Overview of Spontaneous Intracranial Hypotension and Differential Diagnosis with Chiari I Malformation
Abstract
1. Introduction
2. Pathophysiology
3. Classification of SIH
4. Clinical Presentation of SIH
5. SIH: Diagnostic Workup and Imaging Strategy
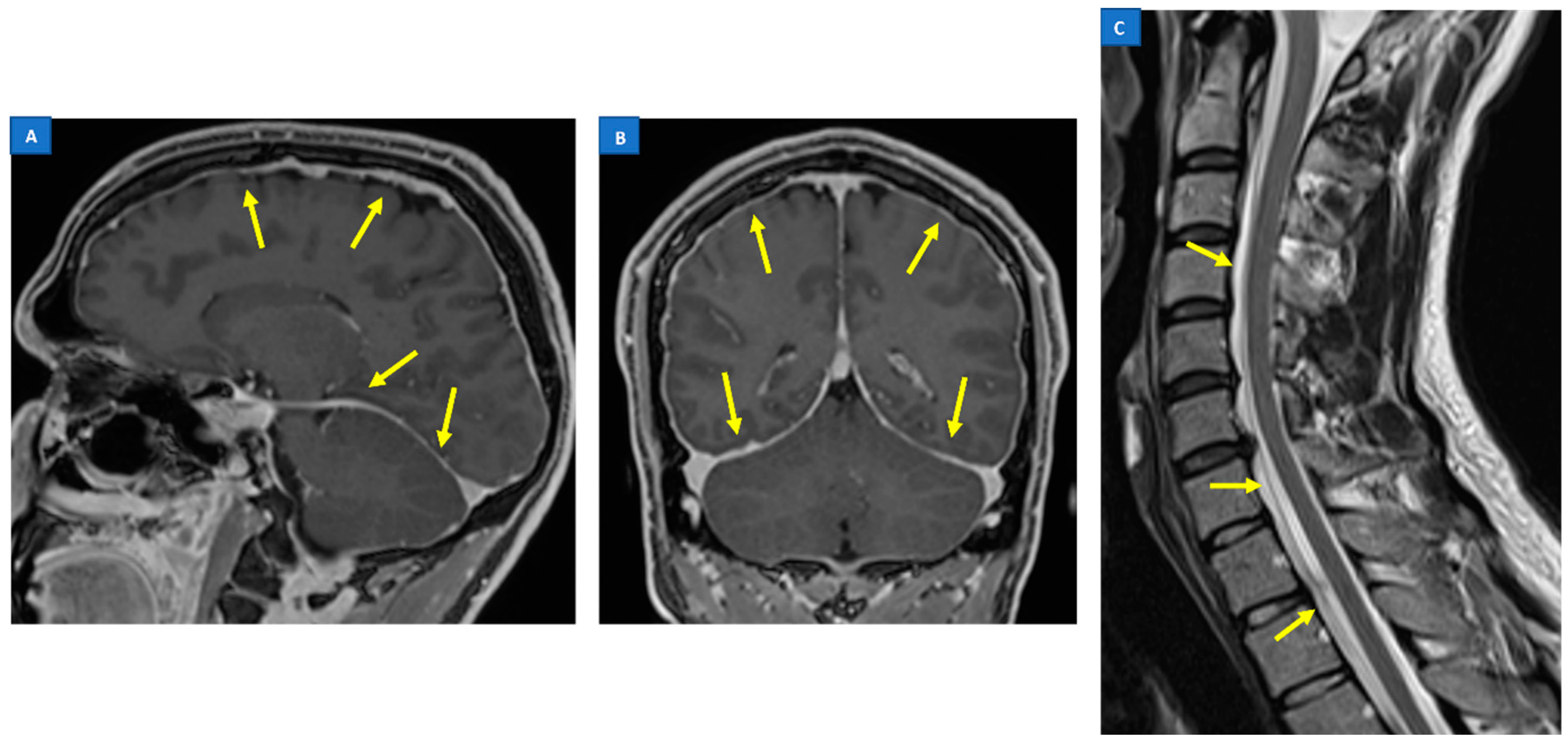
5.1. Head MRI
5.2. Spine MRI
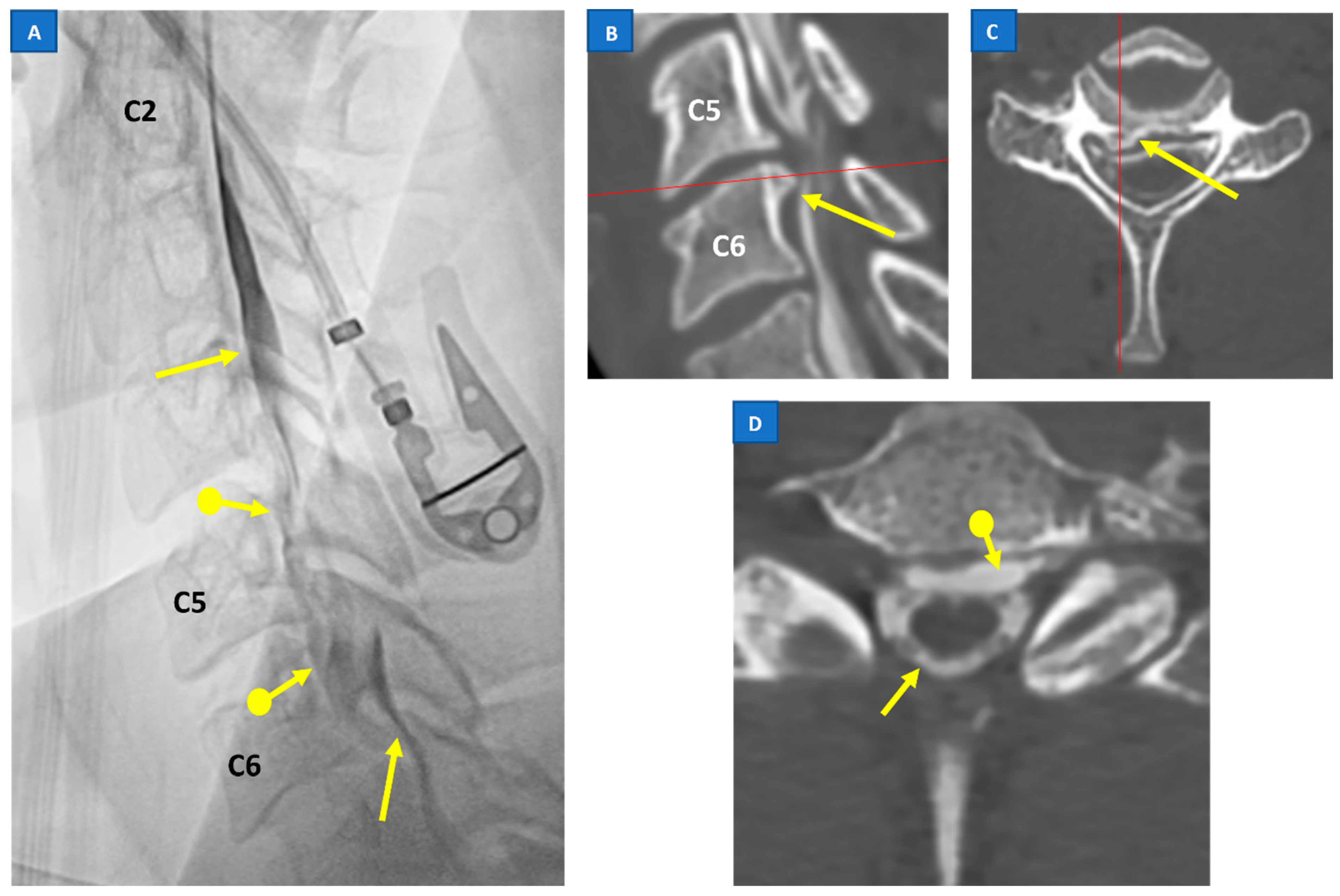
5.3. Digital Subtraction Myelography (DSM)
5.4. CT Myelography
5.5. Radioisotope Cisternography
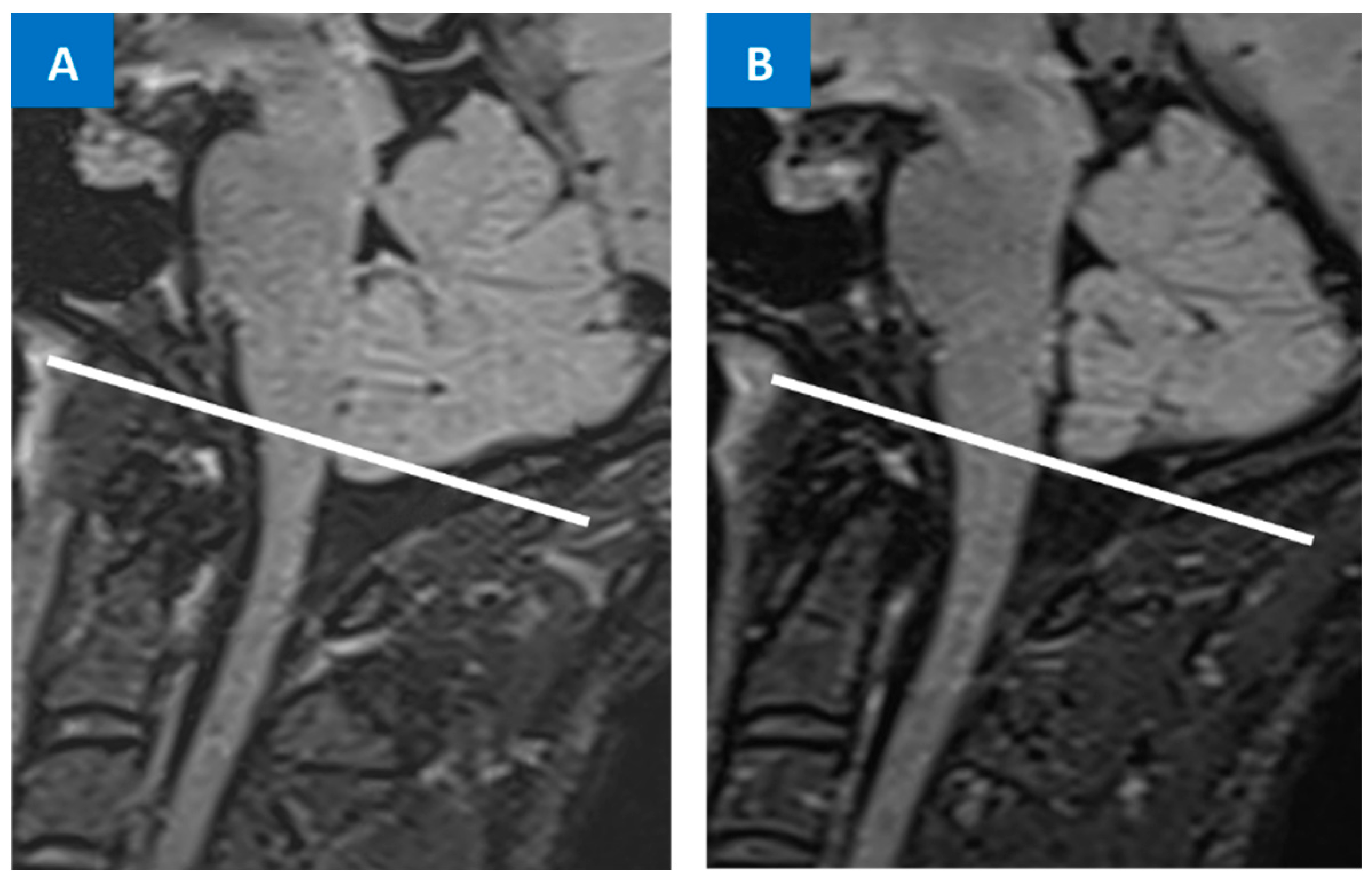
6. Differential Diagnosis between SIH and Chiari Malformation Type I
7. Treatment and Prognosis of SIH
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schaltenbrand, G. Normal and pathological physiology of the cerebrospinal fluid circulation. Lancet 1953, 261, 805–808. [Google Scholar] [CrossRef] [PubMed]
- Schievink, W.I.; Maya, M.; Moser, F.; Tourje, J.; Torbati, S. Frequency of spontaneous intracranial hypotension in the emergency department. J. Headache Pain 2007, 8, 325–328. [Google Scholar] [CrossRef]
- Schievink, W.I. Misdiagnosis of spontaneous intracranial hypotension. Arch. Neurol. 2003, 60, 1713–1718. [Google Scholar] [CrossRef] [PubMed]
- Schievink, W.I.; Maya, M.M.; Louy, C.; Moser, F.G.; Sloninsky, L. Spontaneous intracranial hypotension in childhood and adolescence. J. Pediatr. 2013, 163, 504–510.e3. [Google Scholar] [CrossRef] [PubMed]
- Schievink, W.I.; Meyer, F.B.; Atkinson, J.L.; Mokri, B. Spontaneous spinal cerebrospinal fluid leaks and intracranial hypotension. J. Neurosurg. 1996, 84, 598–605. [Google Scholar] [CrossRef] [PubMed]
- D’Antona, L.; Merchan, M.A.J.; Vassiliou, A.; Watkins, L.D.; Davagnanam, I.; Toma, A.K.; Matharu, M.S. Clinical presentation, investigation findings, and treatment outcomes of spontaneous intracranial hypotension syndrome: A systematic review and meta-analysis. JAMA Neurol. 2021, 78, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Schievink, W.I. Spontaneous spinal cerebrospinal fluid leaks and intracranial hypotension. JAMA 2006, 295, 2286–2296. [Google Scholar] [CrossRef]
