Word Recognition with a Cochlear Implant in Relation to Prediction and Electrode Position
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Audiometric Measures
2.3. Imaging
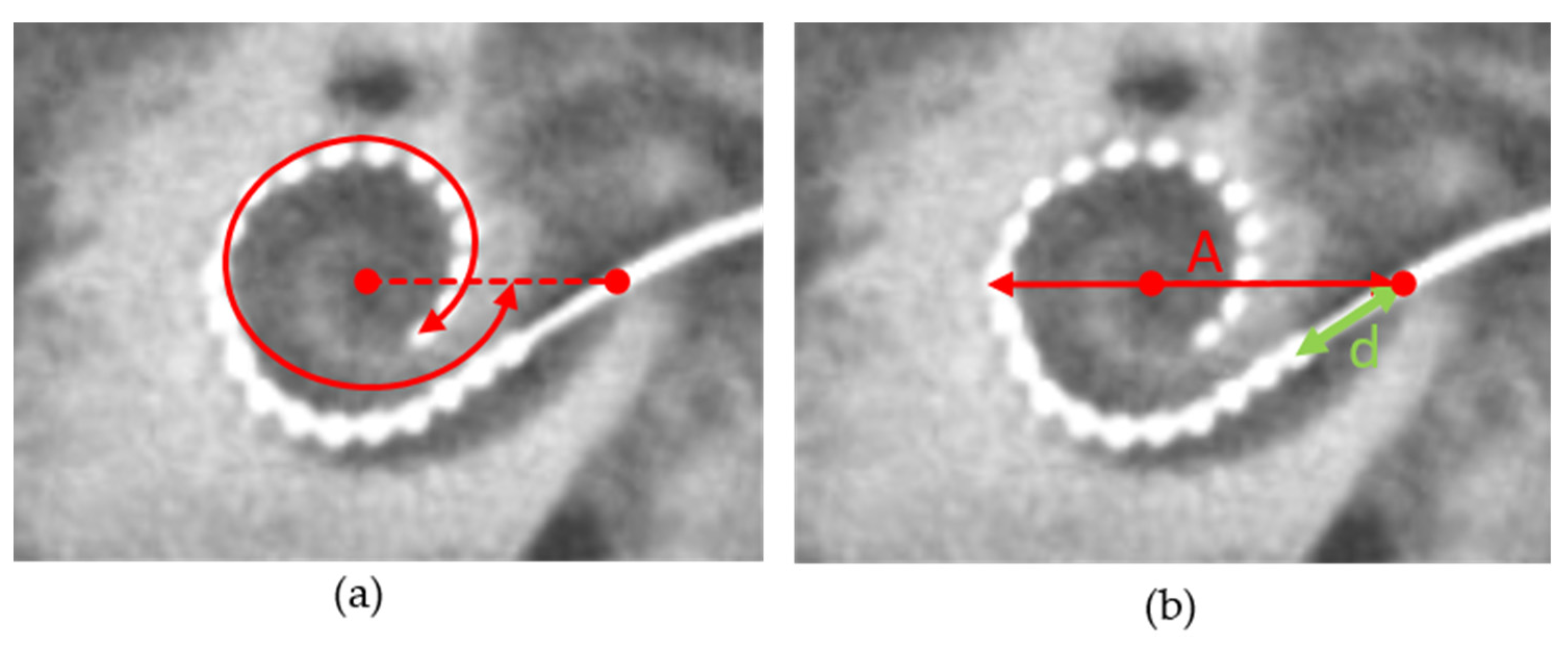
2.4. Measurement of Cochlear Diameter and Electrode Position
2.5. Data Analysis
3. Results
3.1. Study Cases
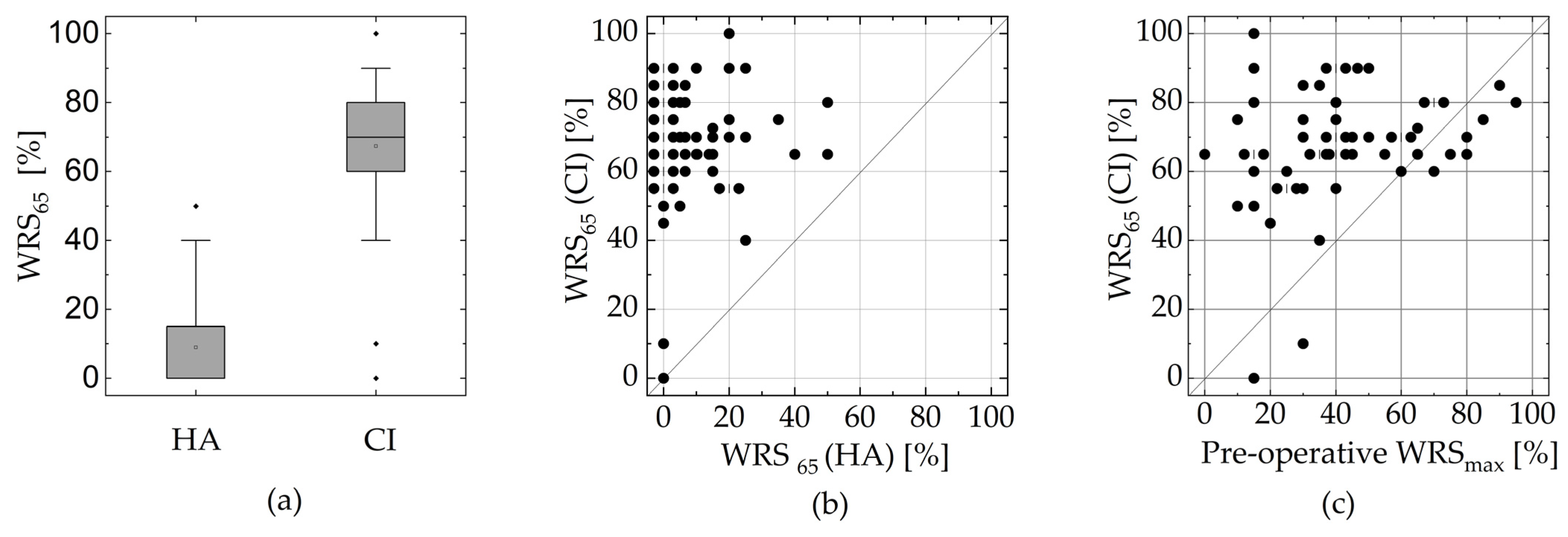
3.2. Insertion Depth and Cochlear Size
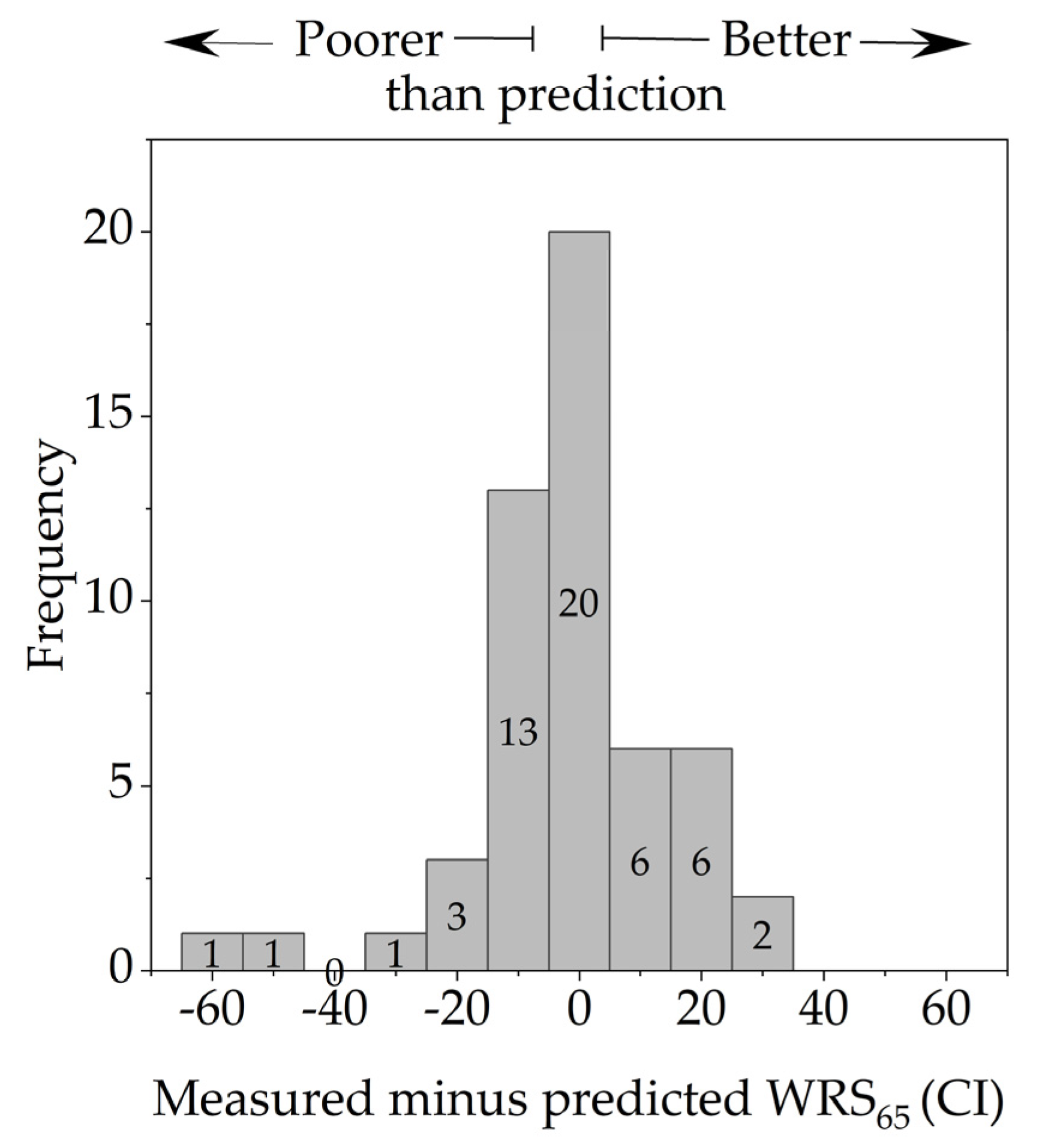
3.3. Dependence of the WRS on the Electrode’s Position and Cochlear Size
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Buchman, C.A.; Gifford, R.H.; Haynes, D.S.; Lenarz, T.; O’Donoghue, G.; Adunka, O.; Biever, A.; Briggs, R.J.; Carlson, M.L.; Dai, P.; et al. Unilateral Cochlear Implants for Severe, Profound, or Moderate Sloping to Profound Bilateral Sensorineural Hearing Loss: A Systematic Review and Consensus Statements. JAMA Otolaryngol. Head Neck Surg. 2020, 146, 942–953. [Google Scholar] [CrossRef] [PubMed]
- Blamey, P.J.; Artieres, F.; Baskent, D.; Bergeron, F.; Beynon, A.; Burke, E.; Dillier, N.; Dowell, R.; Fraysse, B.; Gallégo, S.; et al. Factors affecting auditory performance of postlinguistically deaf adults using cochlear implants: An update with 2251 patients. Audiol. Neurotol. 2013, 18, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Holden, L.K.; Finley, C.C.; Firszt, J.B.; Holden, T.A.; Brenner, C.; Potts, L.G.; Gotter, B.D.; Vanderhoof, S.S.; Mispagel, K.; Heydebrand, G.; et al. Factors affecting open-set word recognition in adults with cochlear implants. Ear Hear. 2013, 34, 342–360. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, U.; Hocke, T.; Hast, A.; Iro, H. Maximum preimplantation monosyllabic score as predictor of cochlear implant outcome. HNO 2019, 67, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, U.; Hocke, T.; Hast, A.; Iro, H. Cochlear Implantation in Candidates with Moderate-to-Severe Hearing Loss and Poor Speech Perception. Laryngoscope 2021, 131, E940–E945. [Google Scholar] [CrossRef] [PubMed]
- Goudey, B.; Plant, K.; Kiral, I.; Jimeno-Yepes, A.; Swan, A.; Gambhir, M.; Büchner, A.; Kludt, E.; Eikelboom, R.H.; Sucher, C.; et al. A MultiCenter Analysis of Factors Associated with Hearing Outcome for 2,735 Adults with Cochlear Implants. Trends Hear. 2021, 25, 23312165211037525. [Google Scholar] [CrossRef]
