Tone Decay Reconsidered: Preliminary Results of a Prospective Study in Hearing-Aid Users with Moderate to Severe Hearing Loss
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Characteristics
2.2. Audiological Parameters
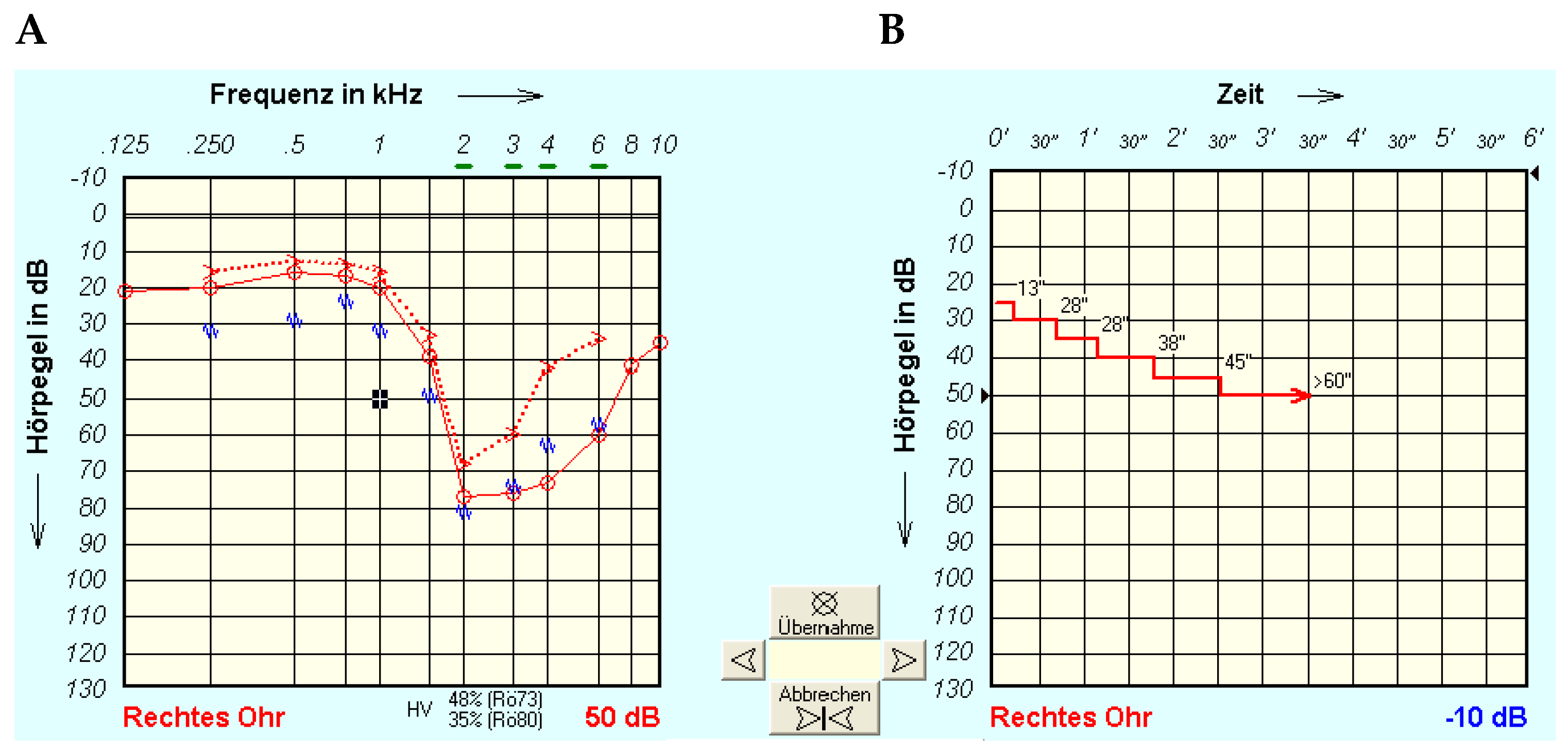
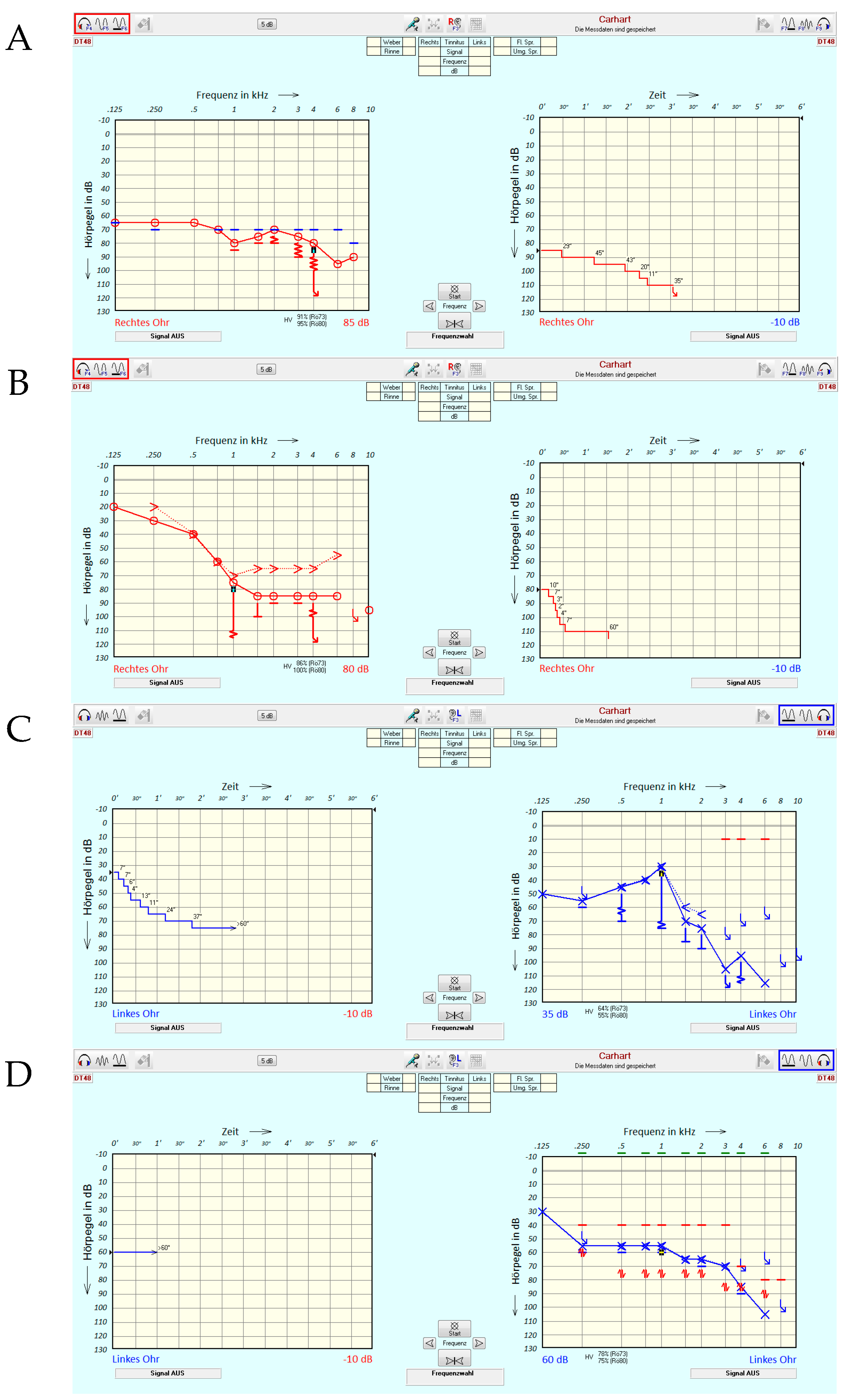
2.3. Tone Decay Test
2.4. Possible Characteristics of Tone Decay Measurements
3. Results
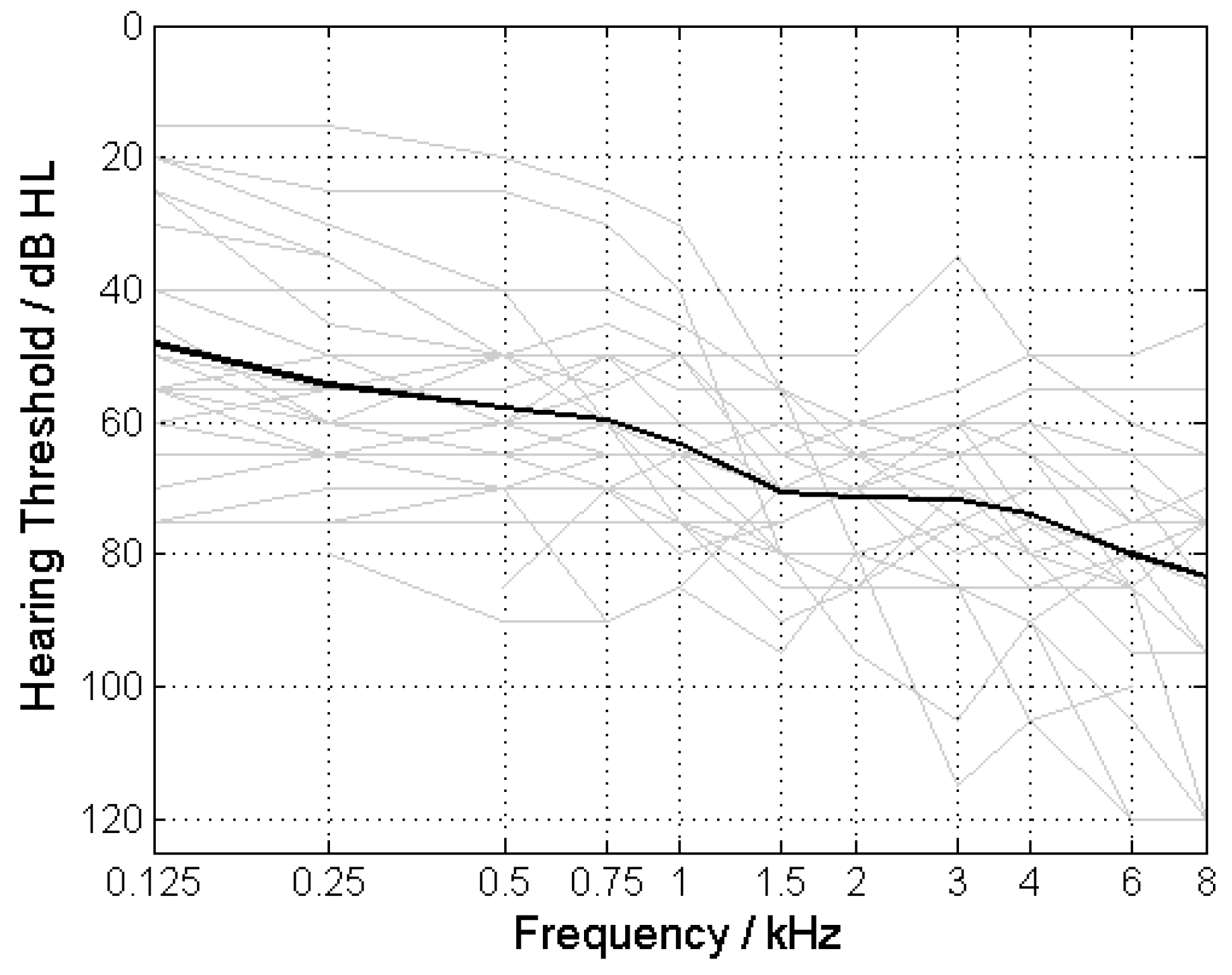
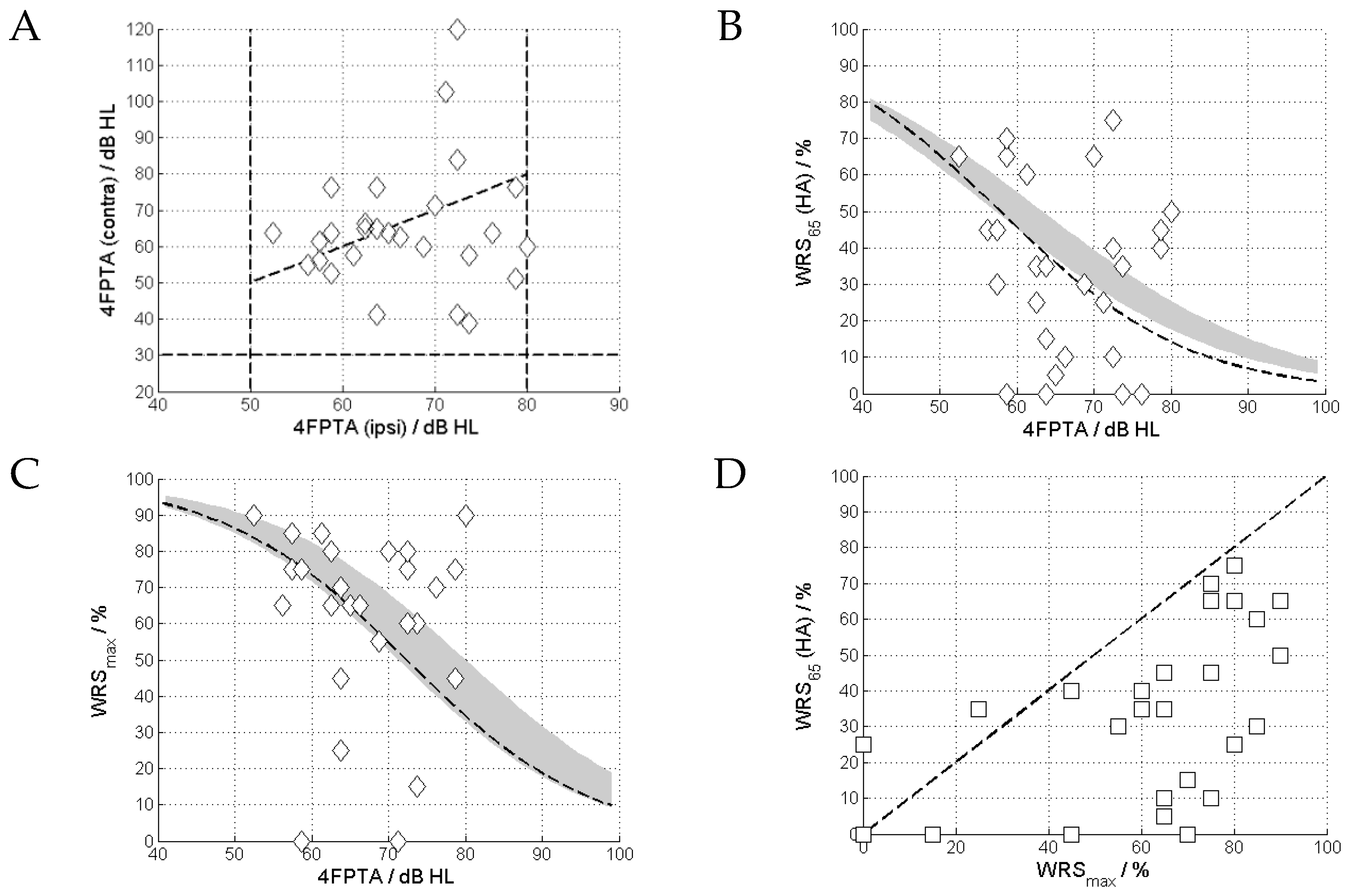
3.1. Pure-Tone and Speech Audiometry
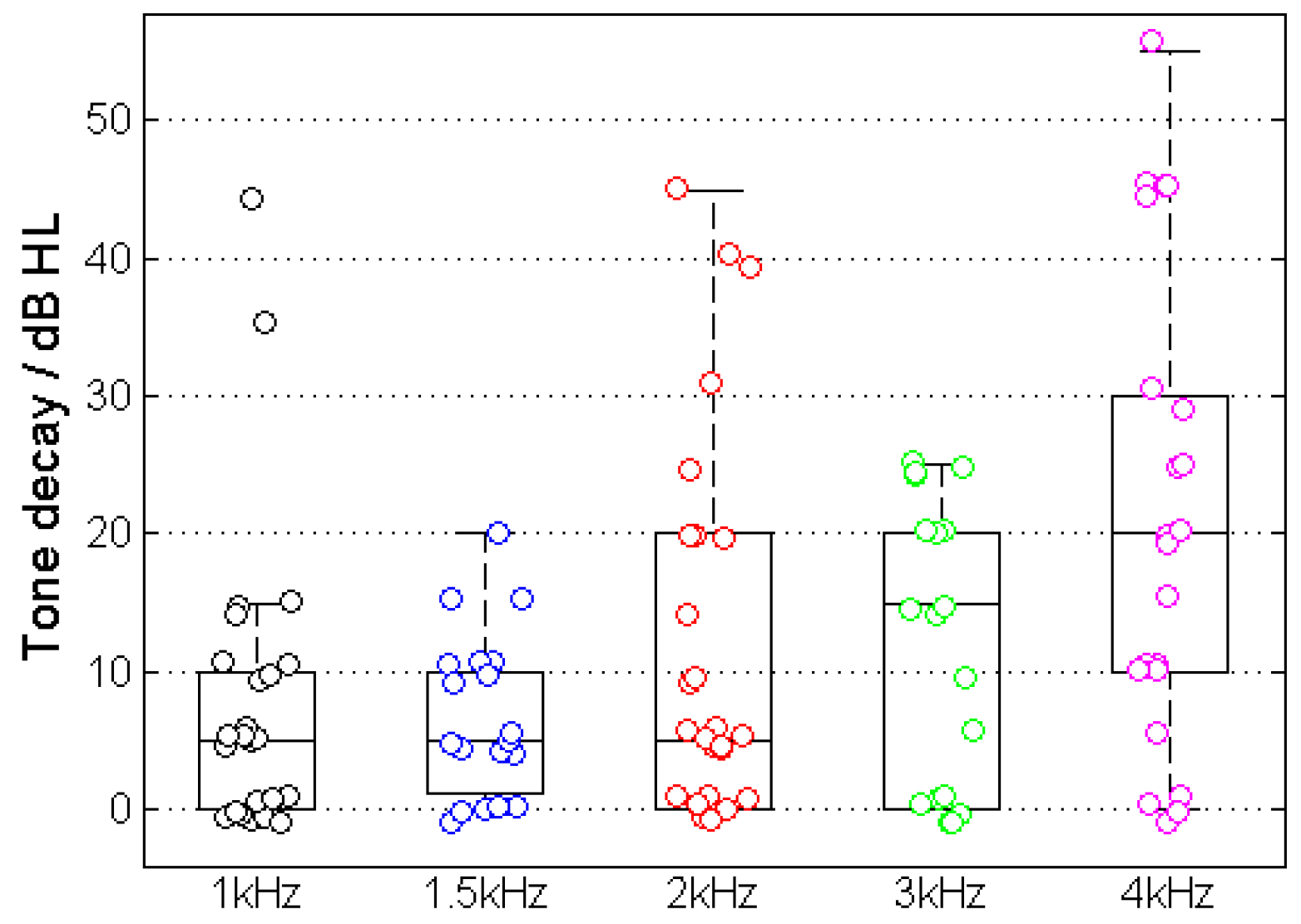
3.2. Tone Decay
4. Discussion
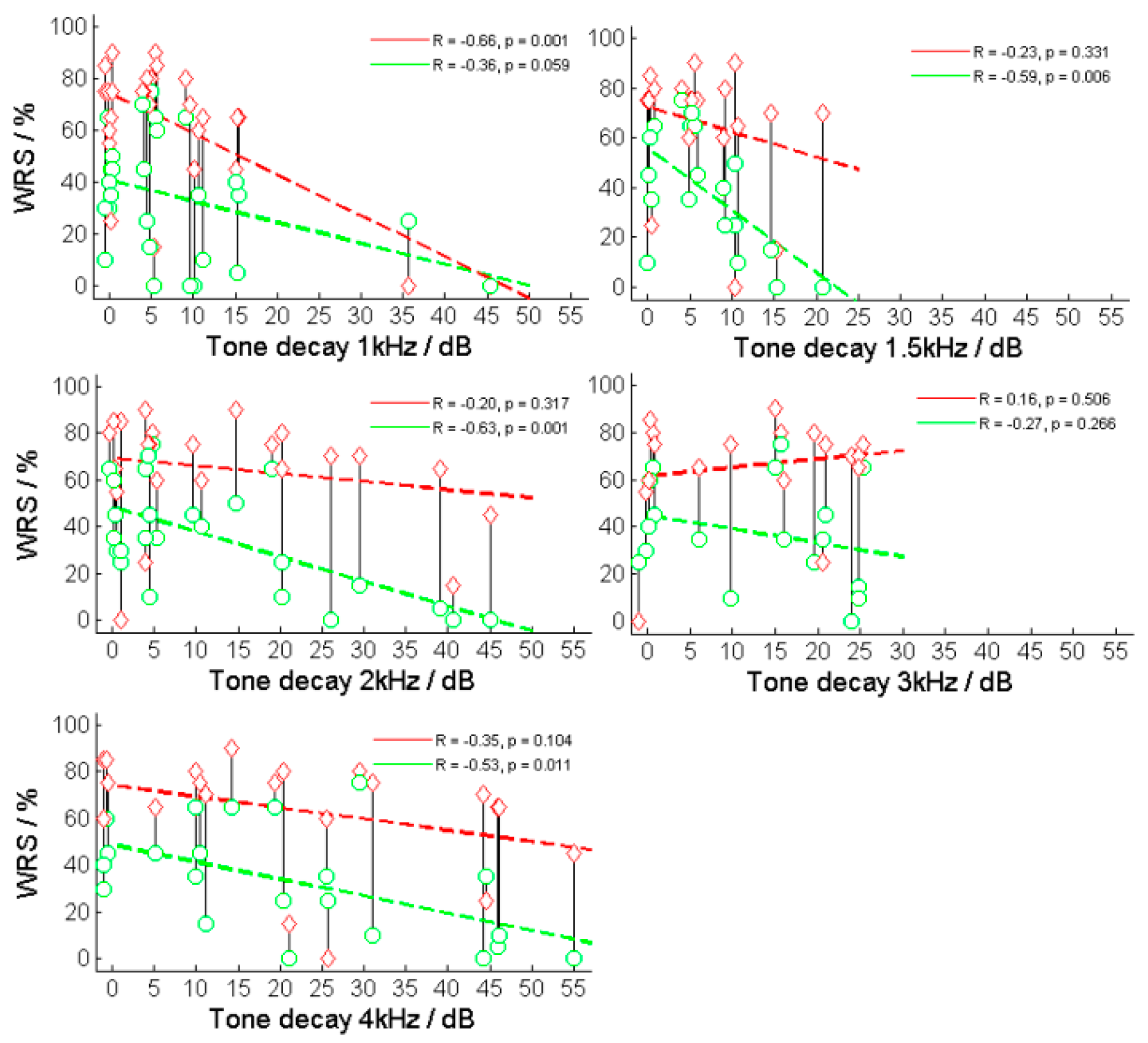
4.1. Tone Decay and Speech Comprehension
4.2. Application of Tone Decay Assessment in a Changing Patient Population
