Diastolic Heart Failure Mechanisms and Assessment Revisited
Abstract
1. Introduction
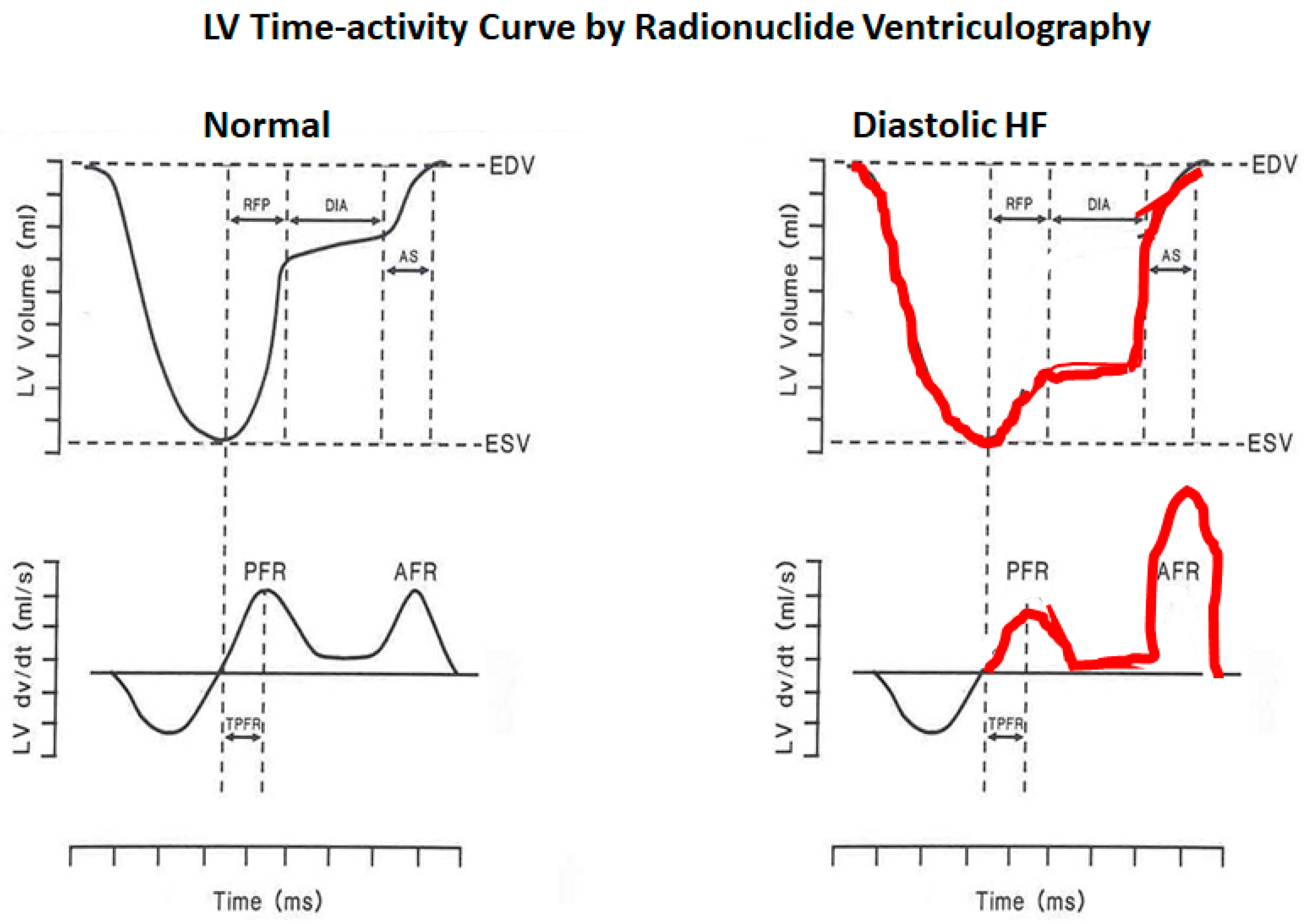
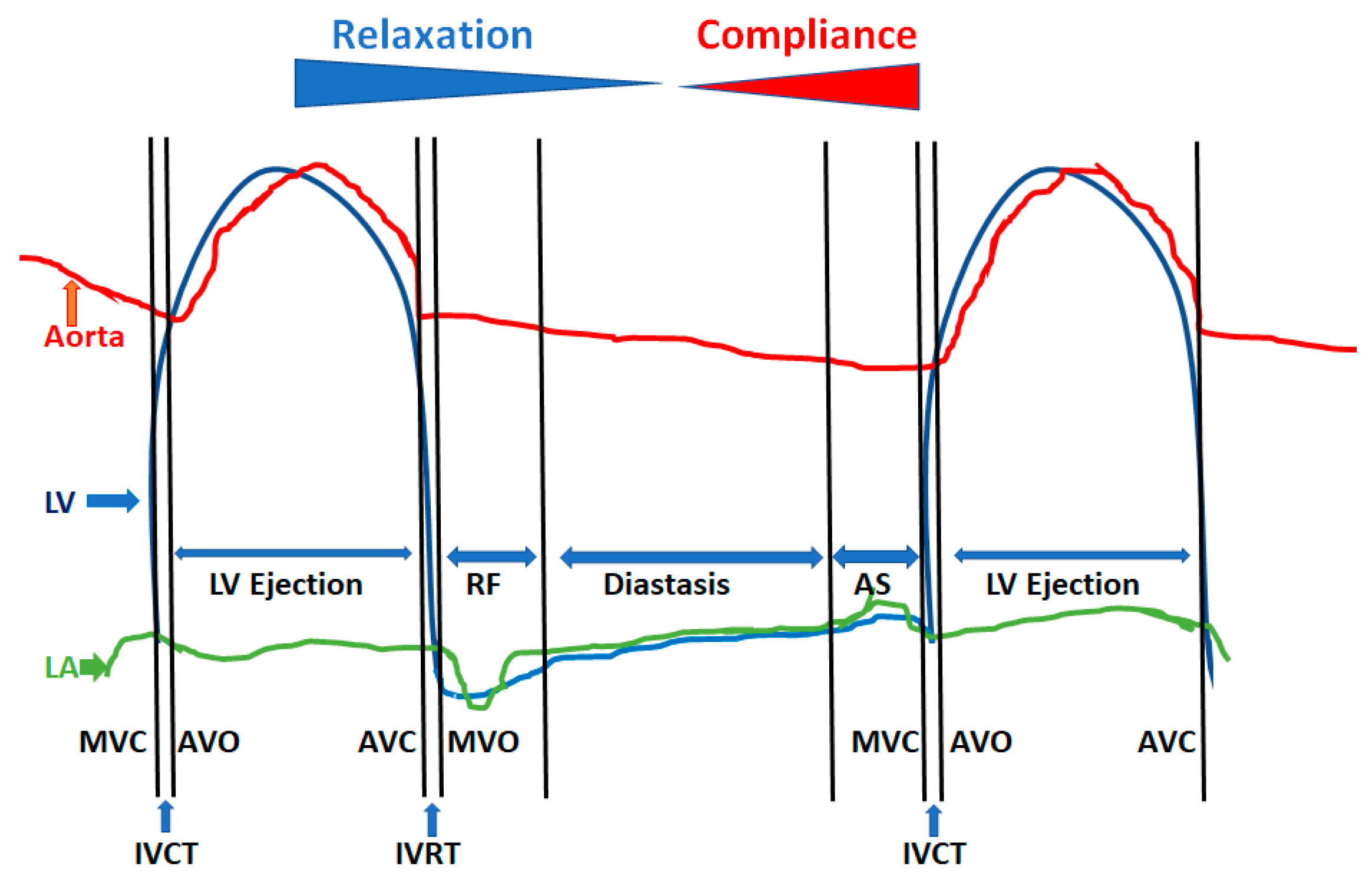
2. Physiology of Diastole
- LV isovolumic relaxation from aortic valve closure to mitral valve opening
- Early phase of rapid passive filling
- Diastasis
- Atrial systole
2.1. LV Relaxation Process
2.2. Early Diastolic Suction Due to LV Recoil
2.3. Left Ventricular Compliance
2.4. Myocardial Stiffness
2.5. Erectile Effects of Coronary Filling
2.6. Factors Affecting Pericardium or Intrapericardial Pressure
2.7. Atrial Systolic Function
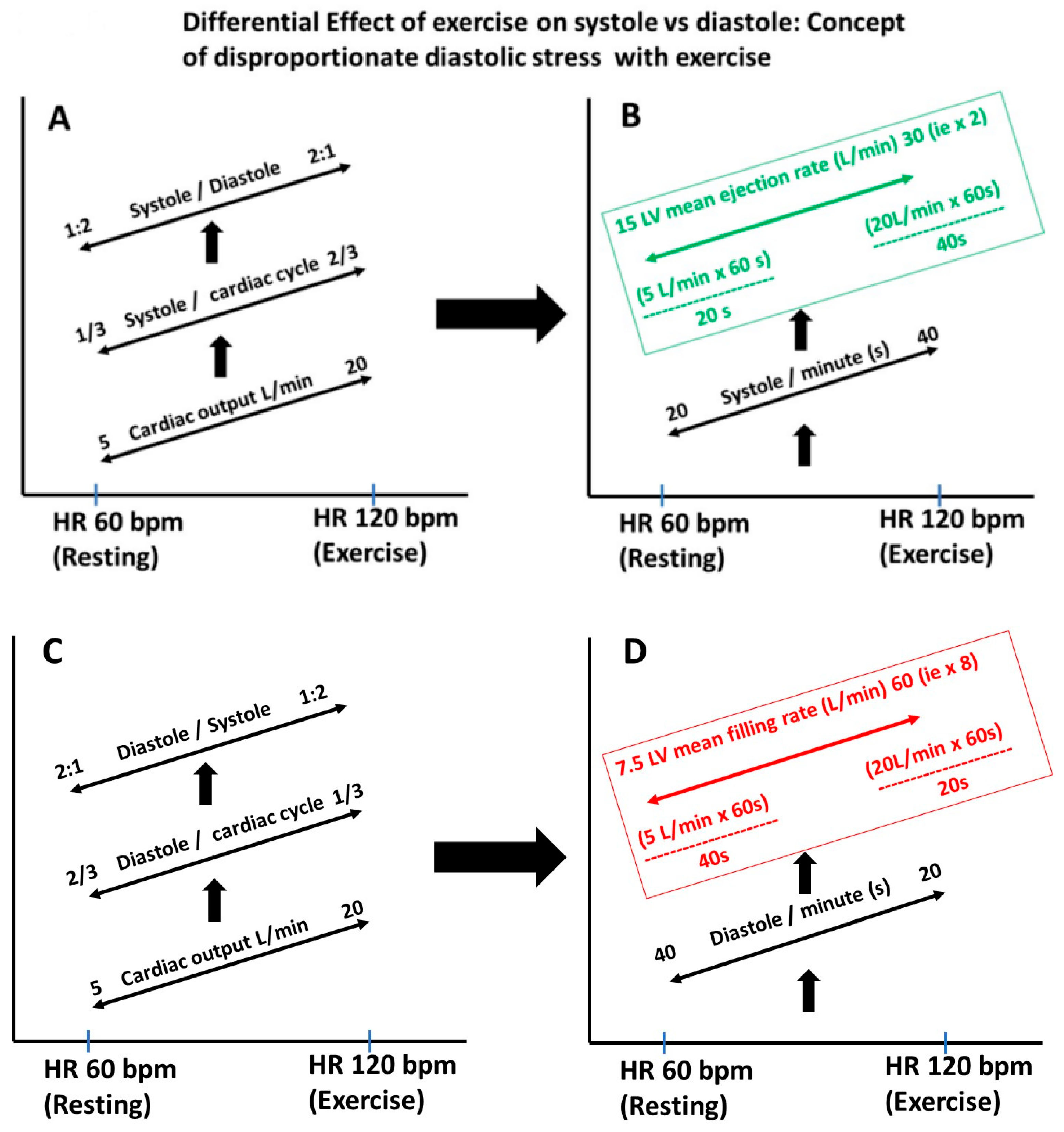
2.8. Heart Rate, Diastolic Filling Rate and Diastolic Stress
2.9. Functional and Structural Basis of Diastolic Impairment
3. Assessment of Diastole
3.1. Invasive Measure
3.1.1. Measurement of LV Relaxation
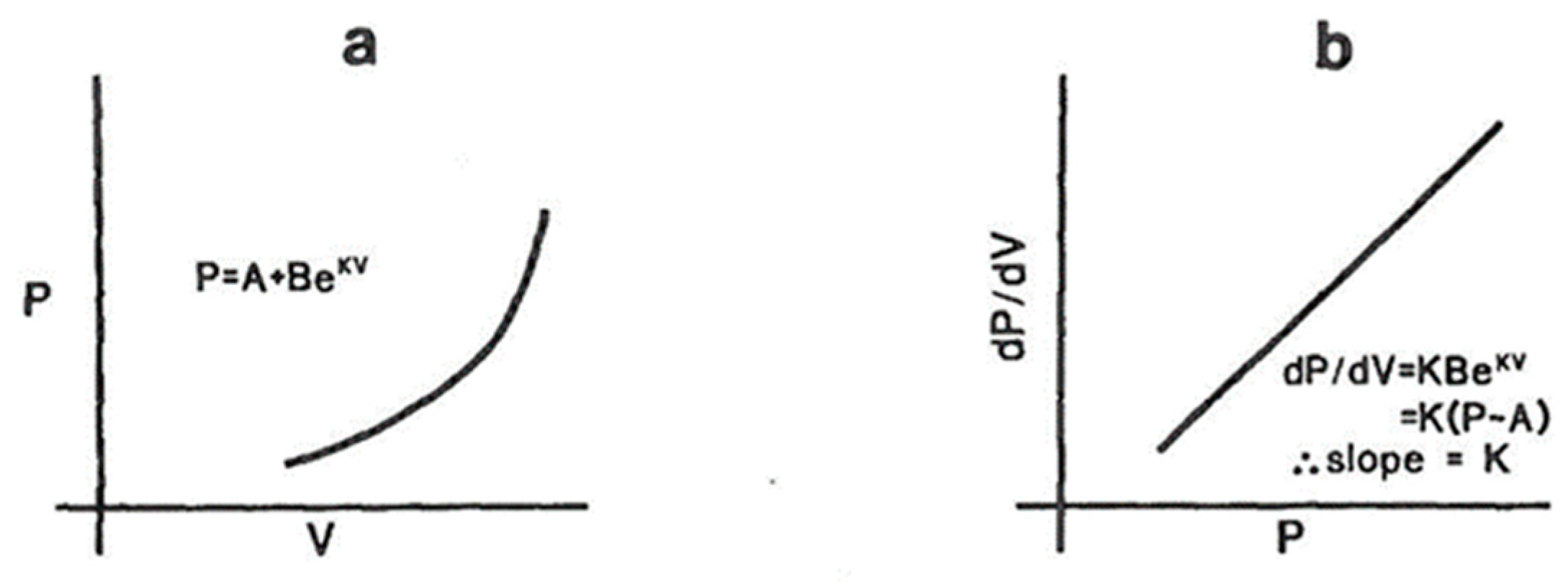
3.1.2. Measurement of Passive Elastic Properties of LV
3.1.3. Measurement of Myocardial Stiffness
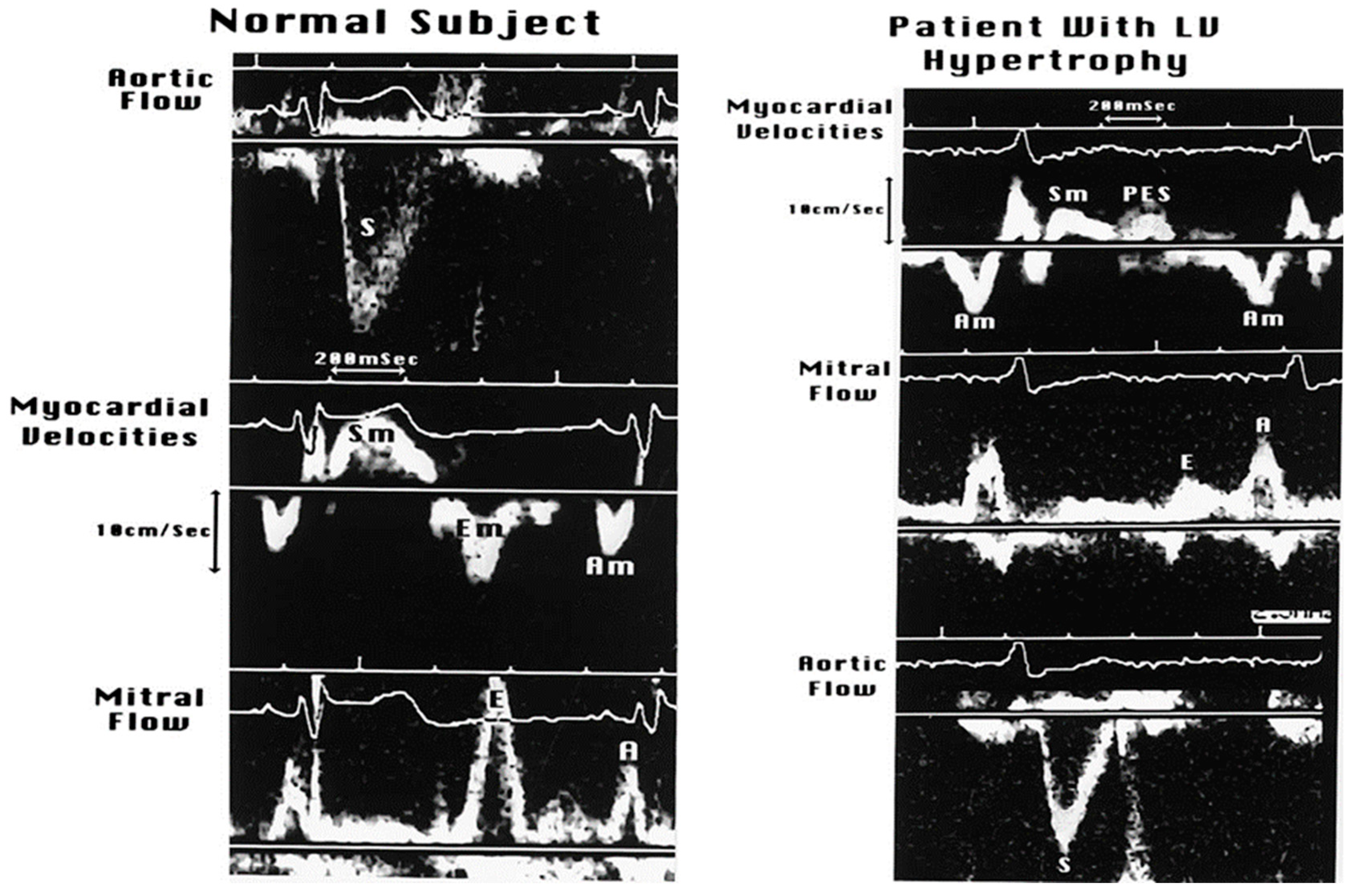
3.2. Echo-Doppler Techniques
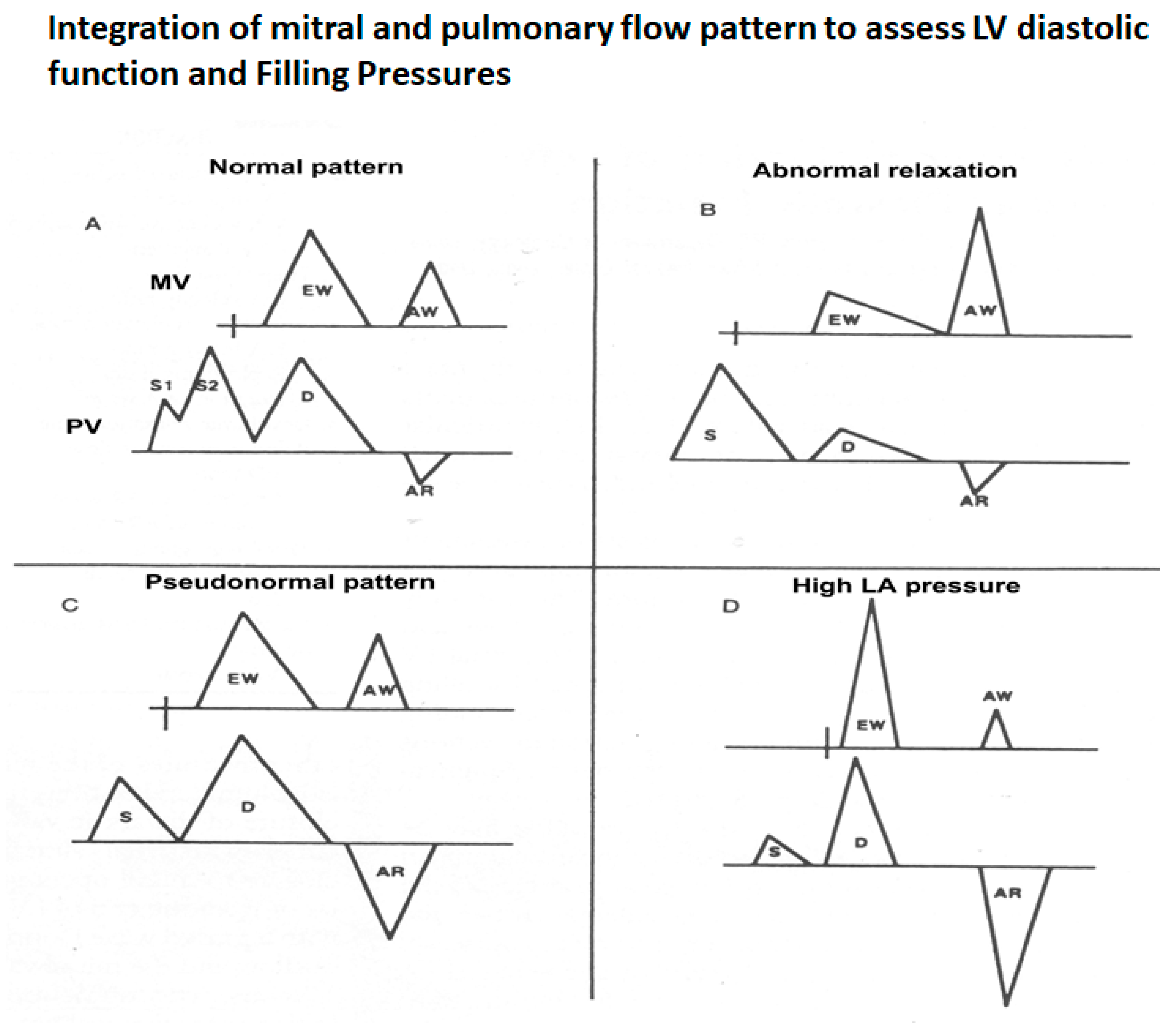
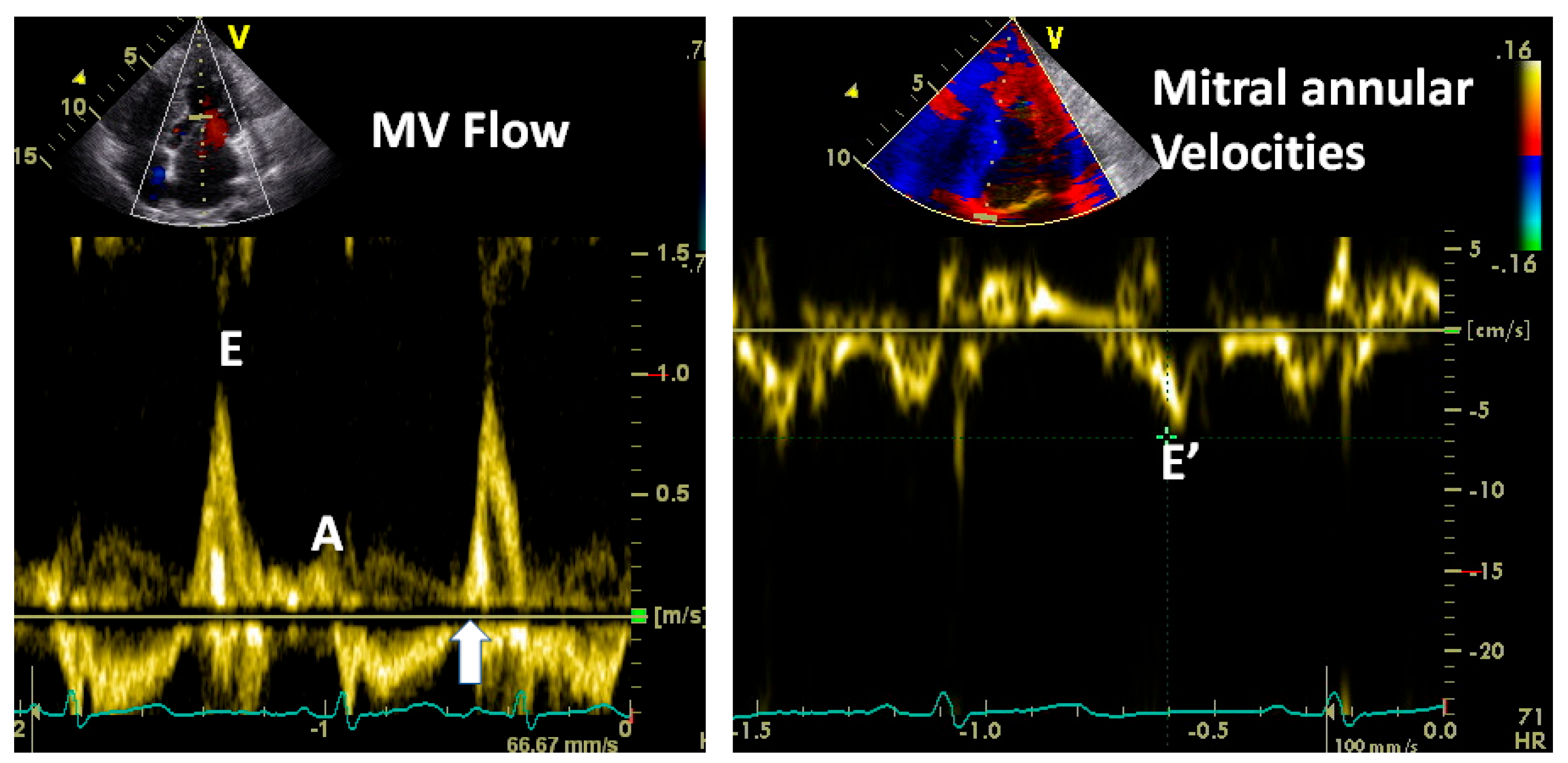
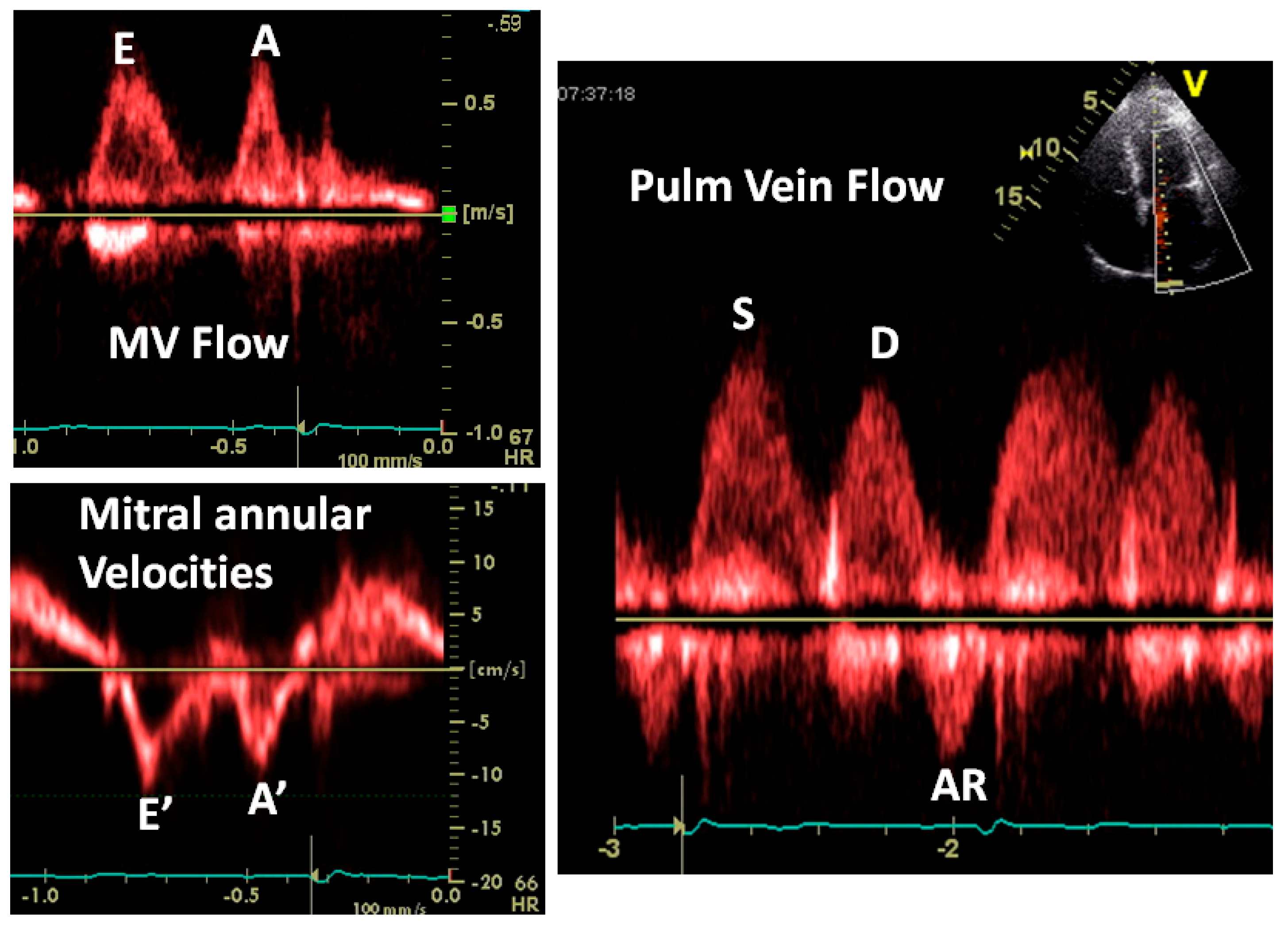
3.2.1. Transmitral Flow
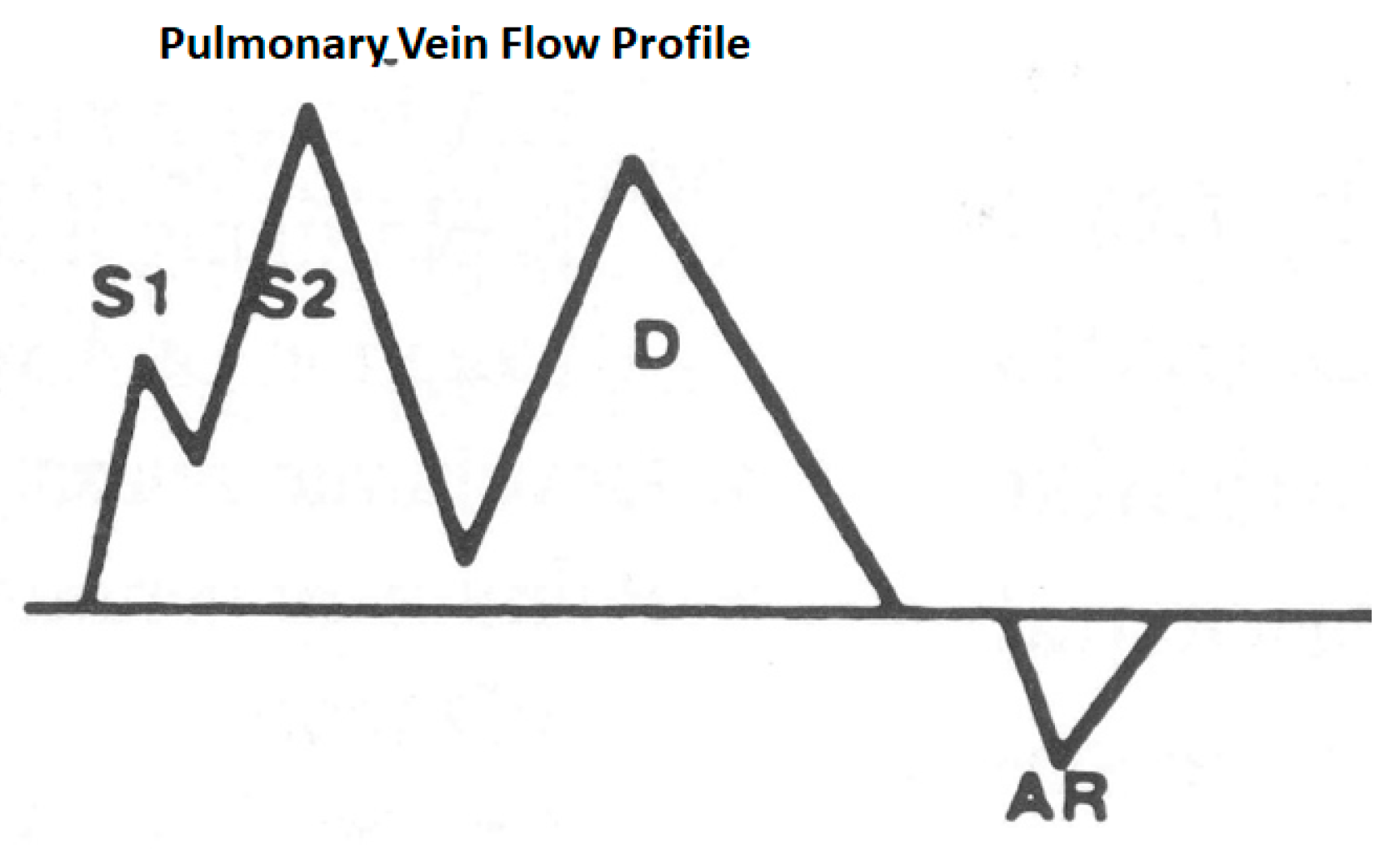
3.2.2. Pulmonary Vein Flow
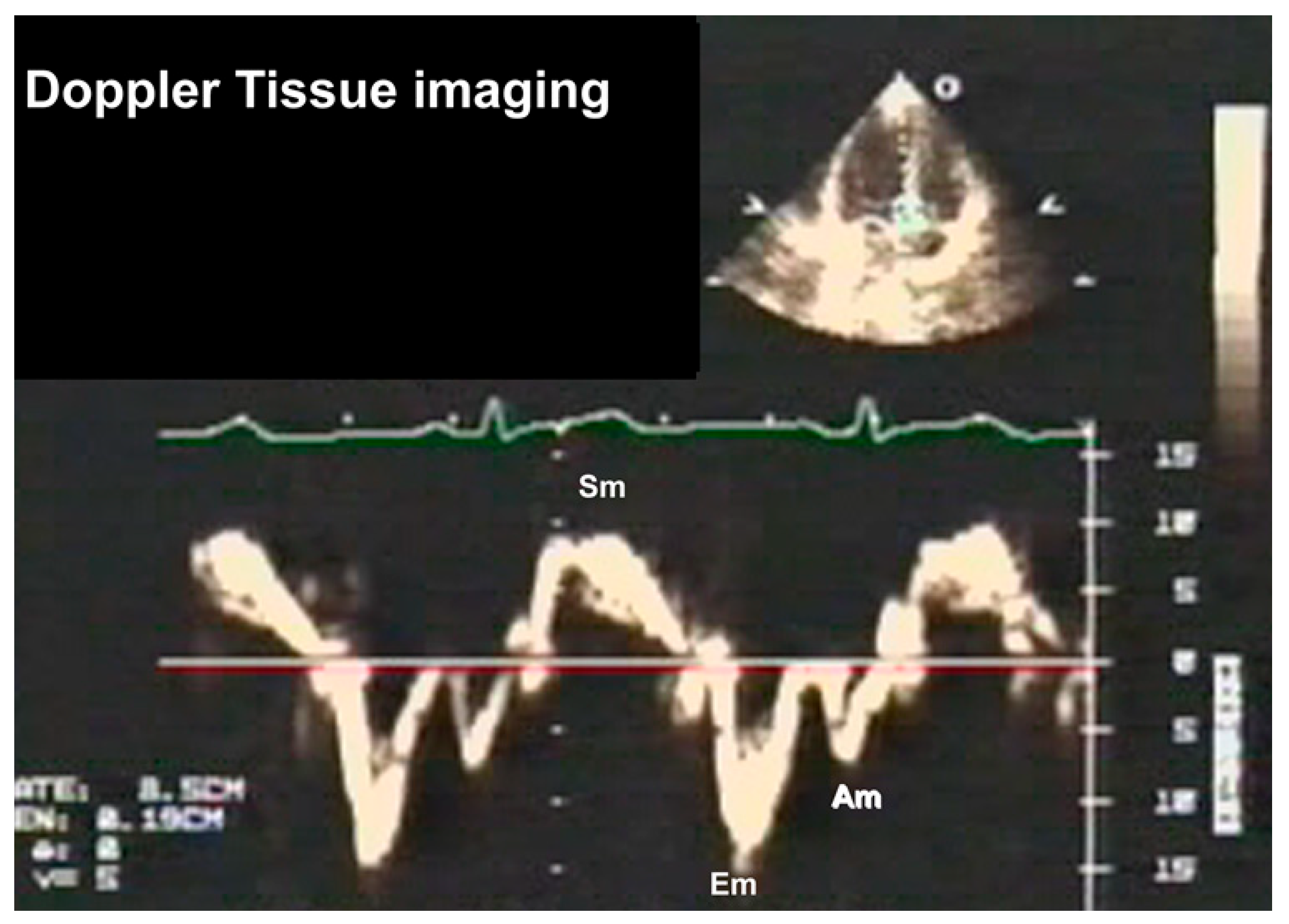
3.2.3. Doppler Tissue Imaging (DTI)
3.2.4. Left Atrial Volume and Dynamics
3.2.5. Mitral E and A Wave Propagation in LV and Their Changing Profiles as Measures of LV Relaxation and Stiffness
3.3. Assessment of Myocardial Structure
