Right-Sided Aortic Arch: A Computed Tomography Angiography Investigation, A Systematic Review with Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Original Study
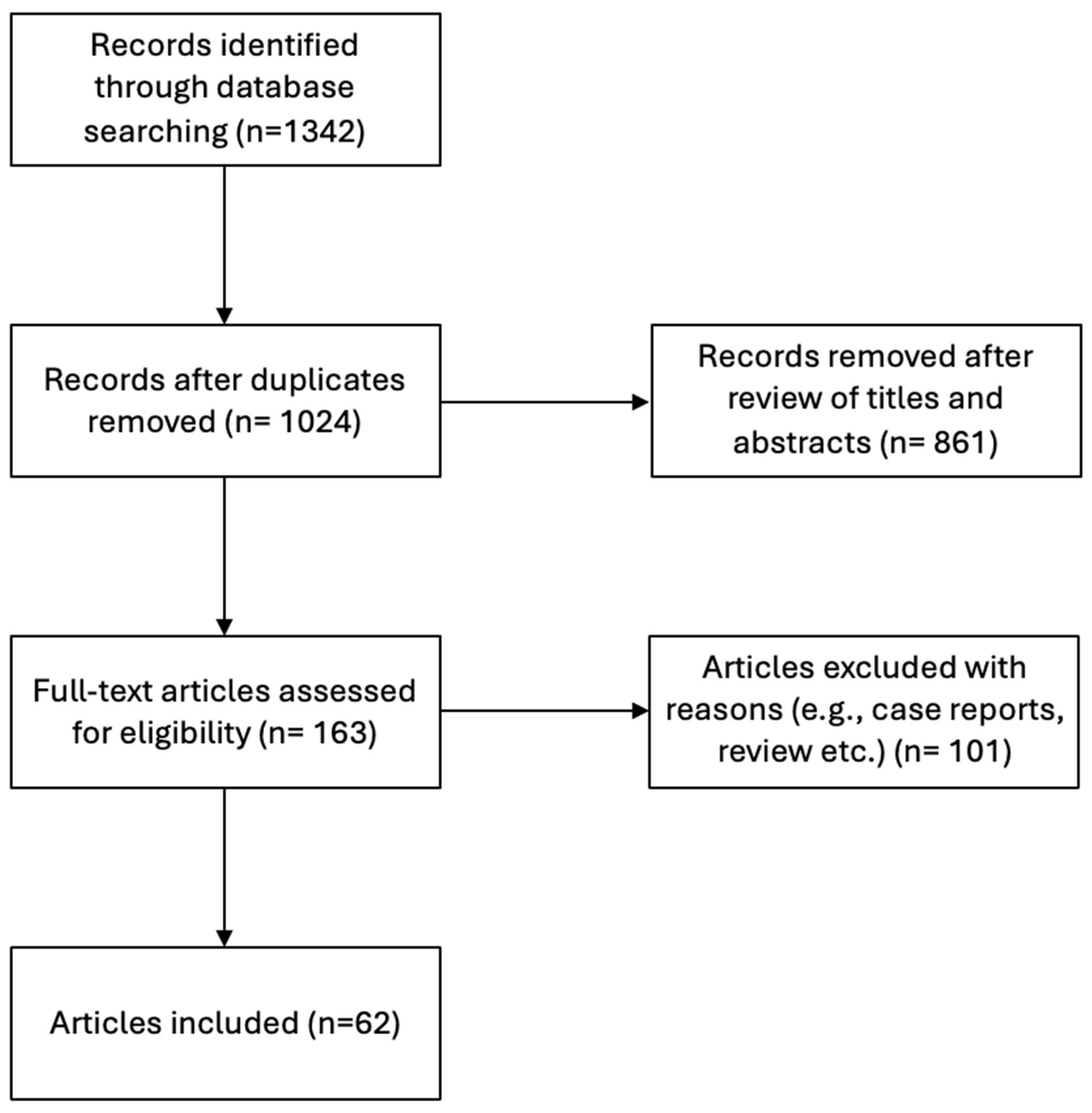
2.2. Search Strategy—Selection Criteria for the Systematic Review Study
2.3. Statistical Analysis
3. Results
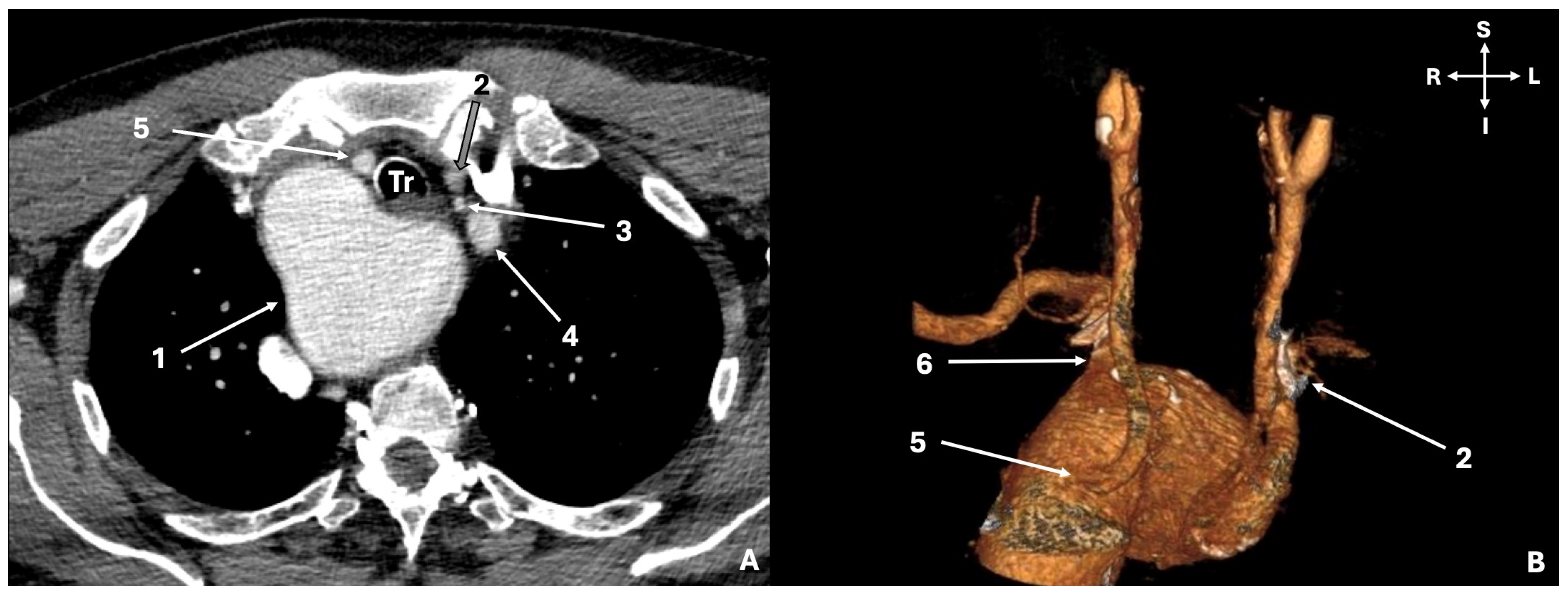
3.1. Original Study Results
3.2. Systematic Review Results
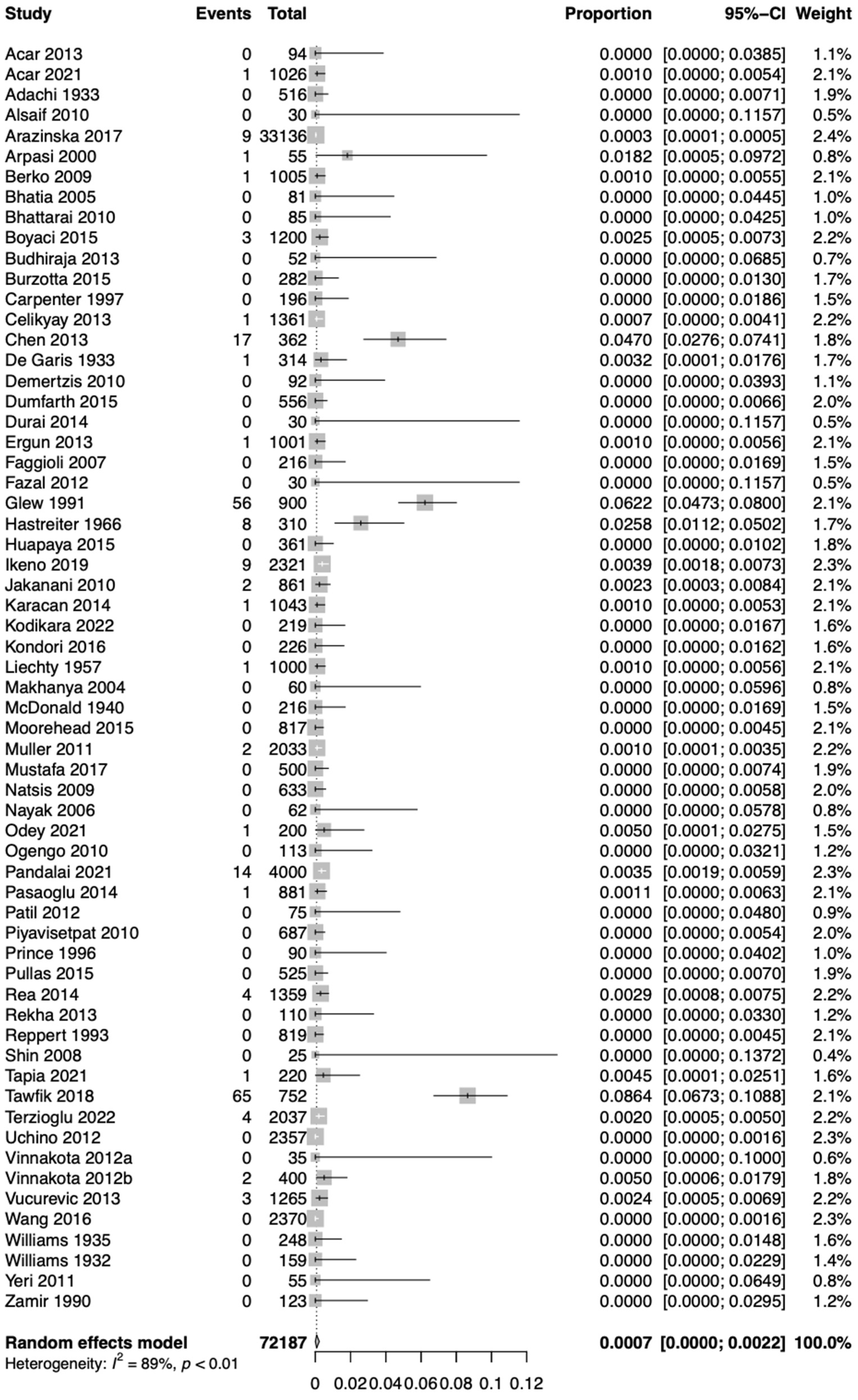
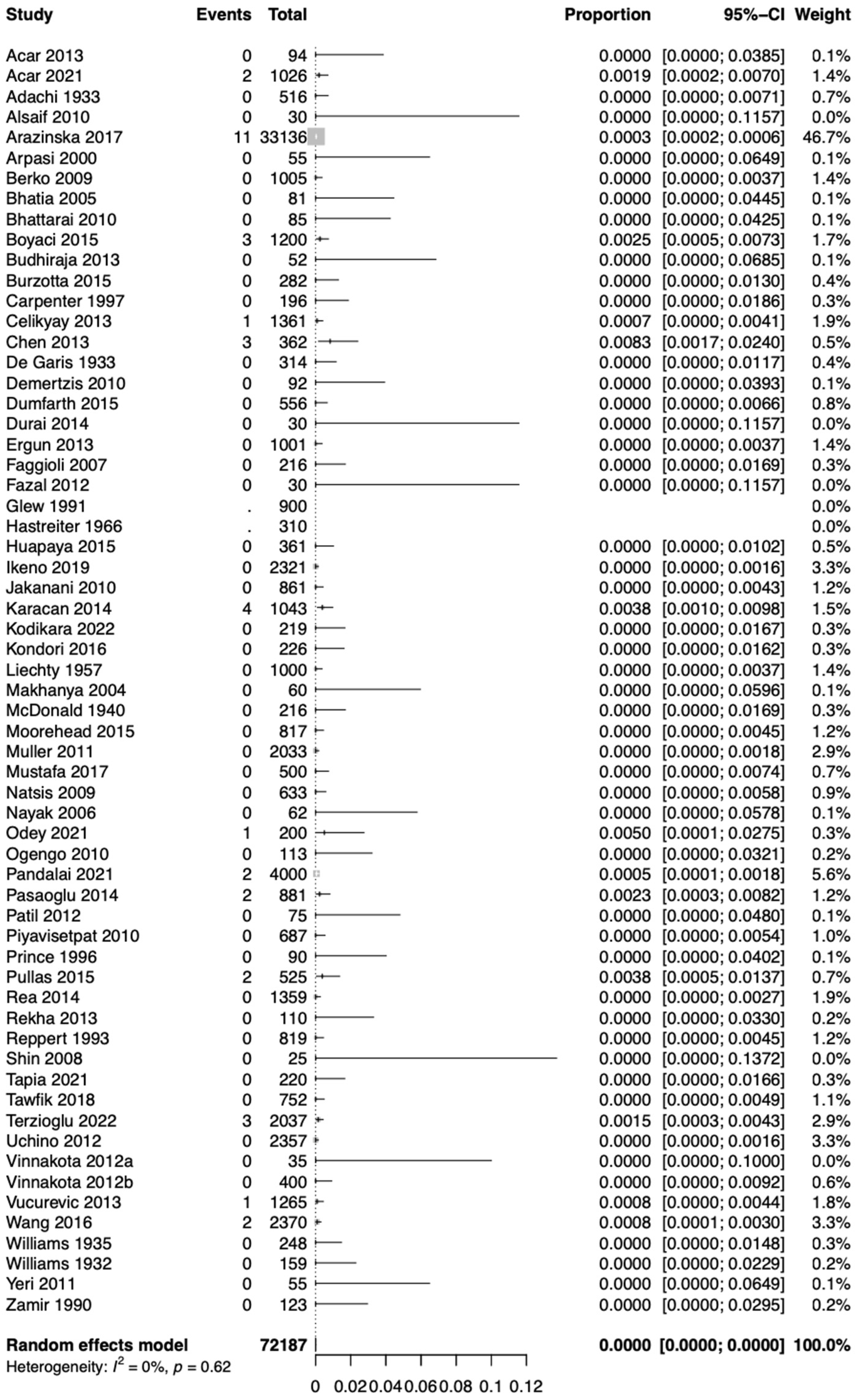
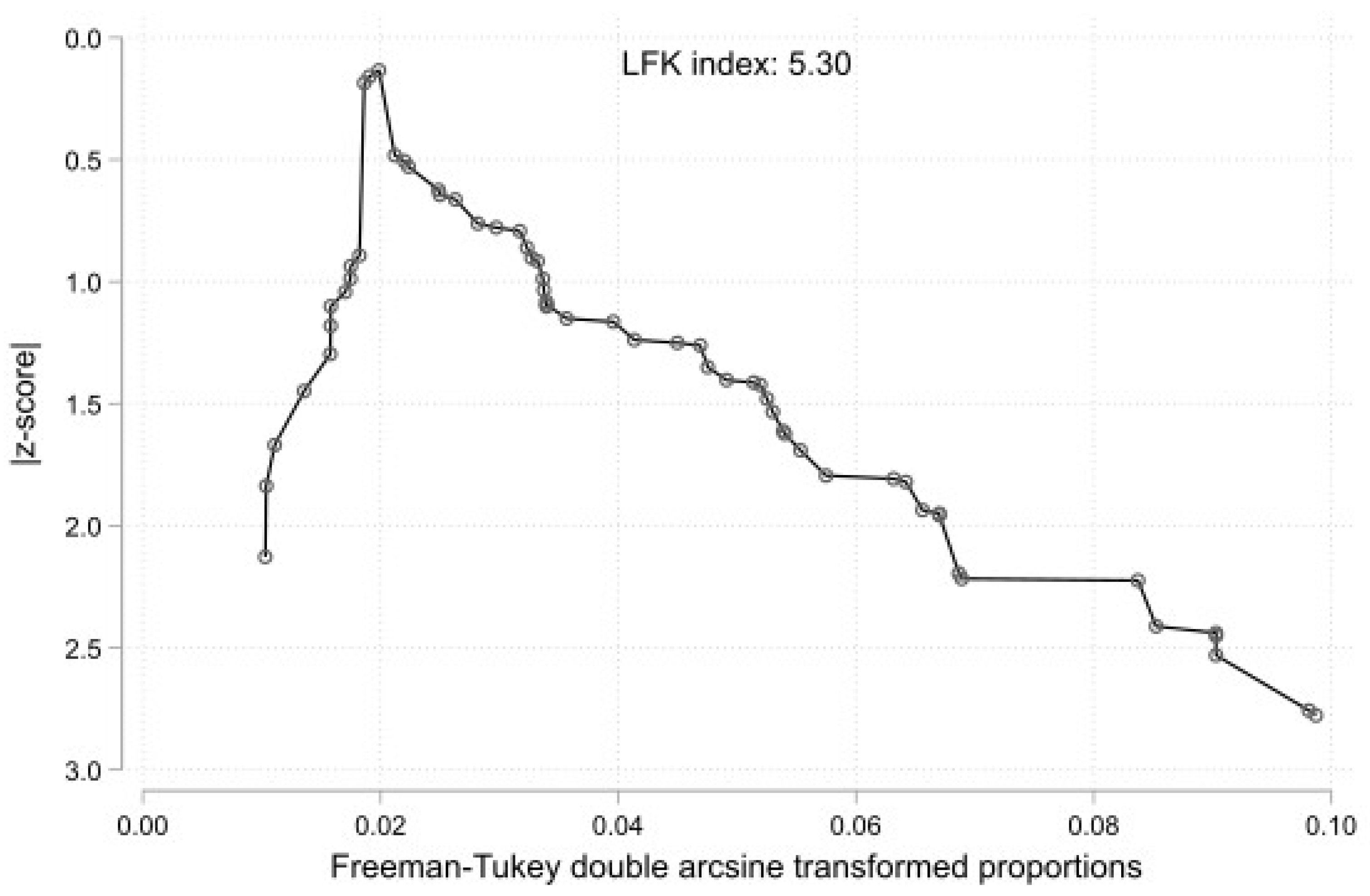
3.3. Statistical Results
4. Discussion
4.1. Embryological Development of the Aortic Arch (AA)
4.2. Morphological Variability of the Left Aortic Arch (LAA)
4.3. Morphological Variability of the Right Aortic Arch (RAA)
4.4. Clinical Implications of the Aortic Arch (AA) Variations
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Popieluszko, P.; Henry, B.M.; Sanna, B.; Hsieh, W.C.; Saganiak, K.; Pękala, P.A.; Walocha, J.A.; Tomaszewski, K.A. A Systematic Review and Meta-Analysis of Variations in Branching Patterns of the Adult Aortic Arch. J. Vasc. Surg. 2018, 68, 298–306.e10. [Google Scholar] [CrossRef]
- Natsis, K.; Piagkou, M.; Lazaridis, N.; Kalamatianos, T.; Chytas, D.; Manatakis, D.; Anastasopoulos, N.; Loukas, M. A Systematic Classification of the Left-Sided Aortic Arch Variants Based on Cadaveric Studies’ Prevalence. Surg. Radiol. Anat. 2021, 43, 327–345. [Google Scholar] [CrossRef]
- Tsiouris, C.; Lazaridis, N.; Piagkou, M.; Duparc, F.; Antonopoulos, I.; Antonitsis, P.; Natsis, K. The Left-Sided Aortic Arch Variants: Prevalence Meta-Analysis of Imaging Studies. Surg. Radiol. Anat. 2022, 44, 673–688. [Google Scholar] [CrossRef]
- Açar, G.; Çiçekcibaşı, A.E.; Uysal, E.; Koplay, M. Anatomical Variations of the Aortic Arch Branching Pattern Using CT Angiography: A Proposal for a Different Morphological Classification with Clinical Relevance. Anat. Sci. Int. 2022, 97, 65–78. [Google Scholar] [CrossRef]
- Ramos-Duran, L.; Nance, J.W.; Schoepf, U.J.; Henzler, T.; Apfaltrer, P.; Hlavacek, A.M. Developmental Aortic Arch Anomalies in Infants and Children Assessed with CT Angiography. Am. J. Roentgenol. 2012, 198, W466–W474. [Google Scholar] [CrossRef]
- Law, M.A.; Mohan, J. Right Aortic Arches; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK431104/ (accessed on 8 August 2023).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Viechtbauer, W.; Cheung, M.W.-L. Outlier and Influence Diagnostics for Meta-Analysis. Res. Synth. Methods 2010, 1, 112–125. [Google Scholar] [CrossRef]