4.3. Limits of this Study and Feasibility
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dörfler, C.; Hocke, T.; Hast, A.; Hoppe, U. Speech recognition with hearing aids for 10 standard audiograms: English version. HNO 2020, 68, 93–99. [Google Scholar] [CrossRef]
- Engler, M.; Digeser, F.; Hoppe, U. Effectiveness of hearing aid provision for severe hearing loss. HNO 2022, 70, 520–532. [Google Scholar] [CrossRef] [PubMed]
- McRackan, T.R.; Ahlstrom, J.B.; Clinkscales, W.B.; Meyer, T.A.; Dubno, J.R. Clinical Implications of Word Recognition Differences in Earphone and Aided Conditions. Otol. Neurotol. 2016, 37, 1475–1481. [Google Scholar] [CrossRef]
- McRackan, T.R.; Fabie, J.E.; Burton, J.A.; Munawar, S.; Holcomb, M.A.; Dubno, J.R. Earphone and Aided Word Recognition Differences in Cochlear Implant Candidates. Otol. Neurotol. 2018, 39, e543–e549. [Google Scholar] [CrossRef] [PubMed]
- Franks, Z.G.; Jacob, A. The speech perception gap in cochlear implant patients. Cochlear Implant. Int. 2019, 20, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Carhart, R. Basic principles of speech audiometry. Acta Otolaryngol. 1951, 40, 62–71. [Google Scholar] [CrossRef]
- Plomp, R. Auditory handicap of hearing impairment and the limited benefit of hearing aids. J. Acoust. Soc. Am. 1978, 63, 533–549. [Google Scholar] [CrossRef]
- Carhart, R. Clinical determination of abnormal auditory adaptation. AMA Arch. Otolaryngol. 1957, 65, 32–39. [Google Scholar] [CrossRef]