4. Summary and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dougherty, A.H.; Naccarelli, G.V.; Gray, E.L.; Hicks, C.H.; Goldstein, R.A. Congestive heart failure with normal systolic function. Am. J. Cardiol. 1984, 54, 778–782. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.M.; Pai, R.G. Diastolic heart failure. Curr. Probl. Cardiol. 1992, 17, 781–868. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Usman, M.S.; Anker, M.S.; Butler, J.; Böhm, M.; Abraham, W.T.; Adamo, M.; Chopra, V.K.; Cicoira, M.; Cosentino, F.; et al. Patient phenotype profiling in heart failure with preserved ejection fraction to guide therapeutic decision making. A scientific statement of the Heart Failure Association, the European Heart Rhythm Association of the European Society of Cardiology, and the European Society of Hypertension. Eur. J. Heart Fail. 2023, 25, 936–955. [Google Scholar] [PubMed]
- Vasan, R.S.; Benjamin, E.J.; Levy, D. Prevalence, clinical features and prognosis of diastolic heart failure: An epidemiologic perspective. J. Am. Coll. Cardiol. 1995, 26, 1565–1574. [Google Scholar] [CrossRef] [PubMed]
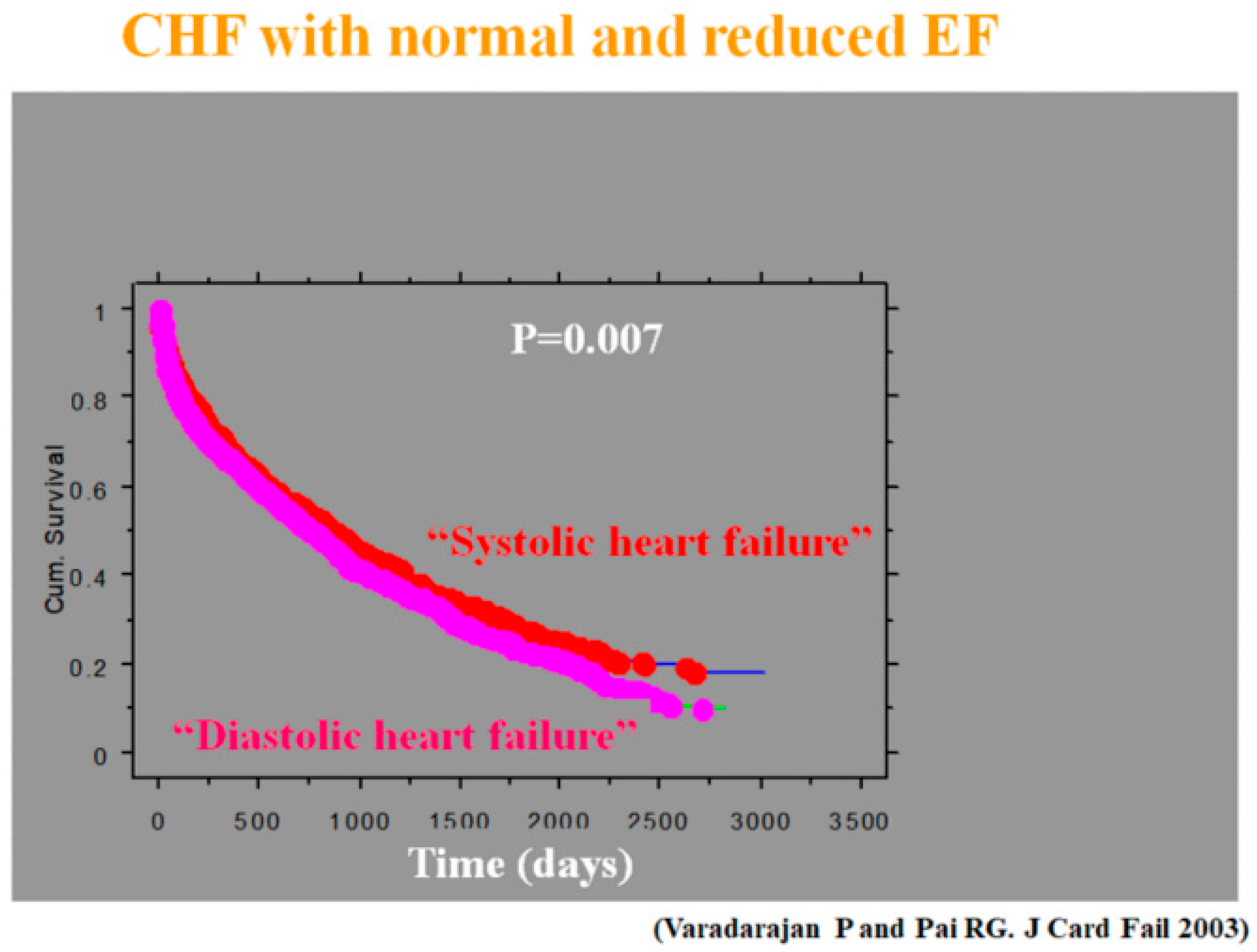
- Varadarajan, P.; Pai, R.G. Prognosis of congestive heart failure in patients with normal versus reduced ejection fractions: Results from a cohort of 2258 hospitalized patients. J. Card. Fail. 2003, 9, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Brutsaert, D.L.; Sys, S.U. Relaxation and diastole of the heart. Physiol. Rev. 1989, 69, 1228–1315. [Google Scholar] [CrossRef]
- Brutsaert, D.L.; Housmans, P.R.; Goethals, M.A. Dual control of relaxation. Its role in the ventricular function in the mammalian heart. Circ. Res. 1980, 47, 637–652. [Google Scholar] [CrossRef] [PubMed]
- Brutsaert, D.L.; Rademakers, F.E.; Sys, S.U. Triple control of relaxation: Implications in cardiac disease. Circulation 1984, 69, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Rapezzi, C.; Aimo, A.; Barison, A.; Emdin, M.; Porcari, A.; Linhart, A.; Keren, A.; Merlo, M.; Sinagra, G. Restrictive cardiomyopathy: Definition and diagnosis. Eur. Heart J. 2022, 43, 4679–4693. [Google Scholar] [CrossRef] [PubMed]
