Challenges of Revisional Metabolic and Bariatric Surgery: A Comprehensive Guide to Unraveling the Complexities and Solutions of Revisional Bariatric Procedures
Abstract
1. Introduction
2. Indications for RMBS
2.1. Index Procedures
2.2. Approach to RMBS: Preoperative Evaluation
3. Surgical Choice
3.1. Revisional Gastric Banding
3.2. Revisional Sleeve Gastrectomy
3.3. Revisional Roux-en-Y Gastric Bypass
3.3.1. Management of Late Complications
3.3.2. Management of WR
Combined TORe and Distalization of the BPL
Distalization of the BPL
Conversion of RYGB to DS
3.4. Revisional Duodenal Switch (DS)
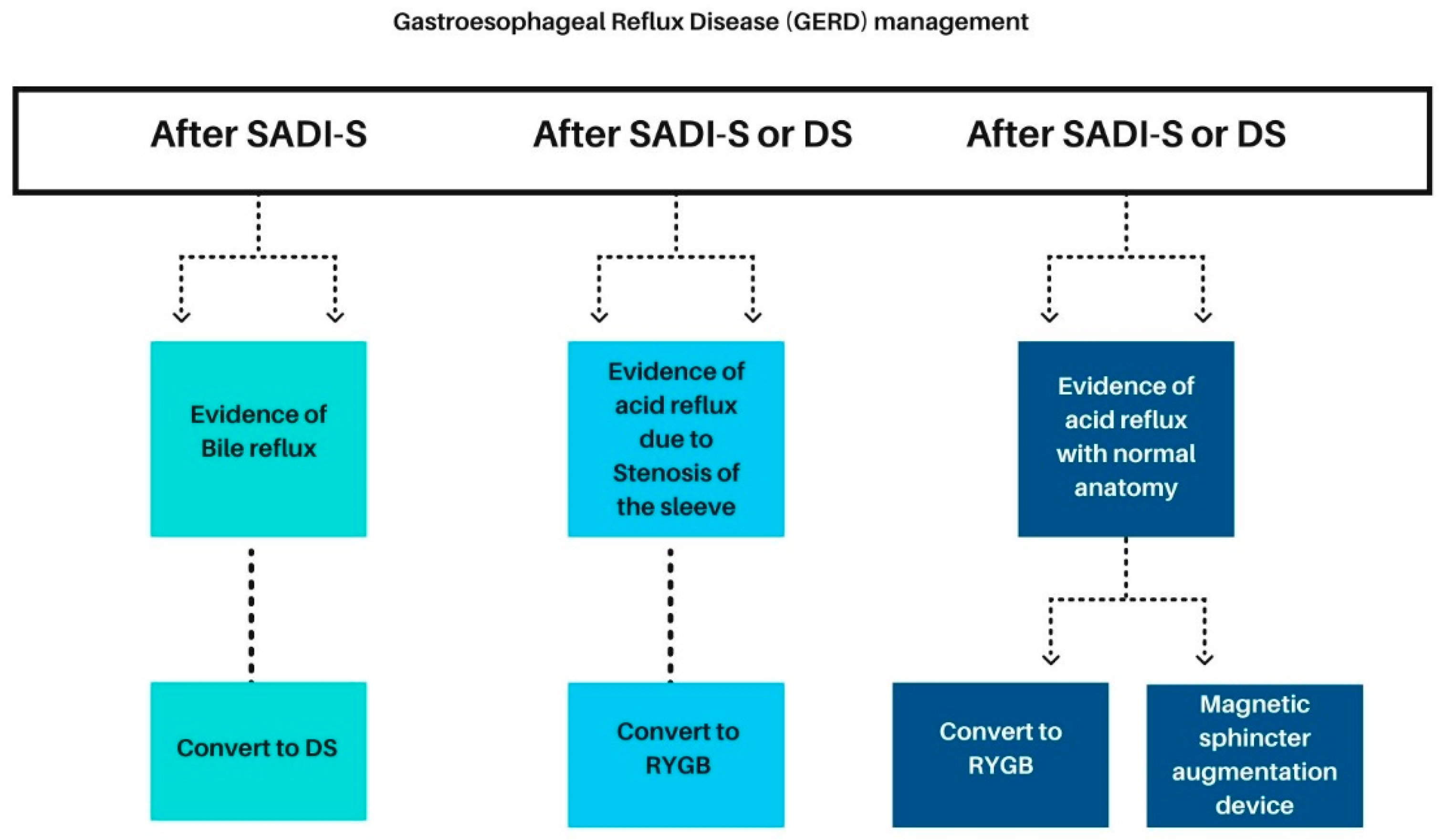
3.4.1. Management of Gastroesophageal Reflux Disease
3.4.2. Management of Malabsorption
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Obesity and Overweight Fact Sheet; WHO: Geneva, Switzerland, 2022; Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight#:~:text=%2Fm2).-,Adults,than%20or%20equal%20to%2030 (accessed on 1 March 2024).
