A Systematic Review of Non-Opioid Pain Management in Chiari Malformation (Type 1) Patients: Current Evidence and Novel Therapeutic Opportunities
Abstract
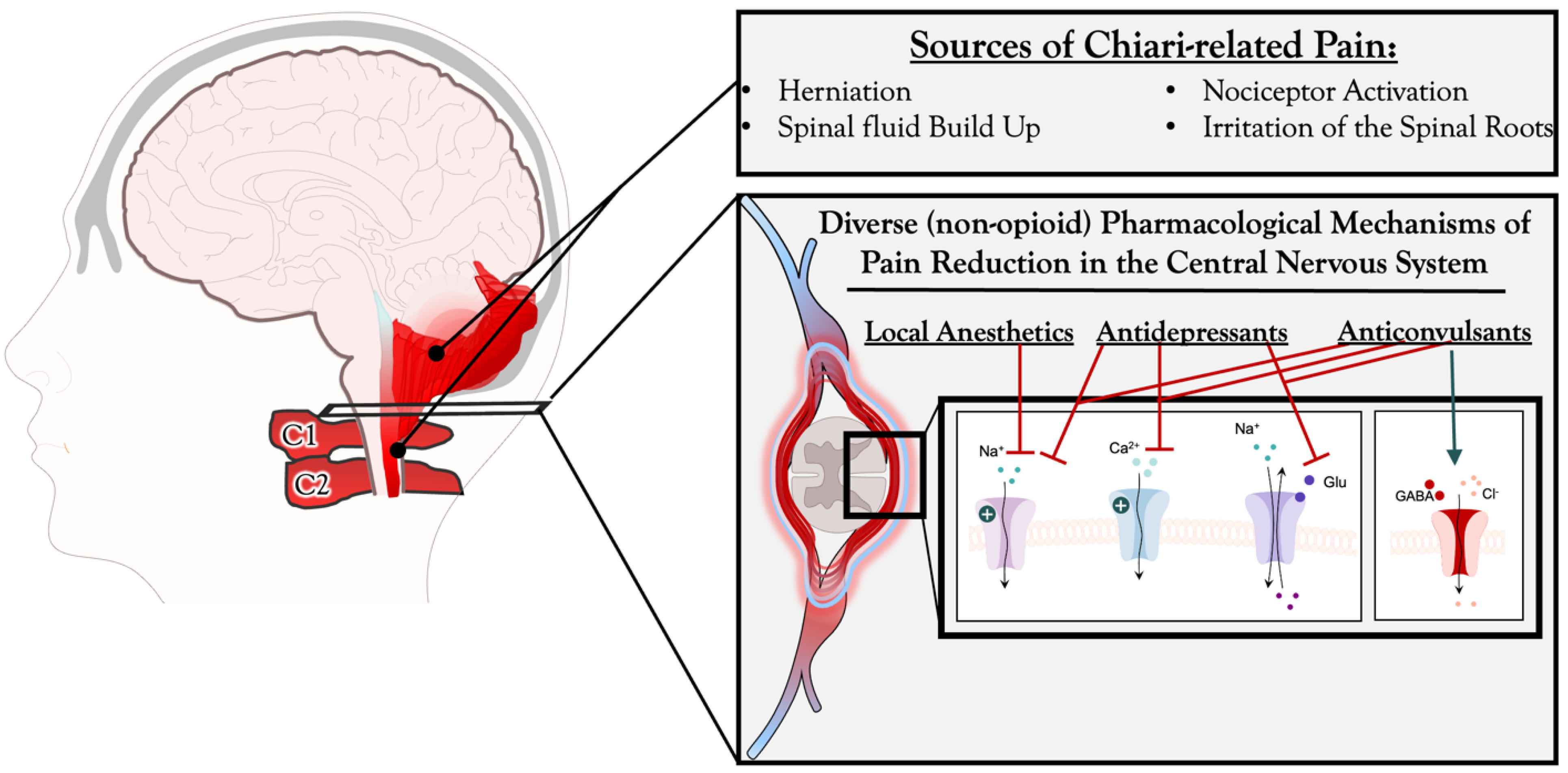
1. Introduction
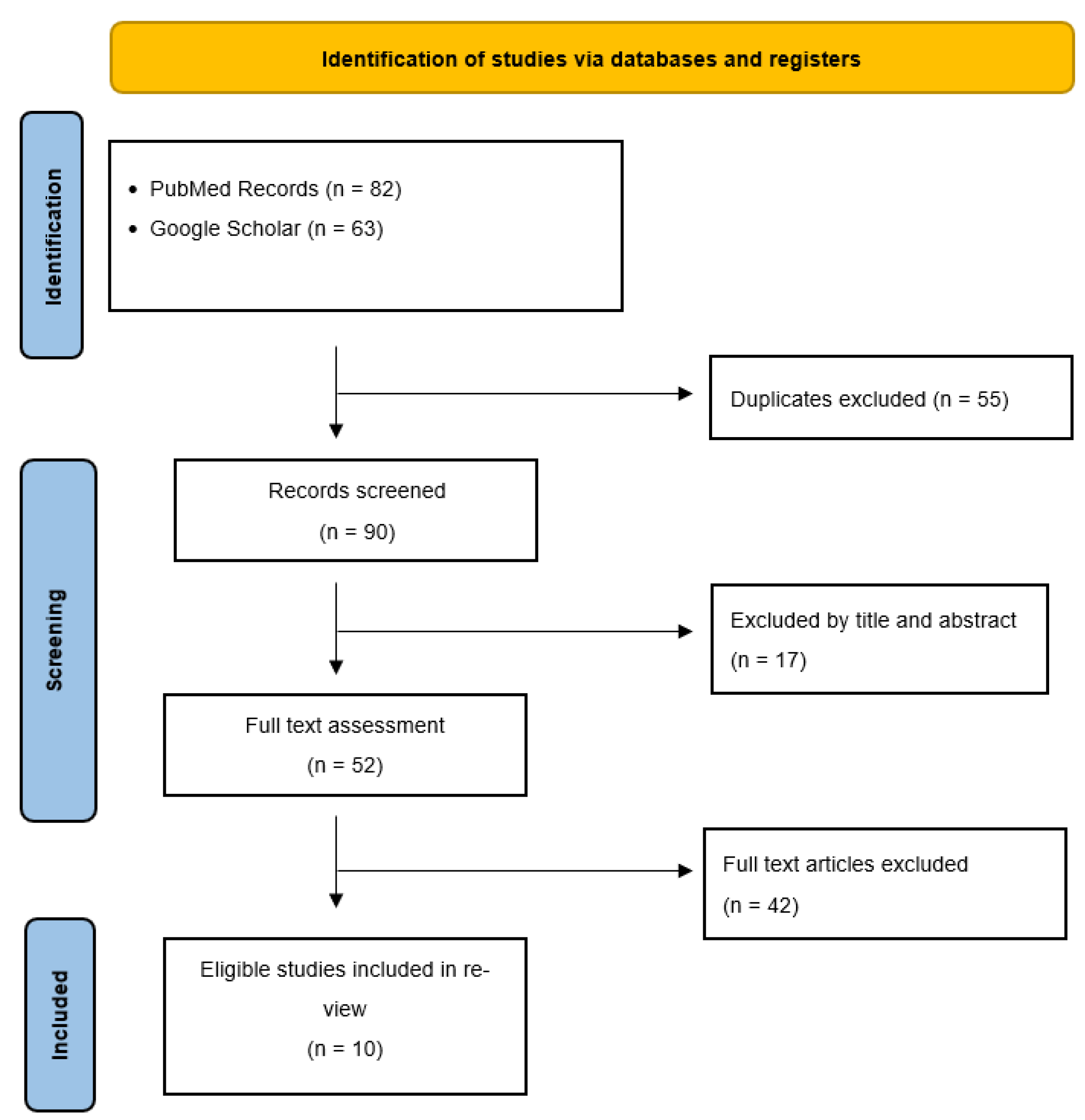
2. Materials and Methods
3. Results
3.1. Anticonvulsants and Diuretics for Pain Management in Chiari Malformation Patients
3.2. Local Anesthetics and Nerve Blocks for Pain Management in CM Patients
3.3. Neurotoxin Therapy for Pain Management in CM Patients
3.4. Cannabinoid Drugs for Pain Management in CM Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hidalgo, J.A.; Tork, C.A.; Varacallo, M. Arnold Chiari Malformation. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2021. [Google Scholar]
- Brugliera, L.; Iannaccone, S.; Nocera, G.; Cimino, P.; D’angelo, G.; Mortini, P.; Capodaglio, P.; Spina, A. Therapeutic cannabis for pain management in a patient with Chiari malformation type I during concomitant SARS-COV-2 infection. J. Neurosurg. Sci. 2023, 67, 129–130. [Google Scholar] [CrossRef]
- Tubbs, R.S.; Beckman, J.; Naftel, R.P.; Chern, J.J.; Wellons, J.C.; Rozzelle, C.J.; Blount, J.P.; Oakes, W.J. Institutional experience with 500 cases of surgically treated pediatric Chiari malformation Type I. J. Neurosurg. Pediatr. 2011, 7, 248–256. [Google Scholar] [CrossRef]
- McClugage, S.G.; Oakes, W.J. The Chiari I malformation. J. Neurosurg. Pediatr. 2019, 24, 217–226. [Google Scholar] [CrossRef]
- Labuda, R.; Nwotchouang, B.S.T.; Ibrahimy, A.; Allen, P.A.; Oshinski, J.N.; Klinge, P.; Loth, F. A new hypothesis for the pathophysiology of symptomatic adult Chiari malformation Type I. Med. Hypotheses 2021, 158, 110740. [Google Scholar] [CrossRef]
- Xu, H.; Chu, L.; He, R.; Ge, C.; Lei, T. Posterior fossa decompression with and without duraplasty for the treatment of Chiari malformation type I—A systematic review and meta-analysis. Neurosurg. Rev. 2016, 40, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Sadler, B.; Kuensting, T.; Strahle, J.; Park, T.S.; Smyth, M.; Limbrick, D.D.; Dobbs, M.B.; Haller, G.; Gurnett, C.A. Prevalence and Impact of Underlying Diagnosis and Comorbidities on Chiari 1 Malformation. Pediatr. Neurol. 