- Gaasch, W.H.; Levine, H.J.; Quinones, M.A.; Alexander, J.K. Left ventricular compliance: Mechanisms and clinical implications. Am. J. Cardiol. 1976, 38, 645–653. [Google Scholar] [CrossRef]
- Yettram, A.L.; Grewal, B.S.; Dawson, J.R.; Gibson, D.G. Factors influencing left-ventricular stiffness. J. Biomed. Eng. 1992, 14, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.H.; Gilmore, J.P.; Sarnoff, S.J. The transport function of the atrium. Factors influencing the relation between mean left atrial pressure and left ventricular end diastolic pressure. Am. J. Cardiol. 1962, 9, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Galderisi, M.; Cosyns, B.; Edvardsen, T.; Cardim, N.; Delgado, V.; Di Salvo, G.; Donal, E.; Sade, L.E.; Ernande, L.; Garbi, M.; et al. Standardization of adult transthoracic echocardiography reporting in agreement with recent chamber quantification, diastolic function, and heart valve disease recommendations: An expert consensus document of the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 1301–1310. [Google Scholar] [PubMed]
- Kumar, S.; Desai, N.; Gona, O.J.; Kumar, V.; Madhu, B. Impact of Updated 2016 ASE/EACVI VIS-À-VIS 2009 ASE Recommendation on the Prevalence of Diastolic Dysfunction and LV Filling Pressures in Patients with Preserved Ejection Fraction. J. Cardiovasc. Imaging 2021, 29, 31–43. [Google Scholar]
- Pai, R.G.; Shah, P.M. Echocardiographic and other noninvasive measurements of cardiac hemodynamics and ventricular function. Curr. Probl. Cardiol. 1995, 20, 681–770. [Google Scholar] [PubMed]