- Huss, M.; Moore, B.C. Tone decay for hearing-impaired listeners with and without dead regions in the cochlea. J. Acoust. Soc. Am. 2003, 114, 3283–3294. [Google Scholar] [CrossRef]
- Scharf, B. Loudness adaptation. In Hearing Research and Theory; Tobias, J.V., Schubert, E.D., Eds.; Academic Press: New York, NY, USA, 1983; Volume 2, pp. 1–56. [Google Scholar]
- Miśkiewicz, A.; Scharf, B.; Hellman, R.; Meiselman, C. Loudness adaptation at high frequencies. J. Acoust. Soc. Am. 1993, 94, 1281–1286. [Google Scholar] [CrossRef]
- Wynne, D.P.; Zeng, F.G.; Bhatt, S.; Michalewski, H.J.; Dimitrijevic, A.; Starr, A. Loudness adaptation accompanying ribbon synapse and auditory nerve disorders. Brain 2013, 136, 1626–1638. [Google Scholar] [CrossRef] [PubMed]
- Arndt, S.; Laszig, R.; Aschendorff, A.; Hassepass, F.; Beck, R.; Wesarg, T. Cochlear implant treatment of patients with single-sided deafness or asymmetric hearing loss. HNO 2017, 65, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Mrowinski, D.; Scholz, G. Audiometrie Eine Anleitung Für Die Praktische Hörprüfung, 5th ed.; Georg Thieme Verlag: Stuttgart, Germany, 2017; p. 58ff. ISBN 978-3-13-240107-5. [Google Scholar]
- Lehnhardt, E. Adaptation und Hörermüdung. In Praxis der Audiometrie, 8th ed.; Lehnhardt, E., Laszig, R., Eds.; Thieme: Stuttgart, Germany, 2001; p. 153ff. [Google Scholar]
- Hoppe, U.; Hast, A.; Hocke, T. Audiometry-Based Screening Procedure for Cochlear Implant Candidacy. Otol. Neurotol. 2015, 36, 1001–1005. [Google Scholar] [CrossRef] [PubMed]
- Kronlachner, M.; Baumann, U.; Stover, T.; Weissgerber, T. Investigation of the quality of hearing aid provision in seniors considering cognitive functions. Laryngorhinootologie 2018, 97, 852–859. [Google Scholar] [CrossRef]
- Beyer, A.; Rieck, J.H.; Mewes, A.; Dambon, J.A.; Hey, M. Extended preoperative speech audiometric diagnostics for cochlear implant treatment. HNO 2023, 71, 779–786. [Google Scholar] [CrossRef] [PubMed]
- AWMF. Leitlinien: Cochlea-Implantat Versorgung und Zentral-Auditorische Implantate. 2020. Available online: https://www.awmf.org/uploads/tx_szleitlinien/017-071l_S2k_Cochlea-Implantat-Versorgung-zentral-auditorische-Implantate_2020-12.pdf (accessed on 1 June 2023).