2020, 106, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Vadivelu, N.; Kai, A.; Tran, D.; Kodumudi, G.; Legler, A.; Ayrian, E. Options for perioperative pain management in neurosurgery. J. Pain Res. 2016, 9, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Kaye, A.D.; Motejunas, M.W.; Cornett, E.M.; Ehrhardt, K.P.; Novitch, M.B.; Class, J.; Siddaiah, H.; Hart, B.M.; Urman, R.D. Emerging Novel Pharmacological Non-opioid Therapies in Headache Management: A Comprehensive Review. Curr. Pain Headache Rep. 2019, 23, 53. [Google Scholar] [CrossRef]
- Golembiewski, J.; Dasta, J. Evolving Role of Local Anesthetics in Managing Postsurgical Analgesia. Clin. Ther. 2015, 37, 1354–1371. [Google Scholar] [CrossRef]
- Türe, H.; Sayin, M.; Karlikaya, G.; Bingol, C.A.; Aykac, B.; Türe, U. The Analgesic Effect of Gabapentin as a Prophylactic Anticonvulsant Drug on Postcraniotomy Pain: A Prospective Randomized Study. Obstet. Anesthesia Dig. 2009, 109, 1625–1631. [Google Scholar] [CrossRef] [PubMed]
- Kundra, S.; Mahendru, V.; Gupta, V.; Choudhary, A. Principles of neuroanesthesia in aneurysmal subarachnoid hemorrhage. J. Anaesthesiol. Clin. Pharmacol. 2014, 30, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Bekker, A.; Cooper, P.R.; Frempong-Boadu, A.; Babu, R.; Errico, T.; Lebovits, A. Evaluation of preoperative administration of the cyclooxygenase-2 inhibitor rofecoxib for the treatment of postoperative pain after lumbar disc surgery. Neurosurgery 2002, 50, 1053–1058. [Google Scholar] [CrossRef]
- McCartney, C.J.L.; Sinha, A.; Katz, J. A Qualitative Systematic Review of the Role of N-Methyl-d-Aspartate Receptor Antagonists in Preventive Analgesia. Obstet. Anesthesia Dig. 2004, 98, 1385–1400. [Google Scholar] [CrossRef] [PubMed]
- Guilfoyle, M.R.; Helmy, A.; Duane, D.; Hutchinson, P.J.A. Regional scalp block for postcraniotomy analgesia: A systematic review and meta-analysis. Obstet. Anesthesia Dig. 2013, 116, 1093–1102. [Google Scholar] [CrossRef] [PubMed]
- Chaki, T.; Sugino, S.; Janicki, P.K.; Ishioka, Y.; Hatakeyama, Y.; Hayase, T.; Kaneuchi-Yamashita, M.; Kohri, N.; Yamakage, M. Efficacy and Safety of a Lidocaine and Ropivacaine Mixture for Scalp Nerve Block and Local Infiltration Anesthesia in Patients Undergoing Awake Craniotomy. J. Neurosurg. Anesthesiol. 2016, 28, 1–5. [Google Scholar] [CrossRef]