- Gorcsan, J., 3rd. Tissue Doppler echocardiography. Curr. Opin. Cardiol. 2000, 15, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Pritchett, A.M.; Jacobsen, S.J.; Mahoney, D.W.; Rodeheffer, R.J.; Bailey, K.R.; Redfield, M.M. Left atrial volume as an index of left atrial size: A population-based study. J. Am. Coll. Cardiol. 2003, 41, 1036–1043. [Google Scholar] [CrossRef]
- Benfari, G.; Essayagh, B.; Nistri, S.; Maalouf, J.; Rossi, A.; Thapa, P.; Michelena, H.I.; Enriquez-Sarano, M. Left Atrial Volumetric/Mechanical Coupling Index: A Novel Predictor of Outcome in Heart Failure with Reduced Ejection Fraction. Circ. Cardiovasc. Imaging 2021, 14, e011608. [Google Scholar] [CrossRef] [PubMed]
- Stugaard, M.; Smiseth, O.A.; Risöe, C.; Ihlen, H. Intraventricular early diastolic filling during acute myocardial ischemia, assessment by multigated color m-mode Doppler echocardiography. Circulation 1993, 88, 2705–2713. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.M.; Piechnik, S.K. CMR Parametric Mapping as a Tool for Myocardial Tissue Characterization. Korean Circ. J. 2020, 50, 658–676. [Google Scholar] [CrossRef] [PubMed]
- Messroghli, D.R.; Moon, J.C.; Ferreira, V.M.; Grosse-Wortmann, L.; He, T.; Kellman, P.; Mascherbauer, J.; Nezafat, R.; Salerno, M.; Schelbert, E.B.; et al. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: A consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). J. Cardiovasc. Magn. Reson. 2017, 19, 75. [Google Scholar] [PubMed]
- Fontana, M.; Martinez-Naharro, A.; Chacko, L.; Rowczenio, D.; Gilbertson, J.A.; Whelan, C.J.; Strehina, S.; Lane, T.; Moon, J.; Hutt, D.F.; et al. Reduction in CMR Derived Extracellular Volume with Patisiran Indicates Cardiac Amyloid Regression. JACC Cardiovasc. Imaging 2021, 14, 189–199. [Google Scholar] [CrossRef]
