Chest Dynamic MRI as Early Biomarker of Respiratory Impairment in Amyotrophic Lateral Sclerosis Patients: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
- -
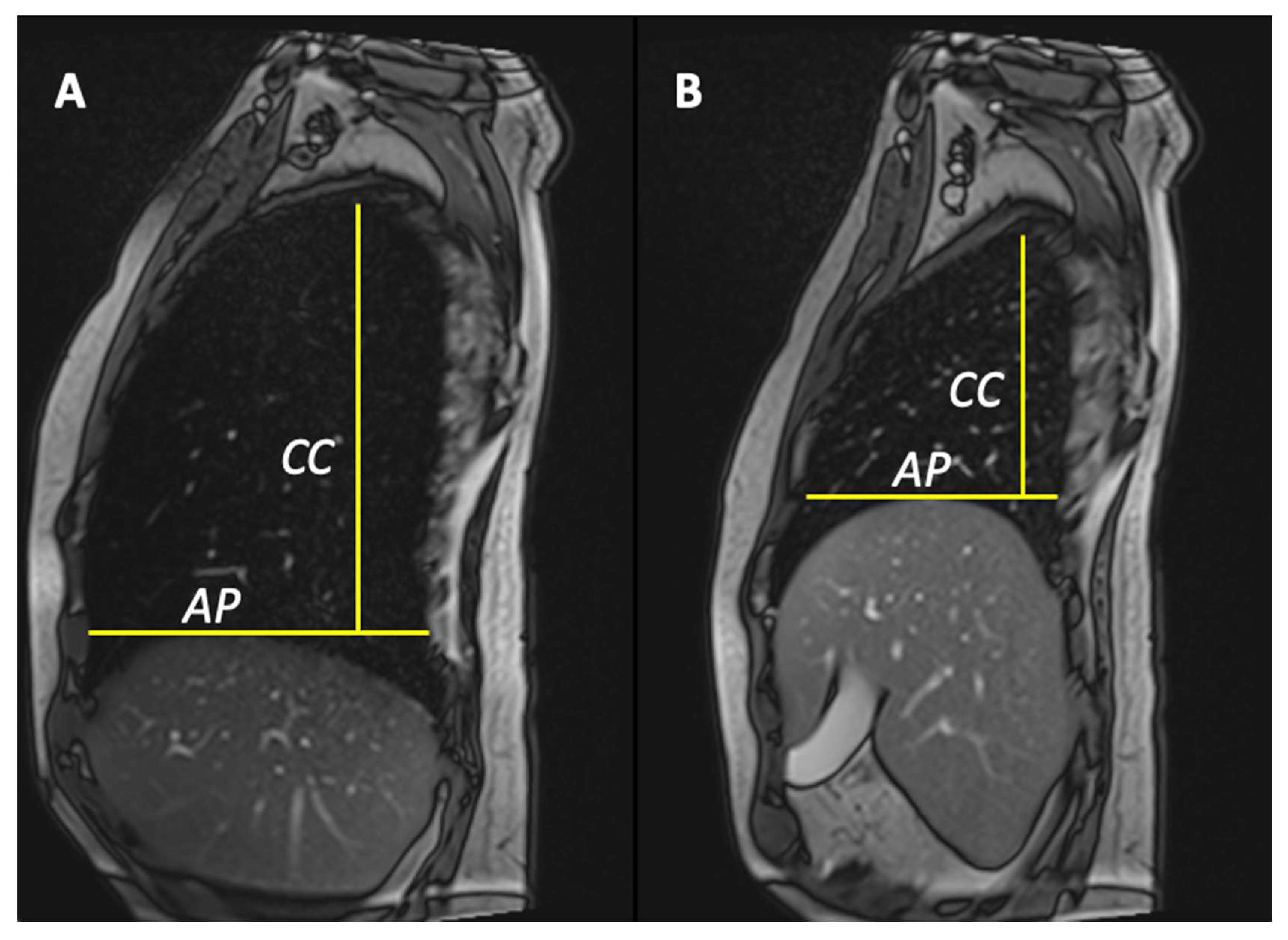
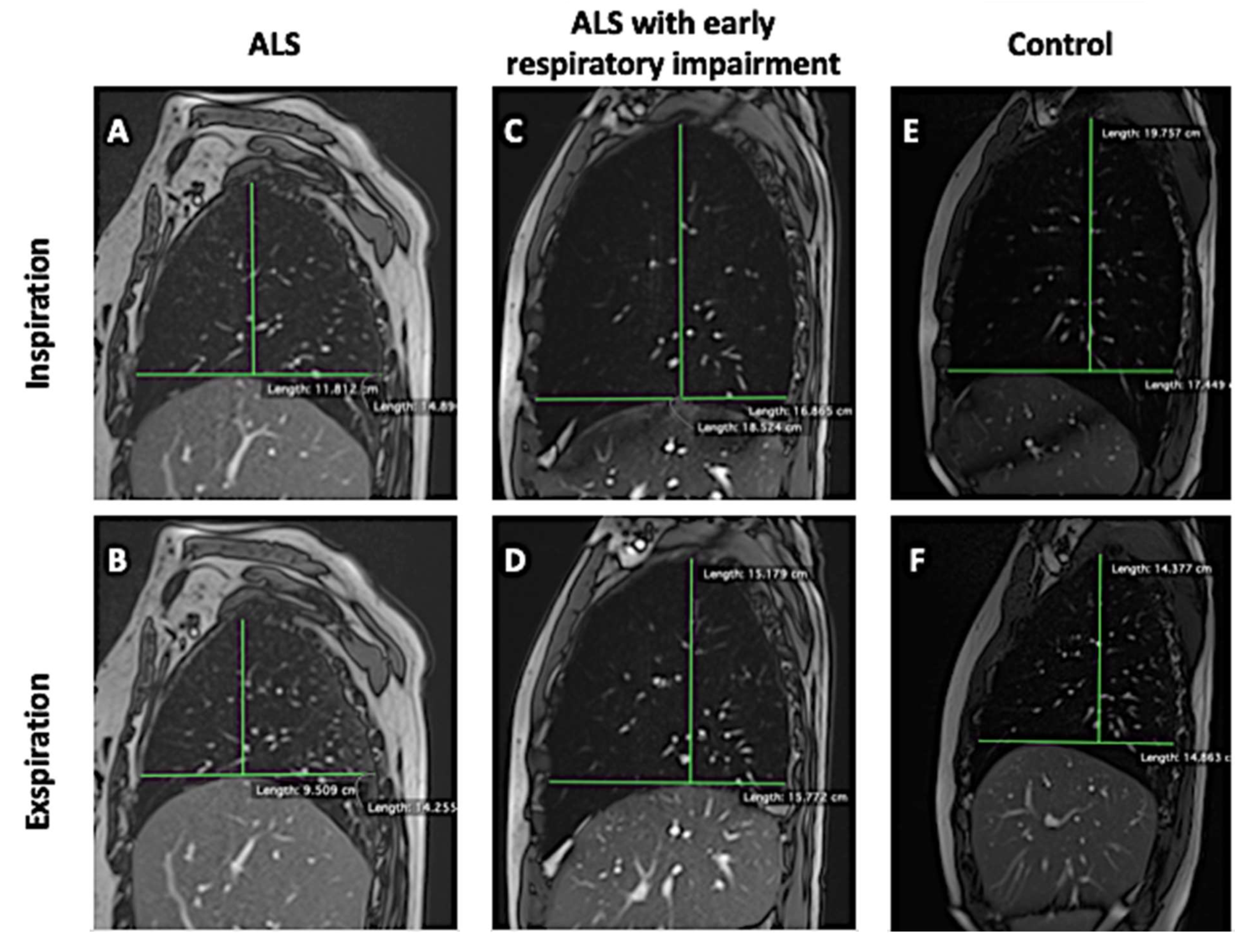
- Anterior–posterior lung diameters delta (anteroposterior delta lung on the right [ΔAPr] and anteroposterior delta lung on the left [ΔAPl]);
- -
- Cranio-caudal lung diameters delta (cranio-caudal delta lung on the right [ΔCCr] and cranio-caudal delta lung on the left [ΔCCl]).
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Available online: https://www.orpha.net/consor/cgi-bin/OC_Exp.php?Lng=IT&Expert=803 (accessed on 1 October 2023).