- Reinstein, E.; Pariani, M.; Bannykh, S.; Rimoin, D.L.; Schievink, W.I. Connective tissue spectrum abnormalities associated with spontaneous cerebrospinal fluid leaks: A prospective study. Eur. J. Hum. Genet. 2013, 21, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Olesen, J.; Dodick, D.; Ducros, A.; Evers, S.; First, M.; Goadsby, P. The International Classification of Headache Disorders, (ICHD-3). Cephalalgia 2018, 38, 1–211. [Google Scholar]
- Holste, K.G.; Muraszko, K.M.; Maher, C.O. Epidemiology of Chiari I Malformation and Syringomyelia. Neurosurg. Clin. N. Am. 2023, 34, 9–15. [Google Scholar] [CrossRef]
- Sadler, B.; Kuensting, T.; Strahle, J.; Park, T.S.; Smyth, M.; Limbrick, D.D.; Dobbs, M.B.; Haller, G.; Gurnett, C.A. Prevalence and impact of underlying diagnosis and comorbidities on Chiari 1 malformation. Pediatr. Neurol. 2020, 106, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Kahn, E.N.; Muraszko, K.M.; Maher, C.O. Prevalence of Chiari I Malformation and Syringomyelia. Neurosurg. Clin. N. Am. 2015, 26, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Ciaramitaro, P.; Garbossa, D.; Peretta, P.; Piatelli, G.; Massimi, L.; Valentini, L.G.; Migliaretti, G.; Baldovino, S.; Roccatello, D.; Kodra, Y.; et al. Syringomyelia and Chiari Syndrome Registry: Advances in epidemiology, clinical phenotypes and natural history based on a North Western Italy cohort. Ann. Dell’istituto Super. Sanità 2020, 56, 48–58. [Google Scholar]
- Bogdanov, E.I.; Faizutdinova, A.T.; Mendelevich, E.G.; Sozinov, A.S.; Heiss, J.D. Epidemiology of Symptomatic Chiari Malformation in Tatarstan: Regional and Ethnic Differences in Prevalence. Neurosurgery 2019, 84, 1090–1097. [Google Scholar] [CrossRef] [PubMed]
- Kranz, P.G.; Gray, L.; Amrhein, T.J. Spontaneous intracranial hypotension: 10 myths and misperceptions. Headache J. Head Face Pain 2018, 58, 948–959. [Google Scholar] [CrossRef]
- Schievink, W.; Maya, M.; Louy, C.; Moser, F.; Tourje, J. Diagnostic criteria for spontaneous spinal CSF leaks and intracranial hypotension. Am. J. Neuroradiol. 2008, 29, 853–856. [Google Scholar] [CrossRef]
- Schievink, W.I.; Schwartz, M.S.; Maya, M.M.; Moser, F.G.; Rozen, T.D. Lack of causal association between spontaneous intracranial hypotension and cranial cerebrospinal fluid leaks. J. Neurosurg. 2012, 116, 749–754. [Google Scholar] [CrossRef]