- Scully, P.R.; Bastarrika, G.; Moon, J.C.; Treibel, T.A. Myocardial Extracellular Volume Quantification by Cardiovascular Magnetic Resonance and Computed Tomography. Curr. Cardiol. Rep. 2018, 20, 15. [Google Scholar] [CrossRef] [PubMed]
- Klem, I. Expanding CT Application to Myocardial Tissue Characterization. JACC Cardiovasc. Imaging 2016, 9, 846–848. [Google Scholar] [CrossRef] [PubMed]
- Weir-McCall, J.R.; Alabed, S. Myocardial Tissue Characterization With CT-Derived Extracellular Volume: Closing the Gap With CMR? JACC Cardiovasc. Imaging 2023, 16, 1318–1320. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Lee, W.N.; Konofagou, E.E. Fundamental performance assessment of 2-D myocardial elastography in a phased-array configuration. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2009, 56, 2320–2327. [Google Scholar] [PubMed]
- Ozturk, A.; Grajo, J.R.; Dhyani, M.; Anthony, B.W.; Samir, A.E. Principles of ultrasound elastography. Abdom. Radiol. 2018, 43, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Pérez, J.E.; Miller, J.G.; Holland, M.R.; Wickline, S.A.; Waggoner, A.D.; Barzilai, B.; Sobel, B.E. Ultrasonic tissue characterization: Integrated backscatter imaging for detecting myocardial structural properties and on-line quantitation of cardiac function. Am. J. Card. Imaging 1994, 8, 106–112. [Google Scholar] [PubMed]
- D’Amato, A.; Prosperi, S.; Severino, P.; Myftari, V.; Labbro Francia, A.; Cestiè, C.; Pierucci, N.; Marek-Iannucci, S.; Mariani, M.V.; Germanò, R.; et al. Current Approaches to Worsening Heart Failure: Pathophysiological and Molecular Insights. Int. J. Mol. Sci. 2024, 25, 1574. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2022, 79, e263–e421. [Google Scholar] [CrossRef] [PubMed]