- Hales, C.M.; Carroll, M.D.; Fryar, C.D.; Ogden, C.L. Prevalence of Obesity and Severe Obesity among Adults: United States, 2017–2018. NCHS Data Brief 2020, 360, 1–8. [Google Scholar] [PubMed]
- Wang, Y.; Beydoun, M.A.; Liang, L.; Caballero, B.; Kumanyika, S.K. Will all Americans become overweight or obese? estimating the progression and cost of the US obesity epidemic. Obesity 2008, 16, 2323–2330. [Google Scholar] [CrossRef] [PubMed]
- Sjöström, L.; Peltonen, M.; Jacobson, P.; Sjöström, C.D.; Karason, K.; Wedel, H.; Ahlin, S.; Anveden, Å.; Bengtsson, C.; Bergmark, G.; et al. Bariatric Surgery and Long-term Cardiovascular Events. JAMA 2012, 307, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Kitahara, C.M.; Flint, A.J.; Berrington de Gonzalez, A.; Bernstein, L.; Brotzman, M.; MacInnis, R.J.; Moore, S.C.; Robien, K.; Rosenberg, P.S.; Singh, P.N.; et al. Association between class III obesity (BMI of 40–59 kg/m2) and mortality: A pooled analysis of 20 prospective studies. PLoS Med. 2014, 11, e1001673. [Google Scholar] [CrossRef] [PubMed]
- Adams, K.F.; Schatzkin, A.; Harris, T.B.; Kipnis, V.; Mouw, T.; Ballard-Barbash, R.; Hollenbeck, A.; Leitzmann, M.F. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N. Engl. J. Med. 2006, 355, 763–778. [Google Scholar] [CrossRef] [PubMed]
- Whitlock, G.; Lewington, S.; Sherliker, P.; Clarke, R.; Emberson, J.; Halsey, J.; Qizilbash, N.; Collins, R.; Peto, R. Body-mass index and cause-specific mortality in 900,000 adults: Collaborative analyses of 57 prospective studies. Lancet 2009, 373, 1083–1096. [Google Scholar] [PubMed]
- Yang, W.; Wen, J.; Wu, F.; Zeng, H.; Guo, B.; Ge, L. Pharmacotherapy weight-loss interventions to prevent type 2 diabetes in overweight or obese adults and older adults: A protocol for systematic review and network meta-analysis. Medicine 2021, 100, e24812. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, I.; Tomassoni, D.; Moruzzi, M.; Traini, E.; Amenta, F.; Tayebati, S.K. Obesity and Metabolic Syndrome Affect the Cholinergic Transmission and Cognitive Functions. CNS Neurol. Disord. Drug Targets 2017, 16, 664–676. [Google Scholar] [CrossRef] [PubMed]
- Mercado, A.; Pham, A.; Wang, Z.; Huang, W.; Chan, P.; Ibrahim, H.; Gogineni, H.; Huang, Y.; Wang, J. Effects of bariatric surgery on drug pharmacokinetics-Preclinical studies. Front. Pharmacol. 2023, 14, 1133415. [Google Scholar] [CrossRef]
- Kissler, H.J.; Settmacher, U. Bariatric surgery to treat obesity. Semin. Nephrol. 2013, 33, 75–89. [Google Scholar] [CrossRef]
- Alalwan, A.A.; Friedman, J.; Park, H.; Segal, R.; Brumback, B.A.; Hartzema, A.G. US national trends in bariatric surgery: A decade of study. Surgery 2021, 170, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, E.S.; Schroeder, R.; Harrison, T.D. Metabolic Surgery for Adult Obesity: Common Questions and Answers. Am. Fam. Physician 2022, 105, 593–601. [Google Scholar] [PubMed]
- Angrisani, L.; Santonicola, A.; Iovino, P.; Vitiello, A.; Zundel, N.; Buchwald, H.; Scopinaro, N. Bariatric Surgery and Endoluminal Procedures: IFSO Worldwide Survey 2014. Obes. Surg. 2017, 27, 2279–2289. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.; Zehetner, J.; Beel, J.; Steffen, R. Long-term outcomes and frequency of reoperative bariatric surgery beyond 15 years after gastric banding: A high band failure rate with safe revisions. Surg. Obes. Relat. Dis. 2019, 15, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Clapp, B.; Ponce, J.; DeMaria, E.; Ghanem, O.; Hutter, M.; Kothari, S.; LaMasters, T.; Kurian, M.; English, W. American Society for Metabolic and Bariatric Surgery 2020 estimate of metabolic and bariatric procedures performed in the United States. Surg. Obes. Relat. Dis. 2022, 18, 1134–1140. [Google Scholar] [CrossRef] [PubMed]
- English, W.J.; DeMaria, E.J.; Hutter, M.M.; Kothari, S.N.; Mattar, S.G.; Brethauer, S.A.; Morton, J.M. American Society for Metabolic and Bariatric Surgery 2018 estimate of metabolic and bariatric procedures performed in the United States. Surg. Obes. Relat. Dis. 2020, 16, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Tan, W.H.; Chang, R.; Eagon, J.C. Perioperative risk and complications of revisional bariatric surgery compared to primary Roux-en-Y gastric bypass. Surg. Endosc. 2015, 29, 1316–1320. [Google Scholar] [CrossRef]
- Park, J.Y.; Song, D.; Kim, Y.J. Causes and outcomes of revisional bariatric surgery: Initial experience at a single center. Ann. Surg. Treat. Res. 2014, 86, 295–301. [Google Scholar] [CrossRef]
- Strong, A.T.; Guerrón, A.D. Revisional bariatric surgery for chronic complications necessitates custom surgical solutions. Mini-Invasive Surg. 2022, 6, 37. [Google Scholar] [CrossRef]
- Sanchez-Cordero, S.; Garcia Ruiz de Gordejuela, A.; Vilallonga, R.; Gonzalez, O.; Ciscar, A.; Ciudin, A.; Zabalegui, A.; Armengol, M. Analysis of the Variability in Different Criteria to Define the Success of Bariatric Surgery: Retrospective Study 5-Year Follow-Up after Sleeve Gastrectomy and Roux-en-Y Gastric Bypass. J. Clin. Med. 2023, 12, 187. [Google Scholar] [CrossRef]