- Shafieibavani, E.; Goudey, B.; Kiral, I.; Zhong, P.; Jimeno-Yepes, A.; Swan, A.; Gambhir, M.; Buechner, A.; Kludt, E.; Eikelboom, R.H.; et al. Predictive models for cochlear implant outcomes: Performance, generalizability, and the impact of cohort size. Trends Hear. 2021, 25, 23312165211066174. [Google Scholar] [CrossRef]
- Thangavelu, K.; Nitzge, M.; Weiß, R.M.; Mueller-Mazzotta, J.; Stuck, B.A.; Reimann, K. Role of cochlear reserve in adults with cochlear implants following post-lingual hearing loss. Eur. Arch. Otorhinolaryngol. 2023, 280, 1063–1071. [Google Scholar] [CrossRef]
- Hoppe, U.; Hast, A.; Hocke, T. Validation of a predictive model for speech discrimination after cochlear impIant provision. HNO 2023, 71, 53–59. [Google Scholar] [CrossRef]
- Franke-Trieger, A.; Jolly, C.; Darbinjan, A.; Zahnert, T.; Murbe, D. Insertion depth angles of cochlear implant arrays with varying length: A temporal bone study. Otol. Neurotol. 2014, 35, 58–63. [Google Scholar] [CrossRef]
- Mewes, A.; Brademann, G.; Hey, M. Comparison of Perimodiolar Electrodes: Imaging and Electrophysiological Outcomes. Otol. Neurotol. 2020, 41, e934–e944. [Google Scholar] [CrossRef] [PubMed]
- Liebscher, T.; Mewes, A.; Hoppe, U.; Hornung, J.; Brademann, G.; Hey, M. Electrode Translocations in Perimodiolar Cochlear Implant Electrodes: Audiological and Electrophysiological Outcome. Z. Med. Phys. 2021, 31, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Risi, F. Considerations and Rationale for Cochlear Implant Electrode Design—Past, Present and Future. J. Int. Adv. Otol. 2018, 14, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Dhanasingh, A. The rationale for FLEX (cochlear implant) electrode with varying array lengths. World J. Otorhinolaryngol. Head Neck Surg. 2021, 7, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Aniket, S.; Litvak, L.; Boyle, P. SPAN: Improved current steering on the Advanced Bionics CII and HiRes90K system. Cochlear Implant. Int. 2010, 11, 465–468. [Google Scholar]