- Nam, K.E.; Kim, J.S.; Hong, B.Y.; Sul, B.; Choi, H.; Jun, S.Y.; Lim, S.H. Botulinum Toxin Type A Injection for Neuropathic Pain in a Patient With a Brain Tumor: A Case Report. Ann. Rehabilitation Med. 2017, 41, 1088–1092. [Google Scholar] [CrossRef]
- Moisset, X.; Bouhassira, D.; Couturier, J.A.; Alchaar, H.; Conradi, S.; Delmotte, M.; Lanteri-Minet, M.; Lefaucheur, J.; Mick, G.; Piano, V.; et al. Pharmacological and non-pharmacological treatments for neuropathic pain: Systematic review and French recommendations. Rev. Neurol. 2020, 176, 325–352. [Google Scholar] [CrossRef]
- Tremont-Lukats, I.W.; Megeff, C.; Backonja, M.-M. Anticonvulsants for neuropathic pain syndromes: Mechanisms of action and place in therapy. Drugs 2000, 60, 1029–1052. [Google Scholar] [CrossRef]
- Vaphiades, M.S.; Braswell, R.; Vaphiades, M.S.; Braswell, R. Resolution of Chiari I Malformation Following Acetazolamide Therapy. Semin. Ophthalmol. 2007, 22, 9–11. [Google Scholar] [CrossRef]
- Vivas, A.C.; Shimony, N.; Jackson, E.M.; Xu, R.; Jallo, G.I.; Rodriguez, L.; Tuite, G.F.; Carey, C.M. Management of hydrocephalus and subdural hygromas in pediatric patients after decompression of Chiari malformation type I: Case series and review of the literature. J. Neurosurg. Pediatr. 2018, 22, 426–438. [Google Scholar] [CrossRef]
- Demols, P.; Vilain, S.; Van Nechel, C. Association hypertension intracranienne idi-opathique-malformation d’Arnold-Chiari: Danger! Bull. Soc. Belge Ophtalmol. 1998, 268, 153–158. (In French) [Google Scholar]
- Klocheva, E.G.; Komiakhov, A.V.; Zhukova, M.V. The use of topiramate at patients with combined craniovertebral anomaly. Zhurnal Nevrol. i psikhiatrii im. S.S. Korsakova 2009, 109, 49–51. (In Russian) [Google Scholar]
- Prasad, G.K.; Khanna, S.; Jaishree, S. Review of adjuvants to local anesthetics in peripheral nerve blocks: Current and future trends. Saudi J. Anaesth. 2020, 14, 77–84. [Google Scholar] [CrossRef]
- Kaye, A.D.; Armstead-Williams, C.; Hyatali, F.; Cox, K.S.; Kaye, R.J.; Eng, L.K.; Anwar, M.A.F.; Patel, P.V.; Patil, S.; Cornett, E.M. Exparel for Postoperative Pain Management: A Comprehensive Review. Curr. Pain Headache Rep. 2020, 24, 73. [Google Scholar] [CrossRef] [PubMed]