- Balduzzi, S.; Rücker, G.; Schwarzer, G. How to Perform a Meta-Analysis with R: A Practical Tutorial. Evid. Based Ment. Health 2019, 22, 153–160. [Google Scholar] [CrossRef]
- Higgins, J.P.T. Measuring Inconsistency in Meta-Analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Schwarzer, G.; Carpenter, J.R.; Rücker, G. Meta-Analysis with R; Springer International Publishing: Cham, Switzerland, 2015; ISBN 978-3-319-21415-3. [Google Scholar]
- Furuya-Kanamori, L.; Barendregt, J.J.; Doi, S.A.R. A New Improved Graphical and Quantitative Method for Detecting Bias in Meta-Analysis. Int. J. Evid. Based Healthc. 2018, 16, 195–203. [Google Scholar] [CrossRef]
- Acar, M.; Ulusoy, M.; Zararsiz, I.; Efe, D. Anatomical variations in the branching of human aortic arch. Biomed. Res. 2013, 24, 531–535. [Google Scholar]
- Adachi, B. Das Arteriensystem der Japaner; Tokyo Kenkyusha Press: Kyoto, Japan, 1928. [Google Scholar]
- Alsaif, H.A.; Ramadan, W.S.; Ramadan, W.; Alsaif, H.A.; Ramadan, W.S. An anatomical study of the aortic arch variations. JKAU Med. Sci. 2010, 17, 37–54. [Google Scholar] [CrossRef]
- Arazińska, A.; Polguj, M.; Szymczyk, K.; Kaczmarska, M.; Trębiński, Ł.; Stefańczyk, L. Right Aortic Arch Analysis—Anatomical Variant or Serious Vascular Defect? BMC Cardiovasc. Disord. 2017, 17, 102. [Google Scholar] [CrossRef]
- Arpasi, P.J.; Bis, K.G.; Shetty, A.N.; White, R.D.; Simonetti, O.P. MR Angiography of the Thoracic Aorta with an Electrocardiographically Triggered Breath-Hold Contrast-Enhanced Sequence. RadioGraphics 2000, 20, 107–120. [Google Scholar] [CrossRef]
- Berko, N.S.; Jain, V.R.; Godelman, A.; Stein, E.G.; Ghosh, S.; Haramati, L.B. Variants and Anomalies of Thoracic Vasculature on Computed Tomographic Angiography in Adults. J. Comput. Assist. Tomogr. 2009, 33, 523–528. [Google Scholar] [CrossRef]
- Bhatia, K.; Ghabriel, M.N.; Henneberg, M. Anatomical Variations in the Branches of the Human Aortic Arch: A Recent Study of a South Australian Population. Folia Morphol. 2005, 64, 217–223. [Google Scholar]
- Bhattarai, C.; Poudel, P.P. Study on the Variation of Branching Pattern of Arch of Aorta in Nepalese. Nepal. Med. Coll. J. 2010, 12, 84–86. [Google Scholar]
- Boyacı, N. Multidetector Computed Tomography Evaluation of Aortic Arch and Branching Variants. Turk. J. Thorac. Cardiovasc. Surg. 2015, 23, 51–57. [Google Scholar] [CrossRef]
- Budhiraja, V.; Rastogi, R.; Jain, V.; Bankwar, V.; Raghuwanshi, S. Anatomical Variations in the Branching Pattern of Human Aortic Arch: A Cadaveric Study from Central India. ISRN Anat. 2013, 2013, 828969. [Google Scholar] [CrossRef]