- Müller, A.; Hocke, T.; Mir-Salim, P. Intraoperative findings on ECAP-measurement: Normal or special case? Int. J. Audiol. 2015, 54, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, U.; Brademann, G.; Stöver, T.; Ramos de Miguel, A.; Cowan, R.; Manrique, M.; Falcón-González, J.C.; Hey, M.; Baumann, U.; Huarte, A.; et al. Evaluation of a Transimpedance Matrix Algorithm to Detect Anomalous Cochlear Implant Electrode Position. Audiol. Neurootol. 2022, 27, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Aschendorff, A.; Kromeier, J.; Klenzner, T.; Laszig, R. Quality control after insertion of the nucleus contour and contour advance electrode in adults. Ear Hear. 2007, 28, 75S–79S. [Google Scholar] [CrossRef]
- Basta, D.; Todt, I.; Ernst, A. Audiological outcome of the pull-back technique in cochlear implantees. Laryngoscope 2010, 120, 1391–1396. [Google Scholar] [CrossRef]
- Riemann, C.; Sudhoff, H.; Todt, I. The Pull-Back Technique for the 532 Slim Modiolar Electrode. Biomed Res. Int. 2019, 2019, 6917084. [Google Scholar] [CrossRef]
- Lailach, S.; Neudert, M.; Zahnert, T. Update cochlear-implantation: Indications and surgical aspects. Laryngorhinootologie 2021, 100, 652–672. [Google Scholar] [CrossRef] [PubMed]
- Dziemba, O.C.; Merz, S.; Hocke, T. Evaluative audiometry after cochlear implant provision. HNO 2023, 71, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Winkler, A.; Holube, I. Test-retest reliability of the Freiburg monosyllabic speech test. HNO 2016, 64, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Verbist, B.M.; Skinner, M.W.; Cohen, L.T.; Leake, P.A.; James, C.; Boëx, C.; Holden, T.A.; Finley, C.C.; Roland, P.S.; Roland, J.T., Jr.; et al. Consensus panel on a cochlear coordinate system applicable in histological, physiological and radiological studies of the human cochlea. Otol. Neurotol. Off. Publ. Am. Otol. Soc. Am. Neurotol. Soc. Eur. Acad. Otol. Neurotol. 2010, 31, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Franke-Trieger, A.; Mürbe, D. Estimation of insertion depth angle based on cochlea diameter and linear insertion depth: A prediction tool for the CI422. Eur. Arch. Otorhinolaryngol. 2015, 272, 3193–3199. [Google Scholar] [CrossRef] [PubMed]
- Rieck, J.H.; Beyer, A.; Mewes, A.; Caliebe, A.; Hey, M. Extended Preoperative Audiometry for Outcome Prediction and Risk Analysis in Patients Receiving Cochlear Implants. J. Clin. Med. 2023, 12, 3262. [Google Scholar] [CrossRef] [PubMed]