- Lu, V.M.; Daniels, D.J.; Haile, D.T.; Ahn, E.S. Effects of intraoperative liposomal bupivacaine on pain control and opioid use after pediatric Chiari I malformation surgery: An initial experience. J. Neurosurg. Pediatr. 2021, 27, 9–15. [Google Scholar] [CrossRef] [PubMed]
- LoPresti, M.A.; Harrell, B.N.M.; Goethe, E.; McClugage, S.; Wyatt, K.M.; Lam, S.K.M. Intrawound Liposomal Bupivacaine in Pediatric Chiari Decompression: A Retrospective Study. Pediatr. Qual. Saf. 2021, 6, e397. [Google Scholar] [CrossRef]
- Levin, D.; Cohen, S.; Mellender, S.; Kiss, G. Sphenopalatine Ganglion Block Successfully Treats Migraines in a Type 1 Arnold Chiari Malformation Pregnant Patient: A Case Report. A A Pract. 2018, 11, 32–34. [Google Scholar] [CrossRef]
- Sandrini, G.; De Icco, R.; Tassorelli, C.; Smania, N.; Tamburin, S. Botulinum neurotoxin type A for the treatment of pain: Not just in migraine and trigeminal neuralgia. J. Headache Pain 2017, 18, 38. [Google Scholar] [CrossRef] [PubMed]
- Felício, A.C.; Godeiro-Junior, C.D.O.; Borges, V.; Silva, S.M.D.A.; Ferraz, H.B. Hemifacial spasm in a patient with neurofibromatosis and Arnold-Chiari malformation: A unique case association. Arq. de Neuro-Psiquiatria 2007, 65, 855–857. [Google Scholar] [CrossRef]
- Felício, A.C.; de Godeiro, C.; Borges, V.; Silva, S.M.D.A.; Ferraz, H.B. Young onset Hemifacial Spasm in patients with Chiari type I malformation. Park. Relat. Disord. 2008, 14, 66–68. [Google Scholar] [CrossRef] [PubMed]
- Bridgeman, M.B.; Abazia, D.T. Medicinal Cannabis: History, Pharmacology, and Implications for the Acute Care Setting. Pharm. Ther. 2017, 42, 180–188. [Google Scholar]
- Avellaneda Fernandez, A.; Isla Guerrero, A.; Izquierdo Martínez, M.; Amado Vázquez, M.E.; Barrón Fernández, J.; Chesa i Octavio, E.; De la Cruz Labrado, J.; Escribano Silva, M.; Fernández de Gamboa Fernández de Araoz, M.; García-Ramos, R.; et al. Malformations of the craniocervical junction (chiari type I and syringomyelia: Classification, diagnosis and treatment). BMC Musculoskelet. Disord. 2009, 10, S1. [Google Scholar] [CrossRef] [PubMed]
- Holly, L.T.; Batzdorf, U. Chiari malformation and syringomyelia: JNSPG 75th Anniversary Invited Review Article. J. Neurosurg. Spine 2019, 31, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.A.; Allen, P.A.; Li, X.; Houston, J.; Loth, F.; Labuda, R.; Delahanty, D.L. An examination of pain, disability, and the psychological correlates of Chiari Malformation pre- and post-surgical correction. Disabil Health J. 2019, 12, 649–656. [Google Scholar] [CrossRef] [PubMed]