- Burzotta, F.; Nerla, R.; Pirozzolo, G.; Aurigemma, C.; Niccoli, G.; Leone, A.M.; Saffioti, S.; Crea, F.; Trani, C. Clinical and Procedural Impact of Aortic Arch Anatomic Variants in Carotid Stenting Procedures. Catheter. Cardiovasc. Interv. 2015, 86, 480–489. [Google Scholar] [CrossRef]
- Carpenter, J.P.; Holland, G.A.; Golden, M.A.; Barker, C.F.; Lexa, F.J.; Gilfeather, M.; Schnall, M.D. Magnetic Resonance Angiography of the Aortic Arch. J. Vasc. Surg. 1997, 25, 145–151. [Google Scholar] [CrossRef]
- Celikyay, Z.R.Y.; Koner, A.E.; Celikyay, F.; Denız, C.; Acu, B.; Firat, M.M. Frequency and Imaging Findings of Variations in Human Aortic Arch Anatomy Based on Multidetector Computed Tomography Data. Clin. Imaging 2013, 37, 1011–1019. [Google Scholar] [CrossRef]
- Chen, X.; Qu, Y.; Peng, Z.; Lu, J.; Ma, X. Diagnosis of Congenital Aortic Arch Anomalies in Chinese Children by Multi-Detector Computed Tomography Angiography. J. Huazhong Univ. Sci. Technol. (Med. Sci.) 2013, 33, 447–451. [Google Scholar] [CrossRef]
- De Garis, C.F.; Black, I.H.; Riemenschneider, E.A. Patterns of the Aortic Arch in American White and Negro Stocks, with Comparative Notes on Certain Other Mammals. J. Anat. 1933, 67, 599–619. [Google Scholar]
- Demertzis, S.; Hurni, S.; Stalder, M.; Gahl, B.; Herrmann, G.; Van den Berg, J. Aortic Arch Morphometry in Living Humans. J. Anat. 2010, 217, 588–596. [Google Scholar] [CrossRef]
- Dumfarth, J.; Chou, A.S.; Ziganshin, B.A.; Bhandari, R.; Peterss, S.; Tranquilli, M.; Mojibian, H.; Fang, H.; Rizzo, J.A.; Elefteriades, J.A. Atypical Aortic Arch Branching Variants: A Novel Marker for Thoracic Aortic Disease. J. Thorac. Cardiovasc. Surg. 2015, 149, 1586–1592. [Google Scholar] [CrossRef]
- Ergun, O. Angiographic Evaluation of Branching Pattern and Anatomy of the Aortic Arch. Turk. Kardiyol. Dern. Ars. Arch. Turk. Soc. Cardiol. 2015, 43, 219–226. [Google Scholar] [CrossRef]
- Faggioli, G.L.; Ferri, M.; Freyrie, A.; Gargiulo, M.; Fratesi, F.; Rossi, C.; Manzoli, L.; Stella, A. Aortic Arch Anomalies Are Associated with Increased Risk of Neurological Events in Carotid Stent Procedures. Eur. J. Vasc. Endovasc. Surg. 2007, 33, 436–441. [Google Scholar] [CrossRef]
- Fazal, M.; Sherke, A.; Suseelamma, D. The variations in the branching pattern of arch of aorta and its embryological correlation. Panacea J. Med. Sci. 2012, 2, 29–31. [Google Scholar]
- Glew, D.; Hartnell, G.G. The Right Aortic Arch Revisited. Clin. Radiol. 1991, 43, 305–307. [Google Scholar] [CrossRef]
- Hastreiter, A.R.; D’Cruz, I.A.; Cantez, T.; Namin, E.P.; Licata, R. Right-Sided Aorta. I. Occurrence of Right Aortic Arch in Various Types of Congenital Heart Disease. II. Right Aortic Arch, Right Descending Aorta, and Associated Anomalies. Heart 1966, 28, 722–739. [Google Scholar] [CrossRef]