- Hardiman, O.; Al-Chalabi, A.; Chio, A.; Corr, E.M.; Logroscino, G.; Robberecht, W.; Shaw, P.J.; Simmons, Z.; van den Berg, L.H. Amyotrophic lateral sclerosis. Nat. Rev. Dis. Primers 2017, 3, 17071, Erratum in Nat. Rev. Dis. Primers 2017, 3, 17085. [Google Scholar] [CrossRef] [PubMed]
- De Marchi, F.; SIgN, O.B.O.; Carrarini, C.; De Martino, A.; Diamanti, L.; Fasano, A.; Lupica, A.; Russo, M.; Salemme, S.; Spinelli, E.G.; et al. Cognitive dysfunction in amyotrophic lateral sclerosis: Can we predict it? Neurol. Sci. 2021, 42, 2211–2222. [Google Scholar] [CrossRef]
- Lombardi, V.; Bombaci, A.; Zampedri, L.; Lu, C.-H.; Malik, B.; Zetterberg, H.; Heslegrave, A.J.; Rinaldi, C.; Greensmith, L.; Hanna, M.G.; et al. Plasma pNfH levels differentiate SBMA from ALS. J. Neurol. Neurosurg. Psychiatry 2019, 91, 215–217. [Google Scholar] [CrossRef] [PubMed]
- Sabatelli, M.; Conte, A.; Zollino, M. Clinical and genetic heterogeneity of amyotrophic lateral sclerosis. Clin. Genet. 2013, 83, 408–416. [Google Scholar] [CrossRef]
- Barbato, F.; Colacicco, G.; Bruno, G.; Ippolito, D.; Siani, F.; Di Masi, A.; Pota, V. Neuromuscular diseases in the pandemic age: 6 months of experience of a newborn clinical center. Neurol. Sci. 2022, 43, 3457–3458. [Google Scholar] [CrossRef]
- da Cunha-Correia, C.; Gama, M.D.P.; Fontana, P.N.; Fantini, F.G.M.M.; Prado, G.F.; Júnior, M.E.T.D.; Schwingel, P.A. Noninvasive mechanical ventilation assistance in amyotrophic lateral sclerosis: A systematic review. Sao Paulo Med. J. 2024, 142, e2022470. [Google Scholar] [CrossRef]
- American Thoracic Society/European Respiratory Society. ATS/ERS Statement on Respiratory Muscle Testing. Am. J. Respir. Crit. Care Med. 2002, 166, 518–624. [Google Scholar] [CrossRef] [PubMed]
- Harlaar, L.; Ciet, P.; van Tulder, G.; Brusse, E.; Timmermans, R.G.; Janssen, W.G.; de Bruijne, M.; van der Ploeg, A.T.; Tiddens, H.A.; van Doorn, P.A.; et al. Diaphragmatic dysfunction in neuromuscular disease, an MRI study. Neuromuscul. Disord. 2021, 32, 15–24. [Google Scholar] [CrossRef]
- Barnard, A.M.; Lott, D.J.; Batra, A.; Triplett, W.T.; Forbes, S.C.; Riehl, S.L.; Willcocks, R.J.; Smith, B.K.; Vandenborne, K.; Walter, G.A. Imaging respiratory muscle quality and function in Duchenne muscular dystrophy. J. Neurol. 2019, 266, 2752–2763. [Google Scholar] [CrossRef]