- Mokri, B. Spontaneous intracranial hypotension. Contin. Lifelong Learn. Neurol. 2015, 21, 1086–1108. [Google Scholar] [CrossRef]
- Schievink, W.I.; Jacques, L. Recurrent spontaneous spinal cerebrospinal fluid leak associated with “nude nerve root” syndrome: Case report. Neurosurgery 2003, 53, 1216–1219. [Google Scholar] [CrossRef]
- Kranz, P.G.; Luetmer, P.H.; Diehn, F.E.; Amrhein, T.J.; Tanpitukpongse, T.P.; Gray, L. Myelographic techniques for the detection of spinal CSF leaks in spontaneous intracranial hypotension. Am. J. Roentgenol. 2016, 206, 8–19. [Google Scholar] [CrossRef]
- Schievink, W.I.; Moser, F.G.; Maya, M.M. CSF–venous fistula in spontaneous intracranial hypotension. Neurology 2014, 83, 472–473. [Google Scholar] [CrossRef] [PubMed]
- Luetmer, P.H.; Schwartz, K.; Eckel, L.; Hunt, C.; Carter, R.; Diehn, F. When should I do dynamic CT myelography? Predicting fast spinal CSF leaks in patients with spontaneous intracranial hypotension. Am. J. Neuroradiol. 2012, 33, 690–694. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.-L.; Hu, X.-Y. Factors affecting cerebrospinal fluid opening pressure in patients with spontaneous intracranial hypotension. J. Zhejiang Univ. Sci. B 2017, 18, 577–585. [Google Scholar] [CrossRef]
- Kranz, P.G.; Tanpitukpongse, T.P.; Choudhury, K.R.; Amrhein, T.J.; Gray, L. How common is normal cerebrospinal fluid pressure in spontaneous intracranial hypotension? Cephalalgia 2016, 36, 1209–1217. [Google Scholar] [CrossRef] [PubMed]
- Mokri, B. Spontaneous cerebrospinal fluid leaks: From intracranial hypotension to cerebrospinal fluid hypovolemia—Evolution of a concept. Mayo Clin. Proc. 1999, 74, 1113–1123. [Google Scholar] [CrossRef]
- Alperin, N.; Lee, S.H.; Sivaramakrishnan, A.; Hushek, S.G. Quantifying the effect of posture on intracranial physiology in humans by MRI flow studies. J. Magn. Reson. Imaging 2005, 22, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Tain, R.W.; Bagci, A.M.; Lam, B.L.; Sklar, E.M.; Ertl-Wagner, B.; Alperin, N. Determination of cranio-spinal canal compliance distribution by MRI: Methodology and early application in idiopathic intracranial hypertension. J. Magn. Reson. Imaging 2011, 34, 1397–1404. [Google Scholar] [CrossRef] [PubMed]