- Jiménez, A.; Pané, A.; Ibarzábal, A.; de Hollanda, A.; Tundidor, D.; Balibrea, J.M.; Andreu, A.; Molero, J.; Cañizares, S.; Obach, A.; et al. Weight-loss thresholds after bariatric surgery and cardiovascular outcomes: More is better. Int. J. Obes. 2022, 46, 279–286. [Google Scholar] [CrossRef] [PubMed]
- El Ansari, W.; Elhag, W. Weight Regain and Insufficient Weight Loss After Bariatric Surgery: Definitions, Prevalence, Mechanisms, Predictors, Prevention and Management Strategies, and Knowledge Gaps-a Scoping Review. Obes. Surg. 2021, 31, 1755–1766. [Google Scholar] [CrossRef] [PubMed]
- Salminen, P.; Kow, L.; Aminian, A.; Kaplan, L.M.; Nimeri, A.; Prager, G.; Behrens, E.; White, K.P.; Shikora, S.; IFSO Experts Panel. IFSO Consensus on Definitions and Clinical Practice Guidelines for Obesity Management-an International Delphi Study. Obes. Surg. 2024, 34, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Lim, R.; Beekley, A.; Johnson, D.C.; Davis, K.A. Early and late complications of bariatric operation. Trauma Surg. Acute Care Open 2018, 3, e000219. [Google Scholar] [CrossRef] [PubMed]
- Thaher, O.; Driouch, J.; Hukauf, M.; Köckerling, F.; Stroh, C. Feasibility and Short-Term Outcomes of One-Step and Two-Step Sleeve Gastrectomy as Revision Procedures for Failed Adjustable Gastric Banding Compared with Those After Primary Sleeve Gastrectomy. Front. Surg. 2021, 8, 752319. [Google Scholar] [CrossRef] [PubMed]
- Switzer, N.J.; Karmali, S.; Gill, R.S.; Sherman, V. Revisional Bariatric Surgery. Surg. Clin. N. Am. 2016, 96, 827–842. [Google Scholar] [CrossRef] [PubMed]
- Clapp, B.; Wynn, M.; Martyn, C.; Foster, C.; O’Dell, M.; Tyroch, A. Long term (7 or more years) outcomes of the sleeve gastrectomy: A meta-analysis. Surg. Obes. Relat. Dis. 2018, 14, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Brissman, M.; Beamish, A.J.; Olbers, T.; Marcus, C. Prevalence of insufficient weight loss 5 years after Roux-en-Y gastric bypass: Metabolic consequences and prediction estimates: A prospective registry study. BMJ Open 2021, 11, e046407. [Google Scholar] [CrossRef] [PubMed]
- Courcoulas, A.P.; Christian, N.J.; Belle, S.H.; Berk, P.D.; Flum, D.R.; Garcia, L.; Horlick, M.; Kalarchian, M.A.; King, W.C.; Mitchell, J.E.; et al. Weight change and health outcomes at 3 years after bariatric surgery among individuals with severe obesity. JAMA 2013, 310, 2416–2425. [Google Scholar] [CrossRef]
- Tolvanen, L.; Christenson, A.; Surkan, P.J.; Lagerros, Y.T. Patients’ Experiences of Weight Regain After Bariatric Surgery. Obes. Surg. 2022, 32, 1498–1507. [Google Scholar] [CrossRef]
- Chahine, E.; Kassir, R.; Dirani, M.; Joumaa, S.; Debs, T.; Chouillard, E. Surgical Management of Gastrogastric Fistula After Roux-en-Y Gastric Bypass: 10-Year Experience. Obes. Surg. 2018, 28, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Di Palma, A.; Liu, B.; Maeda, A.; Anvari, M.; Jackson, T.; Okrainec, A. Marginal ulceration following Roux-en-Y gastric bypass: Risk factors for ulcer development, recurrence and need for revisional surgery. Surg. Endosc. 2021, 35, 2347–2353. [Google Scholar] [CrossRef]
- Diaz-Vico, T.; Elli, E.F. Value of robotic-assisted technique in redo gastrojejunostomy for severe stenosis after gastric bypass. J. Robot. Surg. 2020, 14, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Yimcharoen, P.; Heneghan, H.; Chand, B.; Talarico, J.A.; Tariq, N.; Kroh, M.; Brethauer, S.A. Successful management of gastrojejunal strictures after gastric bypass: Is timing important? Surg. Obes. Relat. Dis. 2012, 8, 151–157. [Google Scholar] [CrossRef]
- Osland, E.; Powlesland, H.; Guthrie, T.; Lewis, C.A.; Memon, M.A. Micronutrient management following bariatric surgery: The role of the dietitian in the postoperative period. Ann. Transl. Med. 2020, 8 (Suppl. S1), S9. [Google Scholar] [CrossRef]
- Mahawar, K.K.; Nimeri, A.; Adamo, M.; Borg, C.M.; Singhal, R.; Khan, O.; Small, P.K. Practices Concerning Revisional Bariatric Surgery: A Survey of 460 Surgeons. Obes. Surg. 2018, 28, 2650–2660. [Google Scholar] [CrossRef]
- Latif, M.A.; Fouda, N.; Omran, E.; Refaey, M.S. Role of imaging in assessment and detection of complications after bariatric surgery. Egypt. J. Radiol. Nucl. Med. 2020, 51, 41. [Google Scholar] [CrossRef]
- Singh, S.S.; Shinde, R.K. Minimally Invasive Gastrointestinal Surgery: A Review. Cureus 2023, 15, e48864. [Google Scholar] [CrossRef] [PubMed]
- Manos, T.; Nedelcu, A.; Noel, P.; Zulian, V.; Danan, M.; Vilallonga, R.; Carandina, S.; Nedelcu, M. Endoscopic Gastric Band Removal. J. Clin. Med. 2023, 12, 617. [Google Scholar] [CrossRef]
- Gonzalez-Heredia, R.; Masrur, M.; Patton, K.; Bindal, V.; Sarvepalli, S.; Elli, E. Revisions after failed gastric band: Sleeve gastrectomy and Roux-en-Y gastric bypass. Surg. Endosc. 2015, 29, 2533–2537. [Google Scholar] [CrossRef]