| Search Keywords | Hits | Included |
|---|---|---|
| (anticonvulsant) AND ((chiari) OR (chiari malformation)) | 38 | 4 |
| (antidepressants) AND ((chiari) OR (chiari malformation)) | 7 | 0 |
| (local anesthetic) AND ((chiari) OR (chiari malformation)) | 18 | 2 |
| (exparel) AND ((chiari) OR (chiari malformation)) | 10 | 1 |
| (bupivacaine liposome) AND ((chiari) OR (chiari malformation)) | 1 | 1 |
| (anticholinergic) AND ((chiari) OR (chiari malformation)) | 6 | 0 |
| ((botox) OR (botulinum toxin)) AND ((chiari) OR (chiari malformation)) | 7 | 2 |
| (cannabinoid) AND ((chiari) OR (chiari malformation)) | 1 | 0 |
| (cannabis) AND ((chiari) OR (chiari malformation)) | 1 | 1 |
| (marijuana) AND ((chiari) OR (chiari malformation)) | 1 | 0 |
| (CBD) AND ((chiari) OR (chiari malformation)) | 0 | 0 |
| (THC) AND ((chiari) OR (chiari malformation)) | 0 | 0 |
| Type | Mode of Action | Drug | Side Effect |
|---|---|---|---|
| Anticonvulsants | Inhibit opening neuronal voltage-dependent channels (Calcium, sodium) and GABA receptor. |
| Hepatotoxicity, drowsiness, fatigue, ataxia, vertigo, gastrointestinal discomfort, headache, blurred vision |
| Antidepressants | Inhibit re-uptake of norepinephrine and serotonin by neurons. |
| Mouth dryness, intense sedation, fatigue, diminished libido, weight loss, nausea, insomnia, headache |
| Local Anesthetics | Inhibit sodium influx through sodium-specific ion channels in the neuronal cell membrane. |
| Dizziness, arrhythmia |
| Analgesics | Act through specific receptors, particularly μ receptors distributed throughout the central and peripheral nervous system blocking them. |
| Nausea, vomiting, sweating, dizziness, mouth dryness sedation, vertigo |
| Diuretic (carbonic anhydrase inhibitors) | Works by causing an accumulation of carbonic acid by preventing its breakdown, resulting in the excretion of sodium, bicarbonate and chloride from the proximal tubule of the kidney |
| Fatigue, nausea, vomiting, abdominal pain, and diarrhea |
| Neurotoxin | Binds pre-synaptically to high-affinity recognition sites on cholinergic nerve terminals and decreases the release of acetylcholine |