- Medina, J.H.; Abrams, K.; Falcone, S.; Bhatia, R.G. Spinal imaging findings in spontaneous intracranial hypotension. Am. J. Roentgenol. 2010, 195, 459–464. [Google Scholar] [CrossRef]
- Alperin, N.; Bagci, A.; Lee, S.; Lam, B. Automated quantitation of spinal CSF volume and measurement of craniospinal CSF redistribution following lumbar withdrawal in idiopathic intracranial hypertension. Am. J. Neuroradiol. 2016, 37, 1957–1963. [Google Scholar] [CrossRef]
- Mokri, B. The Monro–Kellie hypothesis: Applications in CSF volume depletion. Neurology 2001, 56, 1746–1748. [Google Scholar] [CrossRef]
- Cushing, H. The third circulation. In Studies in Intracranial Physiology and Surgery; Oxford University Press: London, UK, 1926; pp. 1–51. [Google Scholar]
- Ciaramitaro, P.; Massimi, L.; Bertuccio, A.; Solari, A.; Farinotti, M.; Peretta, P.; Saletti, V.; Chiapparini, L.; Barbanera, A.; Garbossa, D.; et al. Diagnosis and treatment of Chiari malformation and syringomyelia in adults: International consensus document. Neurol. Sci. 2022, 43, 1327–1342. [Google Scholar] [CrossRef] [PubMed]
- Stovner, L.J.; Bergan, U.; Nilsen, G.; Sjaastad, O. Posterior cranial fossa dimensions in the Chiari I malformation: Relation to pathogenesis and clinical presentation. Neuroradiology 1993, 35, 113–118. [Google Scholar] [CrossRef]
- Buell, T.J.; Heiss, J.D.; Oldfield, E.H. Pathogenesis and Cerebrospinal Fluid Hydrodynamics of the Chiari I Malformation. Neurosurg. Clin. N. Am. 2015, 26, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, S.; Harada, K.; Abe, T.; Yamada, H.; Yokota, A. Syringomyelia secondary to tonsillar herniation caused by posterior fossa tumors. Surg. Neurol. 1995, 43, 470–475, discussion: 475–477. [Google Scholar] [CrossRef] [PubMed]
- Schievink, W.I.; Maya, M.M.; Jean-Pierre, S.; Nuño, M.; Prasad, R.S.; Moser, F.G. A classification system of spontaneous spinal CSF leaks. Neurology 2016, 87, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Farb, R.I.; Forghani, R.; Lee, S.; Mikulis, D.; Agid, R. The venous distension sign: A diagnostic sign of intracranial hypotension at MR imaging of the brain. Am. J. Neuroradiol. 2007, 28, 1489–1493. [Google Scholar] [CrossRef]