- Stroh, C.; Benedix, D.; Weiner, R.; Benedix, F.; Wolff, S.; Knoll, C.; Manger, T.; Obesity Surgery Working Group, Competence Network Obesity. Is a one-step sleeve gastrectomy indicated as a revision procedure after gastric banding? Data analysis from a quality assurance study of the surgical treatment of obesity in Germany. Obes. Surg. 2014, 24, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Silecchia, G.; Rizzello, M.; De Angelis, F.; Raparelli, L.; Greco, F.; Perrotta, N.; Lerose, M.A.; Campanile, F.C. Laparoscopic sleeve gastrectomy as a revisional procedure for failed laparoscopic gastric banding with a “2-step approach”: A multicenter study. Surg. Obes. Relat. Dis. 2014, 10, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Sharples, A.J.; Charalampakis, V.; Daskalakis, M.; Tahrani, A.A.; Singhal, R. Systematic Review and Meta-Analysis of Outcomes After Revisional Bariatric Surgery Following a Failed Adjustable Gastric Band. Obes. Surg. 2017, 27, 2522–2536. [Google Scholar] [CrossRef] [PubMed]
- Marin-Perez, P.; Betancourt, A.; Lamota, M.; Lo Menzo, E.; Szomstein, S.; Rosenthal, R. Outcomes after laparoscopic conversion of failed adjustable gastric banding to sleeve gastrectomy or Roux-en-Y gastric bypass. Br. J. Surg. 2014, 101, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Elnahas, A.; Graybiel, K.; Farrokhyar, F.; Gmora, S.; Anvari, M.; Hong, D. Revisional surgery after failed laparoscopic adjustable gastric banding: A systematic review. Surg. Endosc. 2013, 27, 740–745. [Google Scholar] [CrossRef] [PubMed]
- Felsenreich, D.M.; Langer, F.B.; Bichler, C.; Eilenberg, M.; Jedamzik, J.; Kristo, I.; Vock, N.; Gensthaler, L.; Rabl, C.; Todoroff, A.; et al. Roux-en-Y Gastric Bypass as a Treatment for Barrett’s Esophagus after Sleeve Gastrectomy. Obes Surg. 2020, 30, 1273–1279. [Google Scholar] [CrossRef] [PubMed]
- Akpinar, E.O.; Nienhuijs, S.W.; Liem, R.S.L.; Greve, J.W.M.; Marang-van de Mheen, P.J. Conversion to Roux-en-Y gastric bypass versus one-anastomosis gastric bypass after a failed primary gastric band: A matched nationwide study. Surg. Obes. Relat. Dis. 2022, 18, 948–956. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.J.; Park, A.K.; Witkowski, E.R.; Hutter, M.M. Comparison of Short-term Safety of One Anastomosis Gastric Bypass to Roux-en-Y Gastric Bypass and Sleeve Gastrectomy in the United States: 341 cases from MBSAQIP-accredited Centers. Surg. Obes. Relat. Dis. 2022, 18, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Merz, A.E.; Blackstone, R.B.; Gagner, M.; Torres, A.J.; Himpens, J.; Higa, K.D.; Rosenthal, R.J.; Lloyd, A.; DeMaria, E.J. Duodenal switch in revisional bariatric surgery: Conclusions from an expert consensus panel. Surg. Obes. Relat. Dis. 2019, 15, 894–899. [Google Scholar] [CrossRef]
- Kermansaravi, M.; Parmar, C.; Chiappetta, S.; Shikora, S.; Aminian, A.; Abbas, S.I.; Angrisani, L.; Bashir, A.; Behrens, E.; Bhandari, M.; et al. Best practice approach for redo-surgeries after sleeve gastrectomy, an expert’s modified Delphi consensus. Surg. Endosc. 2023, 37, 1617–1628. [Google Scholar] [CrossRef]
- Bonavina, L.; Saino, G.; Lipham, J.C.; Demeester, T.R. LINX(®) Reflux Management System in chronic gastroesophageal reflux: A novel effective technology for restoring the natural barrier to reflux. Therap. Adv. Gastroenterol. 2013, 6, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Saino, G.; Bonavina, L.; Lipham, J.C.; Dunn, D.; Ganz, R.A. Magnetic Sphincter Augmentation for Gastroesophageal Reflux at 5 Years: Final Results of a Pilot Study Show Long-Term Acid Reduction and Symptom Improvement. J. Laparoendosc. Adv. Surg. Tech. 2015, 25, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Cheung, D.; Switzer, N.J.; Gill, R.S.; Shi, X.; Karmali, S. Revisional bariatric surgery following failed primary laparoscopic sleeve gastrectomy: A systematic review. Obes. Surg. 2014, 24, 1757–1763. [Google Scholar] [CrossRef] [PubMed]
- Huynh, D.; Mazer, L.; Tung, R.; Cunneen, S.; Shouhed, D.; Burch, M. Conversion of laparoscopic sleeve gastrectomy to Roux-en-Y gastric bypass: Patterns predicting persistent symptoms after revision. Surg. Obes. Relat. Dis. 2021, 17, 1681–1688. [Google Scholar] [CrossRef] [PubMed]
- Gautier, T.; Sarcher, T.; Contival, N.; Le Roux, Y.; Alves, A. Indications and mid-term results of conversion from sleeve gastrectomy to Roux-en-Y gastric bypass. Obes. Surg. 2013, 23, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Zadeh, J.R.; Alvarez, R.; Khaitan, L.; Abbas, M. Conversion of gastric sleeve to Roux-en-Y gastric bypass: Overall outcomes and predictors of below-average weight loss. Surg. Obes. Relat. Dis. 2023, 19, 111–117. [Google Scholar] [CrossRef]
- Felsenreich, D.M.; Langer, F.B.; Kefurt, R.; Panhofer, P.; Schermann, M.; Beckerhinn, P.; Sperker, C.; Prager, G. Weight loss, weight regain, and conversions to Roux-en-Y gastric bypass: 10-year results of laparoscopic sleeve gastrectomy. Surg. Obes. Relat. Dis. 2016, 12, 1655–1662. [Google Scholar] [CrossRef]
- Casillas, R.A.; Um, S.S.; Zelada Getty, J.L.; Sachs, S.; Kim, B.B. Revision of primary sleeve gastrectomy to Roux-en-Y gastric bypass: Indications and outcomes from a high-volume center. Surg. Obes. Relat. Dis. 2016, 12, 1817–1825. [Google Scholar] [CrossRef] [PubMed]
- Rayman, S.; Assaf, D.; Azran, C.; Sroka, G.; Assalia, A.; Beglaibter, N.; Elazary, R.; Eldar, S.M.; Romano-Zelekha, O.; Goitein, D. Sleeve Gastrectomy Failure-Revision to Laparoscopic One-Anastomosis Gastric Bypass or Roux-n-Y Gastric Bypass: A Multicenter Study. Obes. Surg. 2021, 31, 2927–2934. [Google Scholar] [CrossRef]
