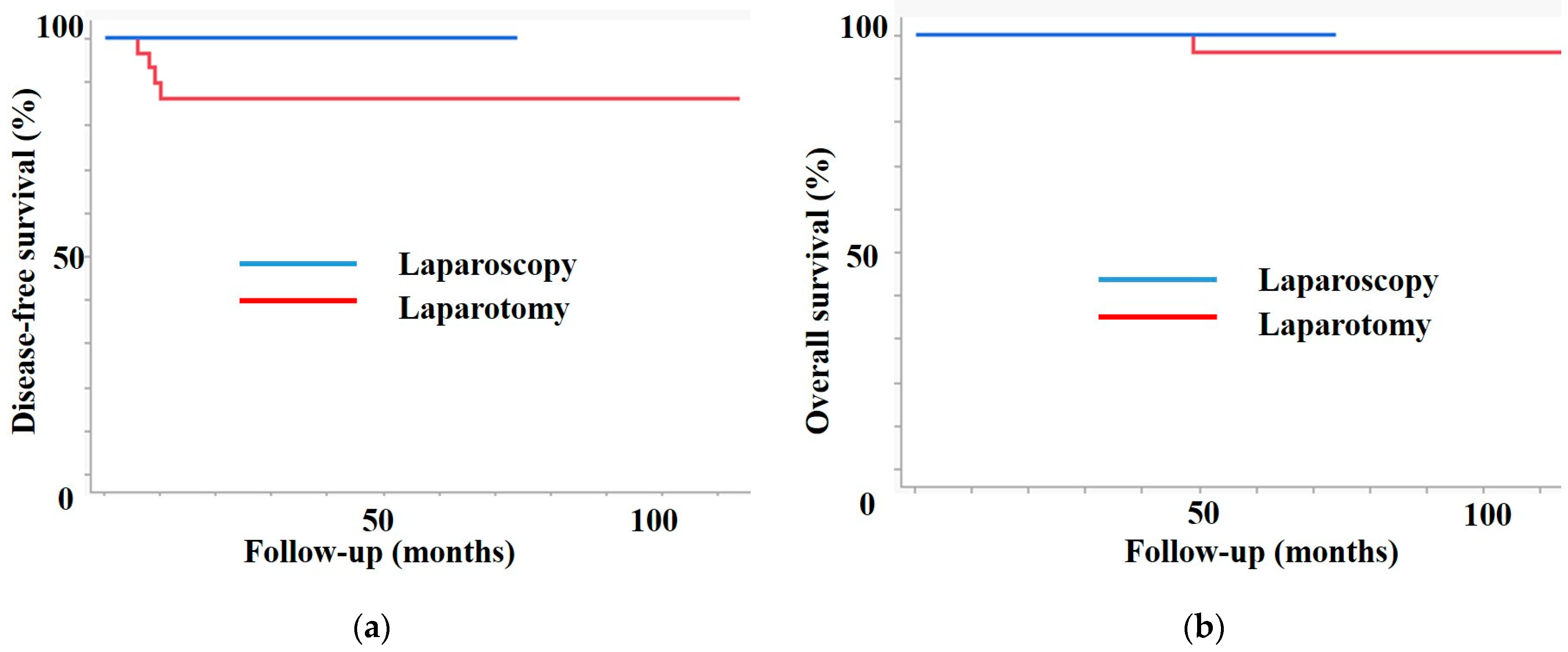
| Recurrence | 0 | 4 | 0.02 |
| 3-year DFS | 100 | 86.4 | 0.02 |
| 3-year OS | 100 | 100 | 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanaka, T.; Nishie, R.; Murakami, H.; Tsuchihashi, H.; Toji, A.; Ueda, S.; Morita, N.; Hashida, S.; Terada, S.; Maruoka, H.; et al. Oncologic Outcomes of Patients with Early-Stage Cervical Cancer after Minimally Invasive Radical Hysterectomy and Sentinel Lymph Node Biopsy. J. Clin. Med. 2024, 13, 3981. https://doi.org/10.3390/jcm13133981
Tanaka T, Nishie R, Murakami H, Tsuchihashi H, Toji A, Ueda S, Morita N, Hashida S, Terada S, Maruoka H, et al. Oncologic Outcomes of Patients with Early-Stage Cervical Cancer after Minimally Invasive Radical Hysterectomy and Sentinel Lymph Node Biopsy. Journal of Clinical Medicine. 2024; 13(13):3981. https://doi.org/10.3390/jcm13133981
Chicago/Turabian StyleTanaka, Tomohito, Ruri Nishie, Hikaru Murakami, Hiromitsu Tsuchihashi, Akihiko Toji, Shoko Ueda, Natsuko Morita, Sousuke Hashida, Shinichi Terada, Hiroshi Maruoka, and et al. 2024. "Oncologic Outcomes of Patients with Early-Stage Cervical Cancer after Minimally Invasive Radical Hysterectomy and Sentinel Lymph Node Biopsy" Journal of Clinical Medicine 13, no. 13: 3981. https://doi.org/10.3390/jcm13133981
APA StyleTanaka, T., Nishie, R., Murakami, H., Tsuchihashi, H., Toji, A., Ueda, S., Morita, N., Hashida, S., Terada, S., Maruoka, H., Taniguchi, K., Komura, K., & Ohmichi, M. (2024). Oncologic Outcomes of Patients with Early-Stage Cervical Cancer after Minimally Invasive Radical Hysterectomy and Sentinel Lymph Node Biopsy. Journal of Clinical Medicine, 13(13), 3981. https://doi.org/10.3390/jcm13133981