- Luetzen, N.; Dovi-Akue, P.; Fung, C.; Beck, J.; Urbach, H. Spontaneous intracranial hypotension: Diagnostic and therapeutic workup. Neuroradiology 2021, 63, 1765–1772. [Google Scholar] [CrossRef] [PubMed]
- Mokri, B. Headache associated with abnormalities in intracranial structure or function: Low cerebrospinal-fluid-pressure headache. In Wolff’s Headache and Other Head Pain; Oxford University Press: Oxford, UK, 2007; pp. 513–531. [Google Scholar]
- Mokri, B. Spontaneous CSF leaks: Low CSF volume syndromes. Neurol. Clin. 2014, 32, 397–422. [Google Scholar] [CrossRef]
- Yamamoto, M.; Suehiro, T.; Nakata, H.; Nishioka, T.; Itoh, H.; Nakamura, T.; Hashimoto, K. Primary low cerebrospinal fluid pressure syndrome associated with galactorrhea. Intern. Med. 1993, 32, 228–231. [Google Scholar] [CrossRef]
- Schievink, W.I.; Nuño, M.; Rozen, T.D.; Maya, M.M.; Mamelak, A.N.; Carmichael, J.; Bonert, V.S. Hyperprolactinemia due to spontaneous intracranial hypotension. J. Neurosurg. 2015, 122, 1020–1025. [Google Scholar] [CrossRef]
- Albayram, S.; Wasserman, B.A.; Yousem, D.M.; Wityk, R. Intracranial hypotension as a cause of radiculopathy from cervical epidural venous engorgement: Case report. Am. J. Neuroradiol. 2002, 23, 618–621. [Google Scholar] [PubMed]
- Farb, R.; Nicholson, P.; Peng, P.; Massicotte, E.; Lay, C.; Krings, T. Spontaneous intracranial hypotension: A systematic imaging approach for CSF leak localization and management based on MRI and digital subtraction myelography. Am. J. Neuroradiol. 2019, 40, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Dobrocky, T.; Grunder, L.; Breiding, P.S.; Branca, M.; Limacher, A.; Mosimann, P.J.; Mordasini, P.; Zibold, F.; Haeni, L.; Jesse, C.M.; et al. Assessing spinal cerebrospinal fluid leaks in spontaneous intracranial hypotension with a scoring system based on brain magnetic resonance imaging findings. JAMA Neurol. 2019, 76, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Mokri, B.; Krueger, B.R.; Miller, G.M.; Piepgras, D.G. Meningeal gadolinium enhancement in low-pressure headaches. J. Neuroimaging 1993, 3, 11–15. [Google Scholar] [CrossRef]
- Schievink, W.I. Spontaneous intracranial hypotension. N. Engl. J. Med. 2021, 385, 2173–2178. [Google Scholar] [CrossRef]