- Felsenreich, D.M.; Steinlechner, K.; Langer, F.B.; Vock, N.; Eichelter, J.; Bichler, C.; Jedamzik, J.; Mairinger, M.; Kristo, I.; Prager, G. Outcome of Sleeve Gastrectomy Converted to Roux-en-Y Gastric Bypass and One-Anastomosis Gastric Bypass. Obes. Surg. 2022, 32, 643–651. [Google Scholar] [CrossRef]
- Wolfe, B.M.; Kvach, E.; Eckel, R.H. Treatment of Obesity: Weight Loss and Bariatric Surgery. Circ. Res. 2016, 118, 1844–1855. [Google Scholar] [CrossRef] [PubMed]
- Angrisani, L.; Santonicola, A.; Iovino, P.; Palma, R.; Kow, L.; Prager, G.; Ramos, A.; Shikora, S.; Collaborative Study Group for the IFSO Worldwide Survey. IFSO Worldwide Survey 2020-2021: Current Trends for Bariatric and Metabolic Procedures. Obes. Surg. 2024, 34, 1075–1085. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.F.; Cohen, L.; Sarmento, L.D.; Rosa, F.M.; Rosado, E.L.; Carneiro, J.R.; Souza, A.A.; Magno, F.C. Effects of Long-Term Roux-En-Y Gastric Bypass on Body Weight and Clinical Metabolic Comorbidities in Bariatric Surgery Service of a University Hospital. Arq. Bras. Cir. Dig. 2016, 29 (Suppl. S1), 20–23. [Google Scholar] [CrossRef] [PubMed]
- Lager, C.J.; Esfandiari, N.H.; Subauste, A.R.; Kraftson, A.T.; Brown, M.B.; Cassidy, R.B.; Nay, C.K.; Lockwood, A.L.; Varban, O.A.; Oral, E.A. Roux-En-Y Gastric Bypass Vs. Sleeve Gastrectomy: Balancing the Risks of Surgery with the Benefits of Weight Loss. Obes. Surg. 2017, 27, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Menzel, C.P.; Flynn, C.R.; English, W.J. Chapter 26—Roux-en-Y gastric bypass: Influence on adipose tissue and metabolic homeostasis. In Visceral and Ectopic Fat; Lamb, H.J., Ed.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 377–389. [Google Scholar]
- Ju, Z.; Anderson, W.; Istfan, N.; Carmine, B.; Carter, C.; Pernar, L.; Marshall, A.; Hess, D.T. Comparison of weight loss outcomes between Roux-en-Y gastric bypass and sleeve gastrectomy in a racially mixed urban patient population. Surg. Obes. Relat. Dis. 2022, 18, 1218–1227. [Google Scholar] [CrossRef] [PubMed]
- Griffith, P.S.; Birch, D.W.; Sharma, A.M.; Karmali, S. Managing complications associated with laparoscopic Roux-en-Y gastric bypass for morbid obesity. Can. J. Surg. 2012, 55, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Tourky, M.; Issa, M.; Salman, M.A.; Salman, A.; Shaaban, H.E.; Safina, A.; Elias, A.A.; Elewa, A.; Noureldin, K.; Mahmoud, A.A.; et al. Nutritional Complications After Laparoscopic Roux-en-Y Gastric Bypass and One-Anastomosis Gastric Bypass: A Comparative Systematic Review and Meta-Analysis. Cureus 2022, 14, e21114. [Google Scholar] [CrossRef] [PubMed]
- Weng, T.C.; Chang, C.H.; Dong, Y.H.; Chang, Y.C.; Chuang, L.M. Anaemia and related nutrient deficiencies after Roux-en-Y gastric bypass surgery: A systematic review and meta-analysis. BMJ Open 2015, 5, e006964. [Google Scholar] [CrossRef]
- Lupoli, R.; Lembo, E.; Saldalamacchia, G.; Avola, C.K.; Angrisani, L.; Capaldo, B. Bariatric surgery and long-term nutritional issues. World J. Diabetes 2017, 8, 464–474. [Google Scholar] [CrossRef]
- Aguas-Ayesa, M.; Yárnoz-Esquíroz, P.; Olazarán, L.; Gómez-Ambrosi, J.; Frühbeck, G. Precision nutrition in the context of bariatric surgery. Rev. Endocr. Metab. Disord. 2023, 24, 979–991. [Google Scholar] [CrossRef]
- Ciobârcă, D.; Cătoi, A.F.; Copăescu, C.; Miere, D.; Crișan, G. Bariatric Surgery in Obesity: Effects on Gut Microbiota and Micronutrient Status. Nutrients 2020, 12, 235. [Google Scholar] [CrossRef]
- Chaparro, C.M.; Suchdev, P.S. Anemia epidemiology, pathophysiology, and etiology in low- and middle-income countries. Ann. N. Y. Acad. Sci. 2019, 1450, 15–31. [Google Scholar] [CrossRef]
- Aarts, E.O.; van Wageningen, B.; Janssen, I.M.; Berends, F.J. Prevalence of Anemia and Related Deficiencies in the First Year following Laparoscopic Gastric Bypass for Morbid Obesity. J. Obes. 2012, 2012, 193705. [Google Scholar] [CrossRef] [PubMed]
- Enani, G.; Bilgic, E.; Lebedeva, E.; Delisle, M.; Vergis, A.; Hardy, K. The incidence of iron deficiency anemia post-Roux-en-Y gastric bypass and sleeve gastrectomy: A systematic review. Surg. Endosc. 2020, 34, 3002–3010. [Google Scholar] [CrossRef] [PubMed]
- de Cleva, R.; Cardia, L.; Riccioppo, D.; Kawamoto, M.; Kanashiro, N.; Santo, M.A. Anemia Before and After Roux-en-Y Gastric Bypass: Prevalence and Evolution on Long-Term Follow-up. Obes. Surg. 2019, 29, 2790–2794. [Google Scholar] [CrossRef]
- Sabate, J.M.; Coupaye, M.; Ledoux, S.; Castel, B.; Msika, S.; Coffin, B.; Jouet, P. Consequences of Small Intestinal Bacterial Overgrowth in Obese Patients Before and After Bariatric Surgery. Obes. Surg. 2017, 27, 599–605. [Google Scholar] [CrossRef]
- Poitou Bernert, C.; Ciangura, C.; Coupaye, M.; Czernichow, S.; Bouillot, J.L.; Basdevant, A. Nutritional deficiency after gastric bypass: Diagnosis, prevention and treatment. Diabetes Metab. 2007, 33, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Bloomberg, R.D.; Fleishman, A.; Nalle, J.E.; Herron, D.M.; Kini, S. Nutritional deficiencies following bariatric surgery: What have we learned? Obes. Surg. 2005, 15, 145–154. [Google Scholar] [CrossRef]