- Barnard, A.M.; Lott, D.J.; Batra, A.; Triplett, W.T.; Willcocks, R.J.; Forbes, S.C.; Rooney, W.D.; Daniels, M.J.; Smith, B.K.; Vandenborne, K.; et al. Characterizing Expiratory Respiratory Muscle Degeneration in Duchenne Muscular Dystrophy Using MRI. Chest 2021, 161, 753–763. [Google Scholar] [CrossRef]
- Gaeta, M.; Musumeci, O.; Mondello, S.; Ruggeri, P.; Montagnese, F.; Cucinotta, M.; Vinci, S.; Milardi, D.; Toscano, A. Clinical and pathophysiological clues of respiratory dysfunction in late-onset Pompe disease: New insights from a comparative study by MRI and respiratory function assessment. Neuromuscul. Disord. 2015, 25, 852–858. [Google Scholar] [CrossRef] [PubMed]
- Wens, S.C.; Ciet, P.; Perez-Rovira, A.; Logie, K.; Salamon, E.; Wielopolski, P.; de Bruijne, M.; E Kruijshaar, M.; Tiddens, H.A.; A van Doorn, P.; et al. Lung MRI and impairment of diaphragmatic function in Pompe disease. BMC Pulm. Med. 2015, 15, 54. [Google Scholar] [CrossRef] [PubMed]
- Harlaar, L.; Ciet, P.; van Tulder, G.; Pittaro, A.; van Kooten, H.A.; van der Beek, N.A.M.E.; Brusse, E.; Wielopolski, P.A.; de Bruijne, M.; van der Ploeg, A.T.; et al. Chest MRI to diagnose early diaphragmatic weakness in Pompe disease. Orphanet J. Rare Dis. 2021, 16, 21. [Google Scholar] [CrossRef] [PubMed]
- Mankodi, A.; Kovacs, W.; Norato, G.; Hsieh, N.; Bandettini, W.P.; Bishop, C.A.; Shimellis, H.; Newbould, R.D.; Kim, E.; Fischbeck, K.H.; et al. Respiratory magnetic resonance imaging biomarkers in Duchenne muscular dystrophy. Ann. Clin. Transl. Neurol. 2017, 4, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Bishop, C.A.; Ricotti, V.; Sinclair, C.D.J.; Evans, M.R.B.; Butler, J.W.; Morrow, J.M.; Hanna, M.G.; Matthews, P.M.; Yousry, T.A.; Muntoni, F.; et al. Semi-Automated Analysis of Diaphragmatic Motion with Dynamic Magnetic Resonance Imaging in Healthy Controls and Non-Ambulant Subjects with Duchenne Muscular Dystrophy. Front. Neurol. 2018, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Pennati, F.; Arrigoni, F.; LoMauro, A.; Gandossini, S.; Russo, A.; D‘Angelo, M.G.; Aliverti, A. Diaphragm Involvement in Duchenne Muscular Dystrophy (DMD): An MRI Study. J. Magn. Reson. Imaging 2019, 51, 461–471. [Google Scholar] [CrossRef]