- Huapaya, J.A.; Chávez-Trujillo, K.; Trelles, M.; Carbajal, R.D.; Espadin, R.F. Anatomic Variations of the Branches of the Aortic Arch in a Peruvian Population. Medwave 2015, 15, e6194. [Google Scholar] [CrossRef]
- Ikeno, Y.; Koide, Y.; Matsueda, T.; Yamanaka, K.; Inoue, T.; Ishihara, S.; Nakayama, S.; Tanaka, H.; Sugimoto, K.; Okita, Y. Anatomical Variations of Aortic Arch Vessels in Japanese Patients with Aortic Arch Disease. Gen. Thorac. Cardiovasc. Surg. 2019, 67, 219–226. [Google Scholar] [CrossRef]
- Jakanani, G.C.; Adair, W. Frequency of Variations in Aortic Arch Anatomy Depicted on Multidetector CT. Clin. Radiol. 2010, 65, 481–487. [Google Scholar] [CrossRef]
- Jalali Kondori, B.; Asadi, M.H.; Rahimian, E.; Tahsini, M.R. Anatomical variations in aortic arch branching pattern. Arch. Iran. Med. 2016, 19, 72–74. [Google Scholar]
- Karacan, A.; Türkvatan, A.; Karacan, K. Anatomical Variations of Aortic Arch Branching: Evaluation with Computed Tomographic Angiography. Cardiol. Young 2014, 24, 485–493. [Google Scholar] [CrossRef]
- Kodikara, I.; Gamage, D.; De Soyza, S.; Ilayperuma, I. Computed Tomographic Analysis of Aortic Arch Branching Patterns: Revisited. SN Compr. Clin. Med. 2022, 4, 17. [Google Scholar] [CrossRef]
- Lale, P.; Toprak, U.; Yagız, G.; Kaya, T.; Uyanık, S.A. Variations in the Branching Pattern of the Aortic Arch Detected with Computerized Tomography Angiography. Adv. Radiol. 2014, 2014, 969728. [Google Scholar] [CrossRef]
- Liechty, J.D.; Shields, T.W.; Anson, B.J. Variations Pertaining to the Aortic Arches and Their Branches; with Comments on Surgically Important Types. Q. Bull. Northwest Univ. Med. Sch. 1957, 31, 136–143. [Google Scholar] [PubMed]
- Makhanya, N.Z.; Mamogale, R.T.; Khan, N. Variants of the left aortic arch branches. S. Afr. J. Radiol. 2004, 8, 10. [Google Scholar] [CrossRef]
- McDonald, J.J.; Anson, B.J. Variations in the Origin of Arteries Derived from the Aortic Arch, in American Whites and Negroes. Am. J. Phys. Anthr. 1940, 27, 91–107. [Google Scholar] [CrossRef]
- Moorehead, P.A.; Kim, A.H.; Miller, C.P.; Kashyap, T.V.; Kendrick, D.E.; Kashyap, V.S. Prevalence of Bovine Aortic Arch Configuration in Adult Patients with and without Thoracic Aortic Pathology. Ann. Vasc. Surg. 2016, 30, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.; Schmitz, B.L.; Pauls, S.; Schick, M.; Röhrer, S.; Kapapa, T.; Schlötzer, W. Variations of the Aortic Arch—A Study on the Most Common Branching Patterns. Acta Radiol. 2011, 52, 738–742. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, A.G.; Allouh, M.Z.; Ghaida, J.H.A.; Al-Omari, M.H.; Mahmoud, W.A. Branching Patterns of the Aortic Arch: A Computed Tomography Angiography-Based Study. Surg. Radiol. Anat. 2017, 39, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Natsis, K.I.; Tsitouridis, I.A.; Didagelos, M.V.; Fillipidis, A.A.; Vlasis, K.G.; Tsikaras, P.D. Anatomical Variations in the Branches of the Human Aortic Arch in 633 Angiographies: Clinical Significance and Literature Review. Surg. Radiol. Anat. 2009, 31, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S.R.; Pai, M.M.; Prabhu, L.V.; D’Costa, S.; Shetty, P. Anatomical organization of aortic arch variations in the India: Embryological basis and review. J. Vasc. Bras. 2006, 5, 95–100. [Google Scholar] [CrossRef]
- Odey, P.A.; Akpaso, M.I.; Ogan, C.A.; Ajang, C.U.; Ikpa, J.O. Variations in the Branching Pattern and Position of the Aortic Arch in Southern Nigeria using Computed Tomography Scans: A Retrospective Study. J. Biomed. Stud. 2021, 1, 102. [Google Scholar]
- Ogeng’o, J.A.; Olabu, B.O.; Kilonzi, J.P. Pattern of Aortic Aneurysms in an African Country. J. Thorac. Cardiovasc. Surg. 2010, 140, 797–800. [Google Scholar] [CrossRef] [PubMed]
- Pandalai, U.; Pillay, M.; Moorthy, S.; Sukumaran, T.T.; Ramakrishnan, S.; Gopalakrishnan, A.; Gopalakrishna Pillai, A.K. Anatomical Variations of the Aortic Arch: A Computerized Tomography-Based Study. Cureus 2021, 13, e13115. [Google Scholar] [CrossRef]
- Pandian, D.K.; Radha, K.; Sundaravadhanam, K.V.K. Study on Branching Pattern of Arch of Aorta in South Indian Population. Int. J. Anat. Res. 2014, 2, 673–676. [Google Scholar] [CrossRef]
- Patil, S.T.; Meshram, M.M.; Kamdi, N.Y.; Kasote, A.P.; Parchand, M.P. Study on Branching Pattern of Aortic Arch in Indian. Anat. Cell Biol. 2012, 45, 203. [Google Scholar] [CrossRef] [PubMed]
- Piyavisetpat, N.; Thaksinawisut, P.; Tumkosit, M. Aortic arch branches’ variations detected on chest CT. Asian Biomed. 2011, 5, 817–823. [Google Scholar]
- Prince, M.R.; Narasimham, D.L.; Jacoby, W.T.; Williams, D.M.; Cho, K.J.; Marx, M.V.; Deeb, G.M. Three-Dimensional Gadolinium-Enhanced MR Angiography of the Thoracic Aorta. Am. J. Roentgenol. 1996, 166, 1387–1397. [Google Scholar] [CrossRef] [PubMed]
- Tapia, G.P.; Zhu, X.; Xu, J.; Liang, P.; Su, G.; Liu, H.; Liu, Y.; Shu, L.; Liu, S.; Huang, C. Incidence of Branching Patterns Variations of the Arch in Aortic Dissection in Chinese Patients. Medicine 2015, 94, e795. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, A.M.; Sobh, D.M.; Ashamallah, G.A.; Batouty, N.M. Prevalence and Types of Aortic Arch Variants and Anomalies in Congenital Heart Diseases. Acad. Radiol. 2019, 26, 930–936. [Google Scholar] [CrossRef] [PubMed]