- Ceneviva, R.; Salgado Júnior, W.; Marchini, J.S. A new revisional surgery for severe protein-calorie malnutrition after Roux-en-Y gastric bypass: Successful duodenojejunal reconstruction using jejunal interposition. Surg. Obes. Relat. Dis. 2016, 12, e21–e23. [Google Scholar] [CrossRef]
- Akusoba, I.; Birriel, T.J.; El Chaar, M. Management of Excessive Weight Loss Following Laparoscopic Roux-en-Y Gastric Bypass: Clinical Algorithm and Surgical Techniques. Obes. Surg. 2016, 26, 5–11. [Google Scholar] [CrossRef]
- Chousleb, E.; Patel, S.; Szomstein, S.; Rosenthal, R. Reasons and operative outcomes after reversal of gastric bypass and jejunoileal bypass. Obes. Surg. 2012, 22, 1611–1616. [Google Scholar] [CrossRef] [PubMed]
- Beran, A.; Shaear, M.; Al-Mudares, S.; Sharma, I.; Matar, R.; Al-Haddad, M.; Salame, M.; Portela, R.; Clapp, B.; Dayyeh, B.K.A.; et al. Predictors of marginal ulcer after gastric bypass: A systematic review and meta-analysis. J. Gastrointest. Surg. 2023, 27, 1066–1077. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Larios, R.; Cornejo, J.; Gunturu, N.S.; Cheng, Y.L.; Elli, E.F. Experience of Robotic Complex Revisional Bariatric Surgery in a High-Volume Center. Obes Surg. 2023, 33, 4034–4041. [Google Scholar] [CrossRef] [PubMed]
- Baumann, A.J.; Mramba, L.K.; Hawkins, R.B.; Carpenter, A.M.; Fleisher, M.S.; Ayzengart, A.L.; Estores, D.S., Jr. Endoscopic Dilation of Bariatric RNY Anastomotic Strictures: A Systematic Review and Meta-analysis. Obes. Surg. 2018, 28, 4053–4063. [Google Scholar] [CrossRef] [PubMed]
- Fayad, L.; Simsek, C.; Oleas, R.; Ichkhanian, Y.; Fayad, G.E.; Ngamreungphong, S.; Schweitzer, M.; Oberbach, A.; Kalloo, A.N.; Khashab, M.A.; et al. Safety and Efficacy of Endoscopically Secured Fully Covered Self-Expandable Metallic Stents (FCSEMS) for Post-Bariatric Complex Stenosis. Obes. Surg. 2019, 29, 3484–3492. [Google Scholar] [CrossRef] [PubMed]
- Corcelles, R.; Jamal, M.H.; Daigle, C.R.; Rogula, T.; Brethauer, S.A.; Schauer, P.R. Surgical management of gastrogastric fistula. Surg. Obes. Relat. Dis. 2015, 11, 1227–1232. [Google Scholar] [CrossRef] [PubMed]
- Istfan, N.W.; Lipartia, M.; Anderson, W.A.; Hess, D.T.; Apovian, C.M. Approach to the Patient: Management of the Post-Bariatric Surgery Patient with Weight Regain. J. Clin. Endocrinol. Metab. 2021, 106, 251–263. [Google Scholar] [CrossRef]
- Matteo, M.V.; Gallo, C.; Pontecorvi, V.; Bove, V.; De Siena, M.; Carlino, G.; Costamagna, G.; Boškoski, I. Weight Recidivism and Dumping Syndrome after Roux-En-Y Gastric Bypass: Exploring the Therapeutic Role of Transoral Outlet Reduction. J. Pers. Med. 2022, 12, 1664. [Google Scholar] [CrossRef]
- Hakiza, L.; Sartoretto, A.; Burgmann, K.; Kumbhari, V.; Matter, C.; Seibold, F.; Staudenmann, D. Transoral Outlet Reduction (TORe) for the Treatment of Weight Regain and Dumping Syndrome after Roux-en-Y Gastric Bypass. Medicina 2023, 59, 125. [Google Scholar] [CrossRef]
- Jirapinyo, P.; de Moura, D.T.H.; Thompson, C.C. Endoscopic submucosal dissection with suturing for the treatment of weight regain after gastric bypass: Outcomes and comparison with traditional transoral outlet reduction (with video). Gastrointest. Endosc. 2020, 91, 1282–1288. [Google Scholar] [CrossRef]
- Heneghan, H.M.; Yimcharoen, P.; Brethauer, S.A.; Kroh, M.; Chand, B. Influence of pouch and stoma size on weight loss after gastric bypass. Surg. Obes. Relat. Dis. 2012, 8, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Lundberg, P.W.; Javier Birriel, T.; Claros, L.; Stoltzfus, J.; El Chaar, M. Revisional Bariatric Surgery for Weight Regain and Refractory Complications in a Single MBSAQIP Accredited Center: What Are We Dealing with? Obes. Surg. 2018, 28, 2789–2795. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.L.; Elli, E.F. Role of Robotic Surgery in Complex Revisional Bariatric Procedures. Obes. Surg. 2021, 31, 2583–2589. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.; Dip, F.; Huaco, J.A.; Moon, R.; Ahmad, H.; LoMenzo, E.; Szomstein, S.; Rosenthal, R. Outcomes of revisional treatment modalities in non-complicated Roux-en-Y gastric bypass patients with weight regain. Obes. Surg. 2015, 25, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Benois, M.; Sebastianelli, L.; Morisot, A.; Amor, I.B.; Gugenheim, J.; Bailly, L.; Iannelli, A. Revisional But Not Conversional Gastric Bypass Surgery Increases the Risk of Leaks: Review of 176 Redo out of 932 Consecutive Cases. Obes. Surg. 2018, 28, 2903–2911. [Google Scholar] [CrossRef] [PubMed]
- Jaruvongvanich, V.; Vantanasiri, K.; Laoveeravat, P.; Matar, R.H.; Vargas, E.J.; Maselli, D.B.; Alkhatry, M.; Fayad, L.; Kumbhari, V.; Fittipaldi-Fernandez, R.J.; et al. Endoscopic full-thickness suturing plus argon plasma mucosal coagulation versus argon plasma mucosal coagulation alone for weight regain after gastric bypass: A systematic review and meta-analysis. Gastrointest. Endosc. 2020, 92, 1164–1175.e6. [Google Scholar] [CrossRef] [PubMed]
- Jirapinyo, P.; Kumar, N.; AlSamman, M.A.; Thompson, C.C. Five-year outcomes of transoral outlet reduction for the treatment of weight regain after Roux-en-Y gastric bypass. Gastrointest. Endosc. 2020, 91, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.D.; Nwokeabia, I.D.; Purnell, S.; Zafar, S.N.; Ortega, G.; Hughes, K.; Fullum, T.M. Revision of Roux-En-Y Gastric Bypass for Weight Regain: A Systematic Review of Techniques and Outcomes. Obes. Surg. 2016, 26, 1627–1634. [Google Scholar] [CrossRef] [PubMed]