- Franks, Z.G.; Jacob, A. The speech perception gap in cochlear implant patients. Cochlear Implant. Int. 2019, 20, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, U.; Hast, A.; Hocke, T. Sprachverstehen mit Hörgeraten in Abhängigkeit vom Tongehör. HNO 2014, 62, 443–448. [Google Scholar] [CrossRef]
- MacPhail, M.E.; Connell, N.T.; Totten, D.J.; Gray, M.T.; Pisoni, D.; Yates, C.W.; Nelson, R.F. Speech Recognition Outcomes in Adults with Slim Straight and Slim Modiolar Cochlear Implant Electrode Arrays. Otolaryngol. Head Neck Surg. 2022, 166, 943–950. [Google Scholar] [CrossRef]
- Moran, M.; Vandali, A.; Briggs, R.J.S.; Dettman, S.; Cowan, R.S.C.; Dowell, R.C. Speech Perception Outcomes for Adult Cochlear Implant Recipients Using a Lateral Wall or Perimodiolar Array. Otol. Neurotol. 2019, 40, 608–616. [Google Scholar] [CrossRef]
- Lauer, G.; Uçta, J.; Decker, L.; Ernst, A.; Mittmann, P. Intracochlear Pressure Changes After Cochlea Implant Electrode Pullback-Reduction of Intracochlear Trauma. Laryngoscope Investig. Otolaryngol. 2019, 4, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Cochlear Nucleus CI622 cochlear implant with slim straight electrode Physicians Guide CI622. Available online: https://mss-p-007-delivery.stylelabs.cloud/api/public/content/75f1036c47a44e88be373bc134305624?v=3a4edc74 (accessed on 27 December 2023).
- Spiegel, J.L.; Polterauer, D.; Hempel, J.M.; Canis, M.; Spiro, J.E.; Müller, J. Variation of the cochlear anatomy and cochlea duct length: Analysis with a new tablet-based software. Eur. Arch. Otorhinolaryngol. 2022, 279, 1851–1861. [Google Scholar] [CrossRef] [PubMed]
- Yukawa, K.; Cohen, L.; Blamey, P.; Pyman, B.; Tungvachirakul, V.; O’Leary, S. Effects of insertion depth of cochlear implant electrodes upon speech perception. Audiol. Neurootol. 2004, 9, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.; Xiang, M.Y.; Li, Y.; Gong, J.M.; Wu, T.; Wang, Y.; Xu, J.; Wang, Y.F.; Li, J. Effect of Electrode Insertion Angle on Cochlear Implantation Outcomes in Adult and Children Patients with Sensorineural Hearing Loss. Oxid. Med. Cell Longev. 2022, 2022, 9914716. [Google Scholar] [CrossRef] [PubMed]