- Metra, M.; Tomasoni, D.; Adamo, M.; Bayes-Genis, A.; Filippatos, G.; Abdelhamid, M.; Adamopoulos, S.; Anker, S.D.; Antohi, L.; Böhm, M.; et al. Worsening of chronic heart failure: Definition, epidemiology, management and prevention. A clinical consensus statement by the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2023, 25, 776–791. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.S.; Lam, C.S.P.; McMurray, J.J.V.; Redfield, M.M. How to Manage Heart Failure with Preserved Ejection Fraction: Practical Guidance for Clinicians. JACC Heart Fail. 2023, 11, 619–636. [Google Scholar] [CrossRef] [PubMed]





| 100 AC | Galen proposed that the heart filled by dilation of the right ventricle |
| 1628 | Harvey proposed that most ventricular filling occurred during atrial systole |
| 1877 | Francois-Franck proposed that most LV filling occurred in early diastole |
| 1906 | Henderson described three phases of diastole |
| 1921 | Wiggers and Katz reported that the atrial contribution to LV filling varied |
| 1927 | Meek proposed that the “contraction remainder” extended into diastole |
| 1949 | Wiggers coined the term “inherent elasticity” to describe the diastolic properties of the myocardium |
| 1970s | Studies were performed on LV relaxation and passive LV properties, and these were characterized |
| 1970s | Extensive studies of hypertrophic cardiomyopathy describing severely abnormal diastole and potential benefits of verapamil |
| 1980s | Diastolic heart failure was described as an entity |
| 1992 | Monograph on “Diastolic heart failure” published by Shah and Pai |
| 2000s | The term “Heart failure with preserved ejection fraction (HFpEF)” was coined because of uncertainties about evaluating LV diastolic function |
| Factors impairing LV relaxation: |
| Aging |