- Smith, B.K.; Allen, S.; Mays, S.; Martin, A.D.; Byrne, B.J. Dynamic respiratory muscle function in late-onset Pompe disease. Sci. Rep. 2019, 9, 19006. [Google Scholar] [CrossRef] [PubMed]
- Pringle, C.E.; Hudson, A.J.; Munoz, D.G.; Kiernan, J.A.; Brown, W.F.; Ebers, G.C. Primary lateral sclerosis: Clinical features, neuropathology and diagnostic criteria. Brain 1992, 115, 495–520. [Google Scholar] [CrossRef]
- Fayssoil, A.; Behin, A.; Ogna, A.; Mompoint, D.; Amthor, H.; Clair, B.; Laforet, P.; Mansart, A.; Prigent, H.; Orlikowski, D.; et al. Diaphragm: Pathophysiology and Ultrasound Imaging in Neuromuscular Disorders. J. Neuromuscul. Dis. 2018, 5, 1–10. [Google Scholar] [CrossRef]
- Papa, G.F.S.; Pellegrino, G.M.; Di Marco, F.; Imeri, G.; Brochard, L.; Goligher, E.; Centanni, S. A Review of the Ultrasound Assessment of Diaphragmatic Function in Clinical Practice. Respiration 2016, 91, 403–411. [Google Scholar] [CrossRef]
- Supinski, G.S.; Morris, P.E.; Dhar, S.; Callahan, L.A. Diaphragm Dysfunction in Critical Illness. Chest 2018, 153, 1040–1051. [Google Scholar] [CrossRef] [PubMed]
- Minami, T.; Manzoor, K.; McCool, F.D. Assessing Diaphragm Function in Chest Wall and Neuromuscular Diseases. Clin. Chest Med. 2018, 39, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Sarwal, A.; Walker, F.O.; Cartwright, M.S. Neuromuscular ultrasound for evaluation of the diaphragm. Muscle Nerve 2013, 47, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Dorst, J.; Ludolph, A.C. Non-invasive ventilation in amyotrophic lateral sclerosis. Ther. Adv. Neurol. Disord. 2019, 12, 1756286419857040. [Google Scholar] [CrossRef] [PubMed]
| ALS | HC | pvalue | |
|---|---|---|---|
| Age | 57.8 ± 12.8 | 56.5 ± 5.7 | 0.119 |
| Sex (M/F) | 5 M; 8 F | 5 M; 6 F | 0.437 * |
| Time onset MRI (months ± SD) | 25.0 ± 17.6 | - | - |
| Time diagnosis MRI (months ± SD) | 19.8 ± 15.5 | - | - |
| FVC (%) ± SD | 59 ± 22 | 97 ± 1.5 | 8.01 × 10−7 |
| FEV1 (%) ± SD | 57 ± 22.2 | 101 ± 4.0 | 8.01 × 10−7 |
| PFC (l/min) ± SD | 173 ± 139 | 459 ± 9.3 | 1.52 × 10−5 |
| ΔAPr (cm) ± SD | 1.1 ± 0.7 | 4.2 ± 0.4 | 8.01 × 10−7 |
| ΔAPl (cm) ± SD | 1.4 ± 0.7 | 4.5 ± 0.9 | 8.01 × 10−7 |
| ΔCCr (cm) ± SD | 2.8 ± 2.0 | 9.0 ± 0.3 | 8.01 × 10−7 |