- Norton, B.C.; Telese, A.; Papaefthymiou, A.; Aslam, N.; Makaronidis, J.; Murray, C.; Haidry, R. Metabolic and Bariatric Endoscopy: A Mini-Review. Life 2023, 13, 1905. [Google Scholar] [CrossRef]
- Brown, A.M.; Spaniolas, K. Distalization of Roux-en-Y Gastric Bypass: Lengthening the Biliopancreatic Limb. J. Gastrointest. Surg. 2020, 24, 2171–2172. [Google Scholar] [CrossRef]
- Runkel, N.; Brydniak, R. Surgical Treatment of Metabolic Syndrome. Visc. Med. 2016, 32, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Mingrone, G.; Panunzi, S.; De Gaetano, A.; Guidone, C.; Iaconelli, A.; Nanni, G.; Castagneto, M.; Bornstein, S.; Rubino, F. Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet 2015, 386, 964–973. [Google Scholar] [CrossRef] [PubMed]
- Bostanjian, D.; Anthone, G.J.; Hamoui, N.; Crookes, P.F. Rhabdomyolysis of gluteal muscles leading to renal failure: A potentially fatal complication of surgery in the morbidly obese. Obes. Surg. 2003, 13, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Hess, D.S. Chapter 30—Biliopancreatic Diversion with Duodenal Switch. In Surgical Management of Obesity; Buchwald, H., Cowan, G.S.M., Pories, W.J., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2007; pp. 252–266. [Google Scholar]
- Admella, V.; Osorio, J.; Sorribas, M.; Sobrino, L.; Casajoana, A.; Pujol-Gebellí, J. Direct and two-step single anastomosis duodenal switch (SADI-S): Unicentric comparative analysis of 232 cases. Cirugía Española 2021, 99, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Nandagopal, R.; Brown, R.J.; Rother, K.I. Resolution of type 2 diabetes following bariatric surgery: Implications for adults and adolescents. Diabetes Technol. Ther. 2010, 12, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Salman, A.A.; Salman, M.A.; Marie, M.A.; Rabiee, A.; Helmy, M.Y.; Tourky, M.S.; Qassem, M.G.; Shaaban, H.E.; Sarhan, M.D. Factors associated with resolution of type-2 diabetes mellitus after sleeve gastrectomy in obese adults. Sci. Rep. 2021, 11, 6002. [Google Scholar] [CrossRef] [PubMed]
- Prachand, V.N.; Ward, M.; Alverdy, J.C. Duodenal switch provides superior resolution of metabolic comorbidities independent of weight loss in the super-obese (BMI > or = 50 kg/m2) compared with gastric bypass. J. Gastrointest. Surg. 2010, 14, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Möller, F.; Hedberg, J.; Skogar, M.; Sundbom, M. Long-term Follow-up 15 Years After Duodenal Switch or Gastric Bypass for Super Obesity: A Randomized Controlled Trial. Obes. Surg. 2023, 33, 2981–2990. [Google Scholar] [CrossRef] [PubMed]
- Marceau, P.; Biron, S.; Hould, F.S.; Lebel, S.; Marceau, S.; Lescelleur, O.; Biertho, L.; Simard, S. Duodenal switch: Long-term results. Obes. Surg. 2007, 17, 1421–1430. [Google Scholar] [CrossRef]
- Parikh, M.; Pomp, A.; Gagner, M. Laparoscopic conversion of failed gastric bypass to duodenal switch: Technical considerations and preliminary outcomes. Surg. Obes. Relat. Dis. 2007, 3, 611–618. [Google Scholar] [CrossRef]
- Lange, J.; Königsrainer, A. Malnutrition as a Complication of Bariatric Surgery—A Clear and Present Danger? Visc. Med. 2019, 35, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Hedberg, J.; Sundström, J.; Sundbom, M. Duodenal switch versus Roux-en-Y gastric bypass for morbid obesity: Systematic review and meta-analysis of weight results, diabetes resolution and early complications in single-centre comparisons. Obes. Rev. 2014, 15, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Oviedo, R.J.; Nayak, T.; Liu, Y.; Zhang, S.; Zhao, F. Laparoscopic Duodenal Switch Versus Roux-en-Y Gastric Bypass at a High-Volume Community Hospital: A Retrospective Cohort Study from a Rural Setting. Obes. Surg. 2021, 31, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Topart, P.A.; Becouarn, G. Revision and reversal after biliopancreatic diversion for excessive side effects or ineffective weight loss: A review of the current literature on indications and procedures. Surg. Obes. Relat. Dis. 2015, 11, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Chierici, A.; Chevalier, N.; Iannelli, A. Postoperative morbidity and weight loss after revisional bariatric surgery for primary failed restrictive procedure: A systematic review and network meta-analysis. Int. J. Surg. 2022, 102, 106677. [Google Scholar]
- Clapp, B.; Badaoui, J.N.; Gamez, J.A.; Vivar, A.; Ghanem, O.M. Reluctance in duodenal switch adoption: An international survey among bariatric surgeons. Surg. Obes. Relat. Dis. 2021, 17, 1760–1765. [Google Scholar] [CrossRef] [PubMed]
- Wargel, Z.M.; Ritchie, T.W.; Shapera, E.; Wheeler, A.A. Laparoscopic Conversion of Single-Anastomosis Duodenal Switch (SADI-S) to Roux-en-Y Gastric Bypass With Concurrent Paraesophageal Hernia Repair for Refractory Biliary Reflux and Paraesophageal Hernia. Cureus 2023, 15, e36205. [Google Scholar] [CrossRef] [PubMed]