| LV hypertrophy |
| Myocardial ischemia |
| LV dyssynchrony; e.g., RV pacing, LV hypertrophy, LBBB |
| Myocardial disarray as in hypertrophic cardiomyopathy |
| Increased LV afterload causing increased myocyte calcium load |
| Factors that improve LV relaxation: |
| Higher sympathetic tone and catecholamines |
| Calcium channel blockers |
| Specific LV lusiotropic agents such as levosimendan and calcium channel blockers in hypertrophic cardiomyopathy |
| Increasing age |
| LV hypertrophy |
| Interstitial fibrosis |
| Myocardial scarring |
| Infiltrative disorders such as cardiac amyloid, sarcoid and other storage disorders |
| Pericardial constriction |
| Acute pericarditis |
| Acute aortic regurgitation |
| Acute mitral regurgitation |
| Acute tricuspid regurgitation |
| Right ventricular infarction |
| Acute massive or submassive pulmonary embolism |
| Large pleural effusions |
| Tension pneumothorax |
| Large mediastinal tumors |
| Use of positive end-expiratory pressure (PEEP) and auto PEEP |
| Force: | An agent that causes a change in momentum |
| Stress: | Force per unit cross-sectional area that causes deformation |
| Strain: | Fractional change from unstressed dimension |
| Creep: | Time-dependent elongation of a material held at constant stress |
| Stress relaxation: | Diminution of stress when held at constant strain |
| Elasticity: | Property of recovery of a material from a stressed state when the stress is removed |
| Viscosity: | Property of the material that retards deformation in response to stress |
| Viscoelasticity: | Property of the material when stress depends upon strain, e.g., myocardium |
| Hookean material: | Material that follows Hooke’s law, which states that the stress–strain relationship is linear |