- Hoppe, U.; Hocke, T.; Hast, A.; Iro, H. Cochlear Implantation in Candidates With Moderate-to-Severe Hearing Loss and Poor Speech Perception. Laryngoscope 2021, 131, E940–E945. [Google Scholar] [CrossRef]
- Thangavelu, K.; Nitzge, M.; Weiß, R.M.; Mueller-Mazzotta, J.; Stuck, B.A.; Reimann, K. Role of cochlear reserve in adults with cochlear implants following post-lingual hearing loss. Eur. Arch. Otorhinolaryngol. 2022, 280, 1063–1071. [Google Scholar] [CrossRef]
- Rieck, J.H.; Beyer, A.; Mewes, A.; Caliebe, A.; Hey, M. Extended Preoperative Audiometry for Outcome Prediction and Risk Analysis in Patients Receiving Cochlear Implants. J. Clin. Med. 2023, 12, 3262. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, U.; Hast, A.; Hocke, T. Validation of a predictive model for speech discrimination after cochlear impIant provision. HNO 2023, 71, 53–59. [Google Scholar] [CrossRef]
- Zwartenkot, J.W.; Snik, A.D.F.M.; Mylanus, E.A.; Mulder, J.J. Amplification options for patients with mixed hearing loss. Otol. Neurotol. 2014, 35, 221–226. [Google Scholar] [CrossRef]
- Rahne, T.; Plontke, S.K. Device-based treatment of mixed hearing loss: An audiological comparison of current hearing systems. HNO 2016, 64, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Rahne, T. Physical audiological principles of implantable hearing systems: About power transmission, coupling and power output. HNO 2021, 69, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Moberly, A.C.; Bates, C.; Harris, M.S.; Pisoni, D.B. The Enigma of Poor Performance by Adults with Cochlear Implants. Otol. Neurotol. 2016, 37, 1522–1528. [Google Scholar] [CrossRef] [PubMed]
- Goudey, B.; Plant, K.; Kiral, I.; Jimeno-Yepes, A.; Swan, A.; Gambhir, M.; Büchner, A.; Kludt, E.; Eikelboom, R.H.; Sucher, C.; et al. A MultiCenter Analysis of Factors Associated with Hearing Outcome for 2,735 Adults with Cochlear Implants. Trends Hear. 2021, 25, 23312165211037525. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Pisa, J.; Hochman, J. Comorbidity associated with worse outcomes in a population of limited cochlear implant performers. Laryngoscope Investig. Otolaryngol. 2023, 8, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Tropitzsch, A.; Schade-Mann, T.; Gamerdinger, P.; Dofek, S.; Schulte, B.; Schulze, M.; Fehr, S.; Biskup, S.; Haack, T.B.; Stöbe, P.; et al. Variability in Cochlear Implantation Outcomes in a Large German Cohort with a Genetic Etiology of Hearing Loss. Ear Hear. 2023, 44, 1464–1484. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, J.; Terkildsen, K. Audiological findings in 125 cases of acoustic neuromas. Acta Otolaryngol. 1975, 80, 353–361. [Google Scholar] [CrossRef]
- Gertner, A.B. Site of lesion testing findings in a routine test battery. Am. J. Otol. 1981, 2, 219–222. [Google Scholar]
- Strasilla, C.; Synchra, V. Imaging-based diagnosis of vestibular schwannoma. HNO 2017, 65, 373–380. [Google Scholar] [CrossRef]
- Hoth, S.; Dziemba, O.C. The Role of Auditory Evoked Potentials in the Context of Cochlear Implant Provision. Otol. Neurotol. 2017, 38, e522–e530. [Google Scholar] [CrossRef]
- Dziemba, O.C.; Hocke, T.; Müller, A. EABR on cochlear implant—Measurements from clinical routine compared to reference values. GMS Z Audiol 2022, 4, Doc05. [Google Scholar] [CrossRef]