- Portela, R.; Marrerro, K.; Vahibe, A.; Galvani, C.; Billy, H.; Abu Dayyeh, B.; Clapp, B.; Ghanem, O.M. Bile Reflux After Single Anastomosis Duodenal-Ileal Bypass with Sleeve (SADI-S): A Meta-analysis of 2,029 Patients. Obes. Surg. 2022, 32, 1516–1522. [Google Scholar] [CrossRef]
- Badaoui, J.N.; Kellogg, T.A.; Abu Dayyeh, B.K.; Maroun, J.W.; McKenzie, T.J.; Harmsen, W.S.; Kendrick, M.L.; Ghanem, O.M. The Outcomes of Laparoscopic Biliopancreatic Diversion with Duodenal Switch on Gastro-esophageal Reflux Disease: The Mayo Clinic Experience. Obes. Surg. 2021, 31, 4363–4370. [Google Scholar] [CrossRef]
- Rao, R.; Mehta, M.; Sheth, D.R.; Hogan, G. Four-Year Nutritional Outcomes in Single-Anastomosis Duodeno-Ileal Bypass with Sleeve Gastrectomy Patients: An Australian Experience. Obes. Surg. 2023, 33, 750–760. [Google Scholar] [CrossRef]
- Chiappetta, S.; Stier, C.; Scheffel, O.; Theodoridou, S.; Weiner, R. The first case report of failed single-anastomosis-duodeno-ileal bypass converted to One anastomosis gastric bypass/Mini-gastric bypass. Int. J. Surg. Case Rep. 2017, 35, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Gebellí, J.P.; Lazzara, C.; de Gordejuela, A.G.R.; Nora, M.; Pereira, A.M.; Sánchez-Pernaute, A.; Osorio, J.; Sobrino, L.; García AJT. Duodenal Switch vs. Single-Anastomosis Duodenal Switch (SADI-S) for the Treatment of Grade IV Obesity: 5-Year Outcomes of a Multicenter Prospective Cohort Comparative Study. Obes. Surg. 2022, 32, 3839–3846. [Google Scholar] [CrossRef] [PubMed]
- Lutsi, B.; Hirano, I. Ambulatory pH Monitoring: New Advances and Indications. Gastroenterol. Hepatol. 2006, 2, 835–842. [Google Scholar]
- Broderick, R.C.; Smith, C.D.; Cheverie, J.N.; Omelanczuk, P.; Lee, A.M.; Dominguez-Profeta, R.; Cubas, R.; Jacobsen, G.R.; Sandler, B.J.; Fuchs, K.H.; et al. Magnetic sphincter augmentation: A viable rescue therapy for symptomatic reflux following bariatric surgery. Surg. Endosc. 2020, 34, 3211–3215. [Google Scholar] [CrossRef] [PubMed]
- Khaitan, L.; Hill, M.; Michel, M.; Chiasson, P.; Woodworth, P.; Bell, R.; Sadek, R.; Hoffman, A.; Loing, K.; Veldhuis, P.; et al. Feasibility and Efficacy of Magnetic Sphincter Augmentation for the Management of Gastroesophageal Reflux Disease Post-Sleeve Gastrectomy for Obesity. Obes. Surg. 2023, 33, 387–396. [Google Scholar] [CrossRef]
- Yashkov, Y.; Bordan, N.; Torres, A.; Malykhina, A.; Bekuzarov, D. SADI-S 250 vs Roux-en-Y Duodenal Switch (RY-DS): Results of 5-Year Observational Study. Obes Surg. 2021, 31, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Finno, P.; Osorio, J.; García-Ruiz-de-Gordejuela, A.; Casajoana, A.; Sorribas, M.; Admella, V.; Serrano, M.; Marchesini, J.B.; Ramos, A.C.; Pujol-Gebellí, J. Single Versus Double-Anastomosis Duodenal Switch: Single-Site Comparative Cohort Study in 440 Consecutive Patients. Obes. Surg. 2020, 30, 3309–3316. [Google Scholar] [CrossRef] [PubMed]
- Burgos, A.M.; Csendes, A.; Braghetto, I. Gastric stenosis after laparoscopic sleeve gastrectomy in morbidly obese patients. Obes. Surg. 2013, 23, 1481–1486. [Google Scholar] [CrossRef]
- Felinska, E.; Billeter, A.; Nickel, F.; Contin, P.; Berlth, F.; Chand, B.; Grimminger, P.; Mikami, D.; Schoppmann, S.F.; Müller-Stich, B. Do we understand the pathophysiology of GERD after sleeve gastrectomy? Ann. N. Y. Acad. Sci. 2020, 1482, 26–35. [Google Scholar] [CrossRef]
- Laffin, M.; Chau, J.; Gill, R.S.; Birch, D.W.; Karmali, S. Sleeve gastrectomy and gastroesophageal reflux disease. J. Obes. 2013, 2013, 741097. [Google Scholar] [CrossRef]
- Saunders, J.; Smith, T. Malnutrition: Causes and consequences. Clin. Med. 2010, 10, 624–627. [Google Scholar] [CrossRef] [PubMed]
- Baltar, J.; Martis-Sueiro, A.; Pardo, M.; Santos, F.; Sartal, M.I.; Crujeiras, A.B.; Peinó, R.; Seoane, L.M.; Bárcena, M.; Bustamante, M. Conversion from Duodenal Switch to Single Anastomosis Duodenal Switch to Deal with Postoperative Malnutrition. Obes. Surg. 2021, 31, 431–436. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Evans, L.A.; Castillo-Larios, R.; Cornejo, J.; Elli, E.F. Challenges of Revisional Metabolic and Bariatric Surgery: A Comprehensive Guide to Unraveling the Complexities and Solutions of Revisional Bariatric Procedures. J. Clin. Med. 2024, 13, 3104. https://doi.org/10.3390/jcm13113104
Evans LA, Castillo-Larios R, Cornejo J, Elli EF. Challenges of Revisional Metabolic and Bariatric Surgery: A Comprehensive Guide to Unraveling the Complexities and Solutions of Revisional Bariatric Procedures. Journal of Clinical Medicine. 2024; 13(11):3104. https://doi.org/10.3390/jcm13113104
Chicago/Turabian StyleEvans, Lorna A., Rocio Castillo-Larios, Jorge Cornejo, and Enrique F. Elli. 2024. "Challenges of Revisional Metabolic and Bariatric Surgery: A Comprehensive Guide to Unraveling the Complexities and Solutions of Revisional Bariatric Procedures" Journal of Clinical Medicine 13, no. 11: 3104. https://doi.org/10.3390/jcm13113104
APA StyleEvans, L. A., Castillo-Larios, R., Cornejo, J., & Elli, E. F. (2024). Challenges of Revisional Metabolic and Bariatric Surgery: A Comprehensive Guide to Unraveling the Complexities and Solutions of Revisional Bariatric Procedures. Journal of Clinical Medicine, 13(11), 3104. https://doi.org/10.3390/jcm13113104