| ΔCCl (cm) ± SD | 3.2 ± 1.7 | 9.4 ± 0.4 | 8.01 × 10−7 |
| ΔPIarea (cm) ± SD | 4.6 ± 4.2 | 40.2 ± 6.4 | 8.01 × 10−7 |
| ΔPIlenght (cm) ± SD | 4.3 ± 2.3 | 13.6 ± 0.8 | 8.01 × 10−7 |
| FVC | FEV1 | PFC | ||||
|---|---|---|---|---|---|---|
| rs | pvalue | r | pvalue | rs | pvalue | |
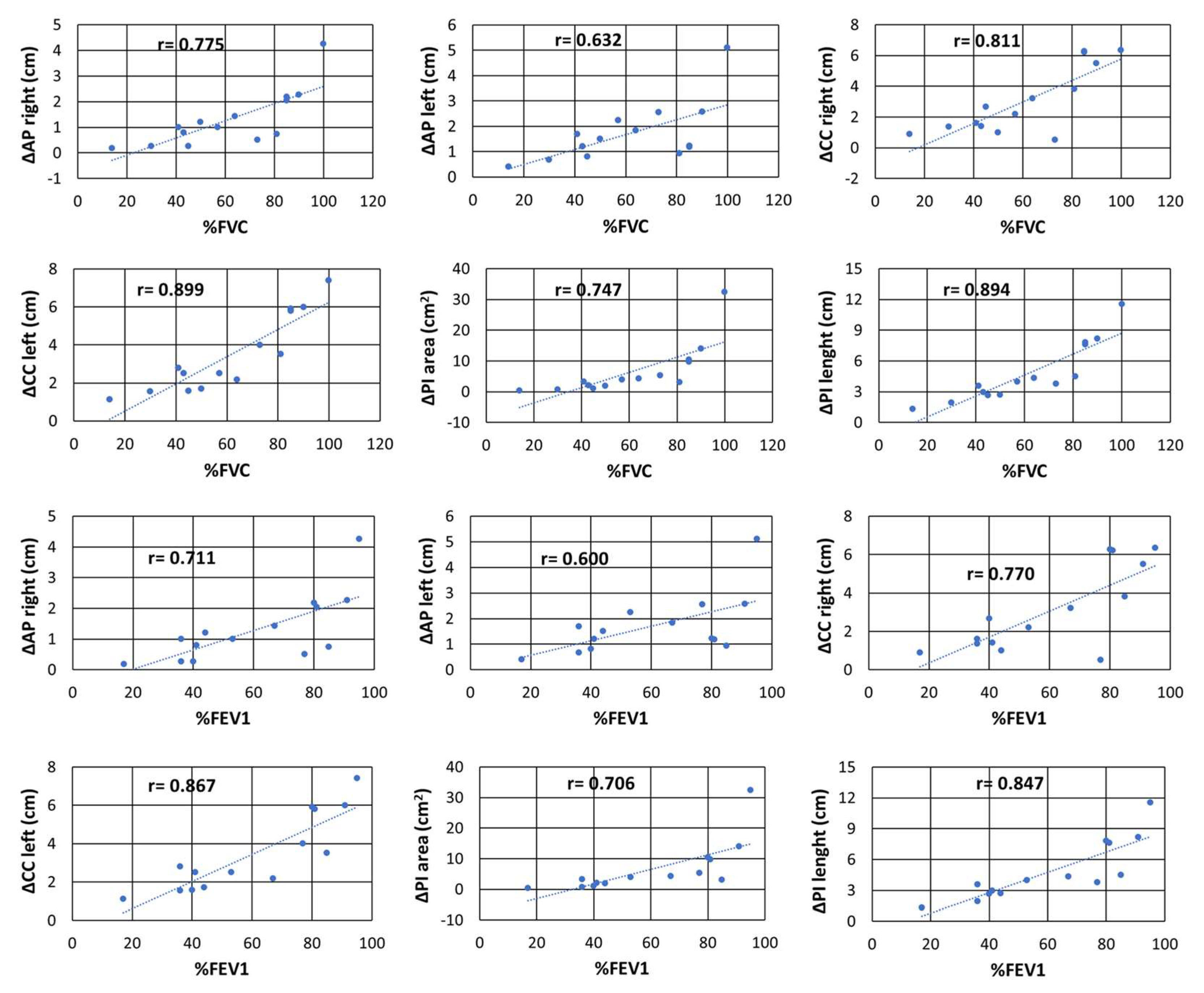
| ΔAPr | 0.775 | 0 | 0.71 | 0 | 0.61 | 0.021 |
| ΔAPl | 0.632 | 0.02 | 0.6 | 0.02 | 0.45 | 0.103 |
| ΔCCr | 0.811 | 0 | 0.77 | 0 | 0.73 | 0.003 |
| ΔCCl | 0.899 | 0 | 0.87 | 0 | 0.77 | 0.001 |
| ΔPIarea | 0.747 | 0 | 0.71 | 0.01 | 0.65 | 0.013 |
| ΔPIlength | 0.894 | 0 | 0.85 | 0 | 0.73 | 0.002 |
| ALSFSRr | 0.745 | 0 | 0.74 | 0 | 0.58 | 0.032 |
| BMI | −0.087 | 0.77 | -0.1 | 0.63 | 0.2 | 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbato, F.; Bombaci, A.; Colacicco, G.; Bruno, G.; Ippolito, D.; Pota, V.; Dongiovanni, S.; Sica, G.; Bocchini, G.; Valente, T.; et al. Chest Dynamic MRI as Early Biomarker of Respiratory Impairment in Amyotrophic Lateral Sclerosis Patients: A Pilot Study. J. Clin. Med. 2024, 13, 3103. https://doi.org/10.3390/jcm13113103
Barbato F, Bombaci A, Colacicco G, Bruno G, Ippolito D, Pota V, Dongiovanni S, Sica G, Bocchini G, Valente T, et al. Chest Dynamic MRI as Early Biomarker of Respiratory Impairment in Amyotrophic Lateral Sclerosis Patients: A Pilot Study. Journal of Clinical Medicine. 2024; 13(11):3103. https://doi.org/10.3390/jcm13113103
Chicago/Turabian StyleBarbato, Francesco, Alessandro Bombaci, Giovanni Colacicco, Giorgia Bruno, Domenico Ippolito, Vincenzo Pota, Salvatore Dongiovanni, Giacomo Sica, Giorgio Bocchini, Tullio Valente, and et al. 2024. "Chest Dynamic MRI as Early Biomarker of Respiratory Impairment in Amyotrophic Lateral Sclerosis Patients: A Pilot Study" Journal of Clinical Medicine 13, no. 11: 3103. https://doi.org/10.3390/jcm13113103
APA StyleBarbato, F., Bombaci, A., Colacicco, G., Bruno, G., Ippolito, D., Pota, V., Dongiovanni, S., Sica, G., Bocchini, G., Valente, T., Scaglione, M., Mainenti, P. P., & Guarino, S. (2024). Chest Dynamic MRI as Early Biomarker of Respiratory Impairment in Amyotrophic Lateral Sclerosis Patients: A Pilot Study. Journal of Clinical Medicine, 13(11), 3103. https://doi.org/10.3390/jcm13113103