- Canfarotta, M.W.; Dillon, M.T.; Brown, K.D.; Pillsbury, H.C.; Dedmon, M.M.; O’Connell, B.P. Insertion Depth and Cochlear Implant Speech Recognition Outcomes: A Comparative Study of 28- and 31.5-mm Lateral Wall Arrays. Otol. Neurotol. 2022, 43, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Roßberg, C.; Timm, M.; Roßberg, W.; Kludt, E.; Bronzlik, P.; Lesinski-Schiedat, A.; Büchner, A.; Lenarz, T. Comparison of speech understanding taking into account the exact electrode position (SRA/MRA/CA). Laryngorhinootologie 2023, 102, 850–855. [Google Scholar] [CrossRef] [PubMed]
- Ketterer, M.C.; Aschendorff, A.; Arndt, S.; Beck, R. Electrode array design determines scalar position, dislocation rate and angle and postoperative speech perception. Eur. Arch. Otorhinolaryngol. 2022, 279, 4257–4267. [Google Scholar] [CrossRef] [PubMed]
- Thimsen, V.; Mantsopoulos, K.; Liebscher, T.; Taha, L.; Eisenhut, F.; Iro, H.; Hoppe, U.; Hornung, J. Association between lateral wall electrode array insertion parameters and audiological outcomes in bilateral cochlear implantation. Eur. Arch. Otorhinolaryngol. 2023, 280, 2707–2714. [Google Scholar] [CrossRef]
- Başkent, D.; Shannon, R.V. Interactions between cochlear implant electrode insertion depth and frequency-place mapping. J. Acoust. Soc. Am. 2005, 117, 1405–1416. [Google Scholar] [CrossRef]
- Finley, C.C.; Holden, T.A.; Holden, L.K.; Whiting, B.R.; Chole, R.A.; Neely, G.J.; Hullar, T.E.; Skinner, M.W. Role of electrode placement as a contributor to variability in cochlear implant outcomes. Otol. Neurotol. 2008, 29, 920–928. [Google Scholar] [CrossRef]
- Heutink, F.; de Rijk, S.R.; Verbist, B.M.; Huinck, W.J.; Mylanus, E.A.M. Angular Electrode Insertion Depth and Speech Perception in Adults with a Cochlear Implant: A Systematic Review. Otol. Neurotol. 2019, 40, 900–910. [Google Scholar] [CrossRef]
- Faulkner, A.; Rosen, S.; Norman, C. The right information may matter more than frequency-place alignment: Simulations of frequency-aligned and upward shifting cochlear implant processors for a shallow electrode array insertion. Ear Hear. 2006, 27, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Suhling, M.C.; Majdani, O.; Salcher, R.; Leifholz, M.; Buchner, A.; Lesinski-Schiedat, A.; Lenarz, T. The Impact of Electrode Array Length on Hearing Preservation in Cochlear Implantation. Otol. Neurotol. 2016, 37, 1006–1015. [Google Scholar] [CrossRef]
- Mewes, A.; Burg, S.; Brademann, G.; Dambon, J.A.; Hey, M. Quality-assured training in the evaluation of cochlear implant electrode position: A prospective experimental study. BMC Med. Educ. 2022, 22, 386. [Google Scholar] [CrossRef] [PubMed]