- Watanabe, A.; Horikoshi, T.; Uchida, M.; Koizumi, H.; Yagishita, T.; Kinouchi, H. Diagnostic value of spinal MR imaging in spontaneous intracranial hypotension syndrome. Am. J. Neuroradiol. 2009, 30, 147–151. [Google Scholar] [CrossRef]
- Chiapparini, L.; Farina, L.; D’Incerti, L.; Erbetta, A.; Pareyson, D.; Carriero, M.; Savoiardo, M. Spinal radiological findings in nine patients with spontaneous intracranial hypotension. Neuroradiology 2002, 44, 143–150. [Google Scholar] [CrossRef]
- Piechowiak, E.I.; Pospieszny, K.; Haeni, L.; Jesse, C.M.; Peschi, G.; Mosimann, P.J.; Kaesmacher, J.; Mordasini, P.; Raabe, A.; Ulrich, C.T.; et al. Role of Conventional Dynamic Myelography for Detection of High-Flow Cerebrospinal Fluid Leaks. Clin. Neuroradiol. 2021, 31, 633–641. [Google Scholar] [CrossRef]
- Kim, D.K.; Carr, C.M.; Benson, J.C.; Diehn, F.E.; Lehman, V.T.; Liebo, G.B.; Morris, J.M.; Morris, P.P.; Verdoorn, J.T.; Cutsforth-Gregory, J.K.; et al. Diagnostic yield of lateral decubitus digital subtraction myelogram stratified by brain MRI findings. Neurology 2021, 96, e1312–e1318. [Google Scholar] [CrossRef]
- Kranz, P.G.; Amrhein, T.J.; Gray, L. CSF venous fistulas in spontaneous intracranial hypotension: Imaging characteristics on dynamic and CT myelography. Am. J. Roentgenol. 2017, 209, 1360–1366. [Google Scholar] [CrossRef]
- Kranz, P.; Gray, L.; Taylor, J. CT-guided epidural blood patching of directly observed or potential leak sites for the targeted treatment of spontaneous intracranial hypotension. Am. J. Neuroradiol. 2011, 32, 832–838. [Google Scholar] [CrossRef] [PubMed]
- Thielen, K.R.; Sillery, J.C.; Morris, J.M.; Hoxworth, J.M.; Diehn, F.E.; Wald, J.T.; Rosebrock, R.E.; Yu, L.; Luetmer, P.H. Ultrafast dynamic computed tomography myelography for the precise identification of high-flow cerebrospinal fluid leaks caused by spiculated spinal osteophytes. J. Neurosurg. Spine 2015, 22, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.L.H.; Vuong, K.; Chugh, T.; Carroll, I. Cerebellar Tonsillar Descent: A Diagnostic Dilemma between Chiari Malformation Type 1 and Spinal Cerebrospinal Fluid Leak. Heliyon 2021, 7, e06795. [Google Scholar] [CrossRef]