- Terzioğlu, E.; Damar, Ç. Evaluation of Aortic Arch Morphologies by Computed Tomographic Angiography in Turkish Population. Turk. J. Thorac. Cardiovasc. Surg. 2022, 30, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Uchino, A.; Saito, N.; Okada, Y.; Kozawa, E.; Nishi, N.; Mizukoshi, W.; Nakajima, R.; Takahashi, M.; Watanabe, Y. Variation of the Origin of the Left Common Carotid Artery Diagnosed by CT Angiography. Surg. Radiol. Anat. 2013, 35, 339–342. [Google Scholar] [CrossRef] [PubMed]
- Vinnakota, S.; Bhattam, N.R. A Study on the Anatomical Organization of the Aortic Arch Anomalies. J. Clin. Diagn. Res. 2012, 6, 1127. [Google Scholar]
- Vučurević, G.; Marinković, S.; Puškaš, L.; Kovačević, I.; Tanasković, S.; Radak, D.; Ilić, A. Anatomy and Radiology of the Variations of Aortic Arch Branches in 1266 Patients. Folia Morphol. 2013, 72, 113–122. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, J.; Xin, S. Morphologic Features of the Aortic Arch and Its Branches in the Adult Chinese Population. J. Vasc. Surg. 2016, 64, 1602–1608.e1. [Google Scholar] [CrossRef]
- Williams, G.D.; Aff, H.M.; Schmeckebier, M.; Edmonds, H.W.; Graul, E.G. Variations in the Arrangement of the Branches Arising from the Aortic Arch in American Whites and Negroes. Anat. Rec. 1932, 54, 247–251. [Google Scholar] [CrossRef]
- Williams, G.D.; Edmonds, H.W. Variations in the Arrangement of the Branches Arising from the Aortic Arch in American Whites and Negroes (a Second Study). Anat. Rec. 1935, 62, 139–146. [Google Scholar] [CrossRef]
- Yeri, L.A.; Gómez, J.E.; Fontaneto, S.; Espósito, M. Variation of the origin of aortic arch branches: In relationship with plates of atheroma. Int. J. Morphol. 2011, 29, 182–186. [Google Scholar] [CrossRef]
- Zamir, M.; Sinclair, P. Continuum Analysis of Common Branching Patterns in the Human Arch of the Aorta. Anat. Embryol. 1990, 181, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.; Gartlehner, G.; Grant, M.; Shamliyan, T.; Sedrakyan, A.; Wilt, T.J.; Griffith, L.; Oremus, M.; Raina, P.; Ismaila, A.; et al. Conducting Quantitative Synthesis When Comparing Medical Interventions: AHRQ and the Effective Health Care Program. J. Clin. Epidemiol. 2011, 64, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Rathke, H. Über die Entwicklung der Arterien, welche bei den Säugetieren von dem Bogen der Aorta ausgehen. Arch. Anat. Physiol. Wiss. Med. 1843, 9, 276–302. [Google Scholar]