- Böttcher-Rebmann, G.; Schell, V.; Zuniga, M.G.; Salcher, R.; Lenarz, T.; Rau, T.S. Preclinical evaluation of a tool for insertion force measurements in cochlear implant surgery. Int. J. Comput. Assist. Radiol. Surg. 2023, 18, 2117–2124. [Google Scholar] [CrossRef] [PubMed]
- Van der Jagt, A.M.A.; Briaire, J.J.; Boehringer, S.; Verbist, B.M.; Frijns, J.H.M. Prolonged Insertion Time Reduces Translocation Rate of a Precurved Electrode Array in Cochlear Implantation. Otol. Neurotol. 2022, 43, e427–e434. [Google Scholar] [CrossRef]
- Barriat, S.; Peigneux, N.; Duran, U.; Camby, S.; Lefebvre, P.P. The Use of a Robot to Insert an Electrode Array of Cochlear Implants in the Cochlea: A Feasibility Study and Preliminary Results. Audiol. Neurootol. 2021, 26, 361–367. [Google Scholar] [CrossRef]
- Lenarz, T.; Buechner, A.; Gantz, B.; Hansen, M.; Tejani, V.D.; Labadie, R.; O’Connell, B.; Buchman, C.A.; Valenzuela, C.V.; Adunka, O.F.; et al. Relationship Between Intraoperative Electrocochleography and Hearing Preservation. Otol. Neurotol. 2022, 43, e72–e78. [Google Scholar] [CrossRef]
- Arweiler-Harbeck, D.; D’Heygere, V.; Meyer, M.; Hans, S.; Waschkies, L.; Lang, S.; Anton, K.; Hessel, H.; Schneider, A.; Heiler, T.; et al. Digital Live Imaging of Intraoperative Electrocochleography—First Description of Feasibility and Hearing Preservation during Cochlear Implantation. Otol. Neurotol. 2021, 42, 1342–1346. [Google Scholar] [CrossRef]
- Harrison, L.; Manjaly, J.G.; Ellis, W.; Lavy, J.A.; Shaida, A.; Khalil, S.S.; Saeed, S.R. Hearing Preservation Outcomes with Standard Length Electrodes in Adult Cochlear Implantation and the Uptake of Electroacoustic Stimulation. Otol. Neurotol. 2020, 41, 1060–1065. [Google Scholar] [CrossRef]
- Spitzer, E.R.; Waltzman, S.B.; Landsberger, D.M.; Friedmann, D.R. Acceptance and Benefits of Electro-Acoustic Stimulation for Conventional-Length Electrode Arrays. Audiol. Neurootol. 2021, 26, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Van de Heyning, P.H.; Dazert, S.; Gavilan, J.; Lassaletta, L.; Lorens, A.; Rajan, G.P.; Skarzynski, H.; Skarzynski, P.H.; Tavora-Vieira, D.; Topsakal, V.; et al. Systematic Literature Review of Hearing Preservation Rates in Cochlear Implantation Associated with Medium- and Longer-Length Flexible Lateral Wall Electrode Arrays. Front. Surg. 2022, 9, 893839. [Google Scholar] [CrossRef] [PubMed]