| Muscle paralysis, headaches, flu-like symptoms, and allergic reactions |
| Cannabinoids | Binds and activates two types of G-protein-coupled receptors, CB1 and CB2 which results in an inhibition of the release of the neurotransmitters acetylcholine and glutamate while indirectly affecting y-aminobutyric acid, N-methyl-D-aspartate, opioid and serotonin receptors. |
| Altered senses, changes in mood, difficulty with cognition, hallucinations, delusions and impaired memory. |
| Name of Study | Journal | Author | Year | Number of Patients | Sex of Patients | Age | Therapy Specifics | Conclusions |
|---|---|---|---|---|---|---|---|---|
| Therapeutic cannabis for pain management in a patient with chiari malformation type i during concomitant SARS-CoV-2 infection | Journal of Neurosurgical Science | Luigia Brugliera et al. [2] | 2023 | 1 | F | 18+ | pharmacological | Support for cannabis in improvement of CM headaches |
| Resolution of Chiari I malformation following acetazolamide therapy | Seminars in Ophthalmology | Michael Vaphides et al. [20] | 2007 | 1 | F | 18+ | pharmacological | CM resolution, supporting CSF flow study for CM patients |
| Management of hydrocephalus and subdural hygromas in pediatric patients after decompression of Chiari malformation type I: case series and review of the literature | JNS Pediatrics | Andrew C. Vivas et al. [21] | 2018 | 5 | M, F | <18 | surgical, then pharmacological | Supporting non-operative management of de novo hydrocephalus following CM decompression |
| Association of idiopathic intracranial hypertension- Arnold-Chiari deformity- danger! | Bull Soc Belge Ophtalmol | P Demols et al. [22] | 1998 | 1 | F | 18+ | surgical | supporting link between IIH and CM |
| The use of topiramate at patients with combined craniovertebral anomaly | Zh Nevrol Psikhiatr Im S S Korsakova | E G Klocheva et al. [23] | 2019 | 28 | M, F | 18+ | pharmacological | supporting use of Topamax for reduction of headache in CM |
| Effects of intraoperative liposomal bupivacaine on pain control and opioid use after pediatric Chiari I malformation surgery: an initial experience | JNS Pediatrics | Victor M Lu et al. [26] | 2020 | 18 | M, F | <18 | pharmacological | supporting intraoperative LB use to reduce pain scores within 24 h post-op |