- Jin, Z.W.; Yamada, T.; Kim, J.H.; Rodríguez-Vázquez, J.F.; Murakami, G.; Arakawa, K. Pathogenesis of Solitary Right Aortic Arch: A Mass Effect Hypothesis Based on Observations of Serial Human Embryonic Sections. Cardiol. Young 2017, 27, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Inaba, Y.; Ito, T.; Hayashi, S.; Takahashi, T.; Misumi, T.; Shimizu, H. A Right Aortic Arch with Abnormal Origin of the Left Vertebral Artery from the Left Common Carotid Artery. J. Vasc. Surg. Cases Innov. Tech. 2018, 4, 128. [Google Scholar] [CrossRef] [PubMed]
- Tong, E.; Rizvi, T.; Hagspiel, K.D. Complex Aortic Arch Anomaly: Right Aortic Arch with Aberrant Left Subclavian Artery, Fenestrated Proximal Right and Duplicated Proximal Left Vertebral Arteries—CT Angiography Findings and Review of the Literature. Neuroradiol. J. 2015, 28, 396–403. [Google Scholar] [CrossRef]
- Luetmer, P.H.; Miller, G.M. Right Aortic Arch with Isolation of the Left Subclavian Artery: Case Report and Review of the Literature. Mayo Clin. Proc. 1990, 65, 407–413. [Google Scholar] [CrossRef]
- Singh, B.; Satyapal, K.S.; Moodley, J.; Rajaruthnam, P. Right Aortic Arch with Isolated Left Brachiocephalic Artery. Clin. Anat. 2001, 14, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Grollman, J.H.; Bedynek, J.L.; Henderson, H.S.; Hall, R.J. Right Aortic Arch with an Aberrant Retroesophageal Innominate Artery: Angiographic Diagnosis. Radiology 1968, 90, 782–783. [Google Scholar] [CrossRef] [PubMed]
- Cinà, C.S.; Althani, H.; Pasenau, J.; Abouzahr, L. Kommerell’s Diverticulum and Right-Sided Aortic Arch: A Cohort Study and Review of the Literature. J. Vasc. Surg. 2004, 39, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Kanne, J.P.; Godwin, J.D. Right Aortic Arch and Its Variants. J. Cardiovasc. Comput. Tomogr. 2010, 4, 293–300. [Google Scholar] [CrossRef] [PubMed]
- D’Antonio, F.; Khalil, A.; Zidere, V.; Carvalho, J.S. Fetuses with Right Aortic Arch: A Multicenter Cohort Study and Meta-analysis. Ultrasound Obstet. Gynecol. 2016, 47, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Faggioni, L.; Gabelloni, M.; Napoli, V.; Iorio, F.; Chella, A.; Caramella, D. Thrombosis of Kommerell’s Diverticulum with Subclavian Steal Phenomenon in a Patient with Non-Small Cell Lung Carcinoma under Chemotherapy. Eur. J. Radiol. Open 2016, 3, 191–194. [Google Scholar] [CrossRef]
- Hurlock, G.S.; Higashino, H.; Mochizuki, T. History of Cardiac Computed Tomography: Single to 320-Detector Row Multislice Computed Tomography. Int. J. Cardiovasc. Imaging 2009, 25, 31–42. [Google Scholar] [CrossRef]

| Morphology | Moderator | Subgroup | Studies’ Number (k=) | Prevalence [95%-CI] | p-Value |
|---|---|---|---|---|---|