- Wable, J.; Frachet, B.; Gallego, S. Tone decay at threshold with auditory electrical stimulation in digisonic cochlear implantees. Audiology 2001, 40, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Wasmann, J.A.; van Eijl, R.H.M.; Versnel, H.; van Zanten, G.A. Assessing auditory nerve condition by tone decay in deaf subjects with a cochlear implant. Int. J. Audiol. 2018, 57, 864–871. [Google Scholar] [CrossRef] [PubMed]
| Subjects | Age | Sex | Ear | Ipsilat. 4FPTA [dB HL] | Contralat. 4FPTA [dB HL] | WRSmax [%] | WRS65(HA) [%] | Tone Decay at 1, 1.5, 2, 3, 4 kHz [dB] |
|---|---|---|---|---|---|---|---|---|
| 1 | 51 | f | L | 58.8 | 76.3 | 0 | 0 | 45—n.c.—n.c.—n.c.—n.c. |
| 2 | 68 | m | L | 68.8 | 60.0 | 55 | 30 | 0—n.c.—0—0—n.c. |
| 3 | 76 | m | L | 80.0 | 60.0 | 90 | 50 | 0—10—15—n.c.—n.c. |
| 4 | 63 | m | R | 62.5 | 65.0 | 65 | 35 | 15—n.c.—0—5—10 |
| 5 | 45 | m | R | 72.5 | 41.3 | 75 | 10 | 0—0—5—10—30 |
| 6 | 59 | m | R | 71.3 | 102.5 | 0 | 25 | 35—10—0—0—25 |
| 7 | 51 | f | R | 78.8 | 76.3 | 45 | 40 | 15—n.c.—n.c.—n.c.—n.c. |
| 8 | 72 | f | R | 58.8 | 63.8 | 75 | 65 | 0—5—20—25—20 |
| 9 | 70 | f | R | 52.5 | 63.8 | 90 | 65 | 5—5—5—15—15 |
| 10 | 41 | m | R | 56.3 | 55.0 | 65 | 45 | 0—n.c.—0—n.c.—5 |
| 10 | 41 | m | L | 57.5 | 56.3 | 85 | 30 | 0—n.c.—0—n.c.—0 |
| 11 | 38 | f | R | 70.0 | 71.3 | 80 | 65 | 10—0—0—0—10 |
| 12 | 76 | f | R | 73.8 | 57.5 | 15 | 0 | 5—15—40—n.c.—20 |
| 13 | 77 | m | R | 78.8 | 51.3 | 75 | 45 | 0—0—10—20—10 |
| 14 | 78 | f | R | 65.0 | 63.8 | 65 | 5 | 15—n.c.—40—n.c.—45 |
| 14 | 78 | f | L | 63.8 | 65.0 | 45 | 0 | 10—n.c.—45—n.c.—55 |
| 15 | 71 | f | L | 72.5 | 120 | 80 | 75 | 5—5—5—15—30 |
| 16 | 76 | f | R | 76.3 | 63.8 | 70 | 0 | 10—20—25—25—45 |
| 16 | 76 | f | L | 63.8 | 76.3 | 70 | 15 | 5—15—30—25—10 |
| 17 | 88 | f | R | 73.8 | 38.8 | 60 | 35 | 10—5—5—15—25 |
| 18 | 79 | f | R | 66.3 | 62.5 | 65 | 10 | 10—10—20—25—45 |
| 18 | 79 | f | L | 62.5 | 66.3 | 80 | 25 | 5—10—20—20—20 |
| 19 | 81 | f | R | 61.3 | 57.5 | 85 | 60 | 5—0—0—0—0 |
| 19 | 81 | m | L | 57.5 | 61.3 | 75 | 45 | 5—5—5—0—0 |
| 20 | 75 | m | L | 58.8 | 52.5 | 75 | 70 | 5—5—5—n.c.—n.c. |
| 21 | 57 | f | L | 72.5 | 83.8 | 60 | 40 | 0—10—10—0—0 |
| 22 | 78 | m | L | 63.8 | 41.3 | 25 | 35 | 0—0—5—20—45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmidt, F.H.; Hocke, T.; Zhang, L.; Großmann, W.; Mlynski, R. Tone Decay Reconsidered: Preliminary Results of a Prospective Study in Hearing-Aid Users with Moderate to Severe Hearing Loss. J. Clin. Med. 2024, 13, 500. https://doi.org/10.3390/jcm13020500
Schmidt FH, Hocke T, Zhang L, Großmann W, Mlynski R. Tone Decay Reconsidered: Preliminary Results of a Prospective Study in Hearing-Aid Users with Moderate to Severe Hearing Loss. Journal of Clinical Medicine. 2024; 13(2):500. https://doi.org/10.3390/jcm13020500
Chicago/Turabian StyleSchmidt, Florian Herrmann, Thomas Hocke, Lichun Zhang, Wilma Großmann, and Robert Mlynski. 2024. "Tone Decay Reconsidered: Preliminary Results of a Prospective Study in Hearing-Aid Users with Moderate to Severe Hearing Loss" Journal of Clinical Medicine 13, no. 2: 500. https://doi.org/10.3390/jcm13020500
APA StyleSchmidt, F. H., Hocke, T., Zhang, L., Großmann, W., & Mlynski, R. (2024). Tone Decay Reconsidered: Preliminary Results of a Prospective Study in Hearing-Aid Users with Moderate to Severe Hearing Loss. Journal of Clinical Medicine, 13(2), 500. https://doi.org/10.3390/jcm13020500