| Non-Hookean material: | Material that does not follow Hooke’s law, e.g., myocardium |
| Modulus of stiffness: | Slope of the stress–strain relationship |
| Compliance: | Change in volume per unit change in pressure for a chamber |
| Stiffness: | Reciprocal of compliance |
| Factors affecting LV IVRT: |
| Rate of LV relaxation increases with impaired relaxation |
| LBBB increases IVRT |
| RV pacing increases IVRT |
| High LA pressure reduces IVRT |
| Large left atrial V wave reduces IVRT |
| Factors affecting E wave amplitude: |
| Impaired LV relaxation reduces E wave amplitude |
| High LA pressure increases its amplitude |
| Exaggerated LV recoil increases its amplitude |
| Factors affecting mitral A wave amplitude: |
| Left atrial contractile function: reduced in atrial cardiomyopathy, post cardioversion |
| Atrial preload: larger LA volume at the time of systole boosts the atrial transport function |
| Atrial afterload: Higher LVEDP or pre-A pressure will reduce atrial contribution |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pai, R.G.; Varadarajan, P. Diastolic Heart Failure Mechanisms and Assessment Revisited. J. Clin. Med. 2024, 13, 3043. https://doi.org/10.3390/jcm13113043
Pai RG, Varadarajan P. Diastolic Heart Failure Mechanisms and Assessment Revisited. Journal of Clinical Medicine. 2024; 13(11):3043. https://doi.org/10.3390/jcm13113043
Chicago/Turabian StylePai, Ramdas G., and Padmini Varadarajan. 2024. "Diastolic Heart Failure Mechanisms and Assessment Revisited" Journal of Clinical Medicine 13, no. 11: 3043. https://doi.org/10.3390/jcm13113043
APA StylePai, R. G., & Varadarajan, P. (2024). Diastolic Heart Failure Mechanisms and Assessment Revisited. Journal of Clinical Medicine, 13(11), 3043. https://doi.org/10.3390/jcm13113043