- Duvall, J.R.; Robertson, C.E.; Cutsforth-Gregory, J.K.; Carr, C.M.; Atkinson, J.L.; Garza, I. Headache due to spontaneous spinal cerebrospinal fluid leak secondary to cerebrospinal fluid-venous fistula: Case series. Cephalalgia 2019, 39, 1847–1854. [Google Scholar] [CrossRef]
- Houk, J.L.; Amrhein, T.J.; Gray, L.; Malinzak, M.D.; Kranz, P.G. Differentiation of Chiari Malformation Type 1 and Spontaneous Intracranial Hypotension Using Objective Measurements of Midbrain Sagging. J. Neurosurg. 2021, 136, 1796–1803. [Google Scholar] [CrossRef]
- Atkinson, J.L.; Weinshenker, B.G.; Miller, G.M.; Piepgras, D.G.; Mokri, B. Acquired Chiari I malformation secondary to spontaneous spinal cerebrospinal fluid leakage and chronic intracranial hypotension syndrome in seven cases. J. Neurosurg. 1998, 88, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Kranz, P.G.; Tanpitukpongse, T.P.; Choudhury, K.R.; Amrhein, T.J.; Gray, L. Imaging signs in spontaneous intracranial hypoten sion: Prevalence and relationship to CSF pressure. AJNR Am. J. Neuroradiol. 2016, 37, 1374–1378. [Google Scholar] [CrossRef] [PubMed]
- Mea, E.; Chiapparini, L.; Leone, M.; Franzini, A.; Messina, G.; Bussone, G. Chronic daily headache in the adults: Differential diagnosis between symptomatic Chiari I malformation and spontaneous intracranial hypotension. Neurol Sci. 2011, 32 (Suppl. 3), S291–S294. [Google Scholar] [CrossRef]
- Nwotchouang, B.S.T.; Eppelheimer, M.S.; Bishop, P.; Biswas, D.; Andronowski, J.M.; Bapuraj, J.R.; Frim, D.; Labuda, R.; Amini, R.; Loth, F. Three-dimensional CT morphometric image analysis of the clivus and sphenoid sinus in chiari malformation type I. Ann. Biomed. Eng. 2019, 47, 2284–2295. [Google Scholar] [CrossRef]
- Brinjikji, W.; Savastano, L.; Atkinson, J.; Garza, I.; Farb, R.; Cutsforth-Gregory, J. A novel endovascular therapy for CSF hypotension secondary to CSF-venous fistulas. Am. J. Neuroradiol. 2021, 42, 882–887. [Google Scholar] [CrossRef]