| RAA Type 1 | Type of Study | Imaging | 42 | 0.0021 [0.0006; 0.0043] | 0.1882 |
| Cadaveric | 20 | 0.0000 [0.0000; 0.0000] | |||
| RAA Type 1 | Nationality | Europe | 12 | 0.0035 [0.0002; 0.0094] | 0.2989 |
| Asia | 28 | 0.0000 [0.0000; 0.0005] | |||
| America | 16 | 0.0000 [0.0000; 0.0003] | |||
| Africa | 5 | 0.0085 [0.0000; 0.0554] | |||
| Oceania | 1 | 0.0000 [0.0000; 0.0211] | |||
| RAA Type 1 | Sample Size | 0–99 cases | 18 | 0.0000 [0.0000; 0.0015] | 0.1830 |
| 100–299 cases | 12 | 0.0004 [0.0000; 0.0022] | |||
| 300–999 cases | 16 | 0.0057 [0.0003; 0.0161] | |||
| 1000 and above cases | 16 | 0.0012 [0.0005; 0.0021] | |||
| RAA Type 2 | Type of Study | Imaging | 40 | 0.0000 [0.0000; 0.0001] | 0.5364 |
| Cadaveric | 20 | 0.0000 [0.0000; 0.0000] | |||
| RAA Type 2 | Nationality | Europe | 10 | 0.0000 [0.0000; 0.0000] | 0.0462 |
| Asia | 28 | 0.0000 [0.0000; 0.0000] | |||
| America | 16 | 0.0000 [0.0000; 0.0000] | |||
| Africa | 5 | 0.0000 [0.0000; 0.0011] | |||
| Oceania | 1 | 0.0000 [0.0000; 0.0211] | |||
| RAA Type 2 | Sample Size | 0–99 cases | 18 | 0.0000 [0.0000; 0.0010] | 0.1193 |
| 100–299 cases | 12 | 0.0000 [0.0000; 0.0012] | |||
| 300–999 cases | 14 | 0.0002 [0.0000; 0.0009] | |||
| 1000 and above cases | 16 | 0.0004 [0.0001; 0.0008] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Triantafyllou, G.; Melissanidis, S.; Vlychou, M.; Tsakotos, G.; Pantazis, N.; Vassiou, K.; Tsiouris, C.; Piagkou, M. Right-Sided Aortic Arch: A Computed Tomography Angiography Investigation, A Systematic Review with Meta-Analysis. J. Clin. Med. 2024, 13, 3105. https://doi.org/10.3390/jcm13113105
Triantafyllou G, Melissanidis S, Vlychou M, Tsakotos G, Pantazis N, Vassiou K, Tsiouris C, Piagkou M. Right-Sided Aortic Arch: A Computed Tomography Angiography Investigation, A Systematic Review with Meta-Analysis. Journal of Clinical Medicine. 2024; 13(11):3105. https://doi.org/10.3390/jcm13113105
Chicago/Turabian StyleTriantafyllou, George, Savvas Melissanidis, Marianna Vlychou, George Tsakotos, Nikos Pantazis, Katerina Vassiou, Christos Tsiouris, and Maria Piagkou. 2024. "Right-Sided Aortic Arch: A Computed Tomography Angiography Investigation, A Systematic Review with Meta-Analysis" Journal of Clinical Medicine 13, no. 11: 3105. https://doi.org/10.3390/jcm13113105
APA StyleTriantafyllou, G., Melissanidis, S., Vlychou, M., Tsakotos, G., Pantazis, N., Vassiou, K., Tsiouris, C., & Piagkou, M. (2024). Right-Sided Aortic Arch: A Computed Tomography Angiography Investigation, A Systematic Review with Meta-Analysis. Journal of Clinical Medicine, 13(11), 3105. https://doi.org/10.3390/jcm13113105