| Intrawound Liposomal Bupivacaine in Pediatric Chiari Decompression: A Retrospective Study | Pediatr Qual Saf | Melissa A LoPresti et al. [27] | 2021 | 30 | M, F | <18 | pharmacological | supporting LB use for decreased opioid use and pain following CM decompression |
| Sphenopalatine Ganglion Block Successfully Treats Migraines in a Type 1 Arnold Chiari Malformation Pregnant Patient: A Case Report | A&A Practice | Danielle Levin et al. [28] | 2018 | 1 | F | 18+ | pharmacological | supporting transnasal sphenopalatine ganglion blocks in resolution of CM headaches |
| Hemifacial spasm in a patient with neurofibromatosis and Arnold-Chiari malformation: a unique case association | Arq Neuropsiquiatr | Andre Carvalho Felicio et al. [30] | 2007 | 1 | F | 18+ | pharmacological | supporting botulinum toxin for HFS in CM |
| Young onset Hemifacial Spasm in patients with Chiari type I malformation | Parkinsonism Relat Disord | Andre Carvalho Felicio et al. [31] | 2008 | 5 | M, F | 18+ | pharmacological | supporting botulinum toxin for HFS in CM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barpujari, A.; Kiley, A.; Ross, J.A.; Veznedaroglu, E. A Systematic Review of Non-Opioid Pain Management in Chiari Malformation (Type 1) Patients: Current Evidence and Novel Therapeutic Opportunities. J. Clin. Med. 2023, 12, 3064. https://doi.org/10.3390/jcm12093064
Barpujari A, Kiley A, Ross JA, Veznedaroglu E. A Systematic Review of Non-Opioid Pain Management in Chiari Malformation (Type 1) Patients: Current Evidence and Novel Therapeutic Opportunities. Journal of Clinical Medicine. 2023; 12(9):3064. https://doi.org/10.3390/jcm12093064
Chicago/Turabian StyleBarpujari, Awinita, Alina Kiley, Jennifer A. Ross, and Erol Veznedaroglu. 2023. "A Systematic Review of Non-Opioid Pain Management in Chiari Malformation (Type 1) Patients: Current Evidence and Novel Therapeutic Opportunities" Journal of Clinical Medicine 12, no. 9: 3064. https://doi.org/10.3390/jcm12093064
APA StyleBarpujari, A., Kiley, A., Ross, J. A., & Veznedaroglu, E. (2023). A Systematic Review of Non-Opioid Pain Management in Chiari Malformation (Type 1) Patients: Current Evidence and Novel Therapeutic Opportunities. Journal of Clinical Medicine, 12(9), 3064. https://doi.org/10.3390/jcm12093064