- Dalbert, A.; Huber, A.; Baumann, N.; Veraguth, D.; Roosli, C.; Pfiffner, F. Hearing Preservation After Cochlear Implantation May Improve Long-term Word Perception in the Electric-only Condition. Otol. Neurotol. 2016, 37, 1314–1319. [Google Scholar] [CrossRef] [PubMed]
- Buchner, A.; Illg, A.; Majdani, O.; Lenarz, T. Investigation of the effect of cochlear implant electrode length on speech comprehension in quiet and noise compared with the results with users of electro-acoustic-stimulation, a retrospective analysis. PLoS ONE 2017, 12, e0174900. [Google Scholar] [CrossRef]
- Rader, T.; Doms, P.; Adel, Y.; Weissgerber, T.; Strieth, S.; Baumann, U. A method for determining precise electrical hearing thresholds in cochlear implant users. Int. J. Audiol. 2018, 57, 502–509. [Google Scholar] [CrossRef]
- Plesch, J.; Ernst, B.P.; Strieth, S.; Rader, T. A psychoacoustic application for the adjustment of electrical hearing thresholds in cochlear implant patients. PLoS ONE 2019, 14, e0223625. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franke-Trieger, A.; Lailach, S.; Shetty, J.; Murrmann, K.; Zahnert, T.; Neudert, M. Word Recognition with a Cochlear Implant in Relation to Prediction and Electrode Position. J. Clin. Med. 2024, 13, 183. https://doi.org/10.3390/jcm13010183
Franke-Trieger A, Lailach S, Shetty J, Murrmann K, Zahnert T, Neudert M. Word Recognition with a Cochlear Implant in Relation to Prediction and Electrode Position. Journal of Clinical Medicine. 2024; 13(1):183. https://doi.org/10.3390/jcm13010183
Chicago/Turabian StyleFranke-Trieger, Annett, Susen Lailach, Joshua Shetty, Katrin Murrmann, Thomas Zahnert, and Marcus Neudert. 2024. "Word Recognition with a Cochlear Implant in Relation to Prediction and Electrode Position" Journal of Clinical Medicine 13, no. 1: 183. https://doi.org/10.3390/jcm13010183
APA StyleFranke-Trieger, A., Lailach, S., Shetty, J., Murrmann, K., Zahnert, T., & Neudert, M. (2024). Word Recognition with a Cochlear Implant in Relation to Prediction and Electrode Position. Journal of Clinical Medicine, 13(1), 183. https://doi.org/10.3390/jcm13010183