- Mamlouk, M.D.; Shen, P.Y.; Sedrak, M.F.; Dillon, W.P. CT-guided fibrin glue occlusion of cerebrospinal fluid–venous fistulas. Radiology 2021, 299, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Pagani-Estévez, G.L.; Cutsforth-Gregory, J.K.; Morris, J.M.; Mokri, B.; Piepgras, D.G.; Mauck, W.D.; Eldrige, J.S.; Watson, J.C. Procedural predictors of epidural blood patch efficacy in spontaneous intracranial hypotension. Reg. Anesth. Pain Med. 2019, 44, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Bergui, M.; Mistretta, F.; Bosco, G.; Cester, G.; Chioffi, F.; Gambino, A.; Molinaro, S.; Russo, R.; Sorarù, G.; Causin, F. CSF-venous leak responsible for spontaneous intracranial hypotension treated by endovascular venous route: First cases in Italy. Interv. Neuroradiol. 2022. [Google Scholar] [CrossRef] [PubMed]
| Morphological Type of Leak | Location of Leak | |
|---|---|---|
| Type 1 (60% of cases) - 1a - 1b | Dural tear Dural tear | Ventral dura Posterolateral dura |
| Type 2 (20% of cases) | ||
| - 2a | Simple single or multiple meningeal diverticula | Lateral dura |
| - 2b | Complex meningeal diverticula or dural ectasia | Lateral dura |
| Type 3 (20% of cases) | Direct CSF-venous fistula | Distal nerve root sleeve |
| Type 4 | Indeterminate origin |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bin Wan Hassan, W.M.N.; Mistretta, F.; Molinaro, S.; Russo, R.; Bosco, G.; Gambino, A.; Bergui, M. Overview of Spontaneous Intracranial Hypotension and Differential Diagnosis with Chiari I Malformation. J. Clin. Med. 2023, 12, 3287. https://doi.org/10.3390/jcm12093287
Bin Wan Hassan WMN, Mistretta F, Molinaro S, Russo R, Bosco G, Gambino A, Bergui M. Overview of Spontaneous Intracranial Hypotension and Differential Diagnosis with Chiari I Malformation. Journal of Clinical Medicine. 2023; 12(9):3287. https://doi.org/10.3390/jcm12093287
Chicago/Turabian StyleBin Wan Hassan, Wan Muhammad Nazief, Francesco Mistretta, Stefano Molinaro, Riccardo Russo, Giovanni Bosco, Andrea Gambino, and Mauro Bergui. 2023. "Overview of Spontaneous Intracranial Hypotension and Differential Diagnosis with Chiari I Malformation" Journal of Clinical Medicine 12, no. 9: 3287. https://doi.org/10.3390/jcm12093287
APA StyleBin Wan Hassan, W. M. N., Mistretta, F., Molinaro, S., Russo, R., Bosco, G., Gambino, A., & Bergui, M. (2023). Overview of Spontaneous Intracranial Hypotension and Differential Diagnosis with Chiari I Malformation. Journal of Clinical Medicine, 12(9), 3287. https://doi.org/10.3390/jcm12093287






