Post-Operative Complications after Foramen Magnum Decompression with Duraplasty Using Different Graft Materials in Adults Patients with Chiari I Malformation: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Data Collection
2.3. Outcomes
2.4. Quality Scoring
2.5. Statistical Analysis
3. Results
3.1. Literature Review
3.2. Quality of Studies
3.3. Demographic Data and Clinical Characteristics
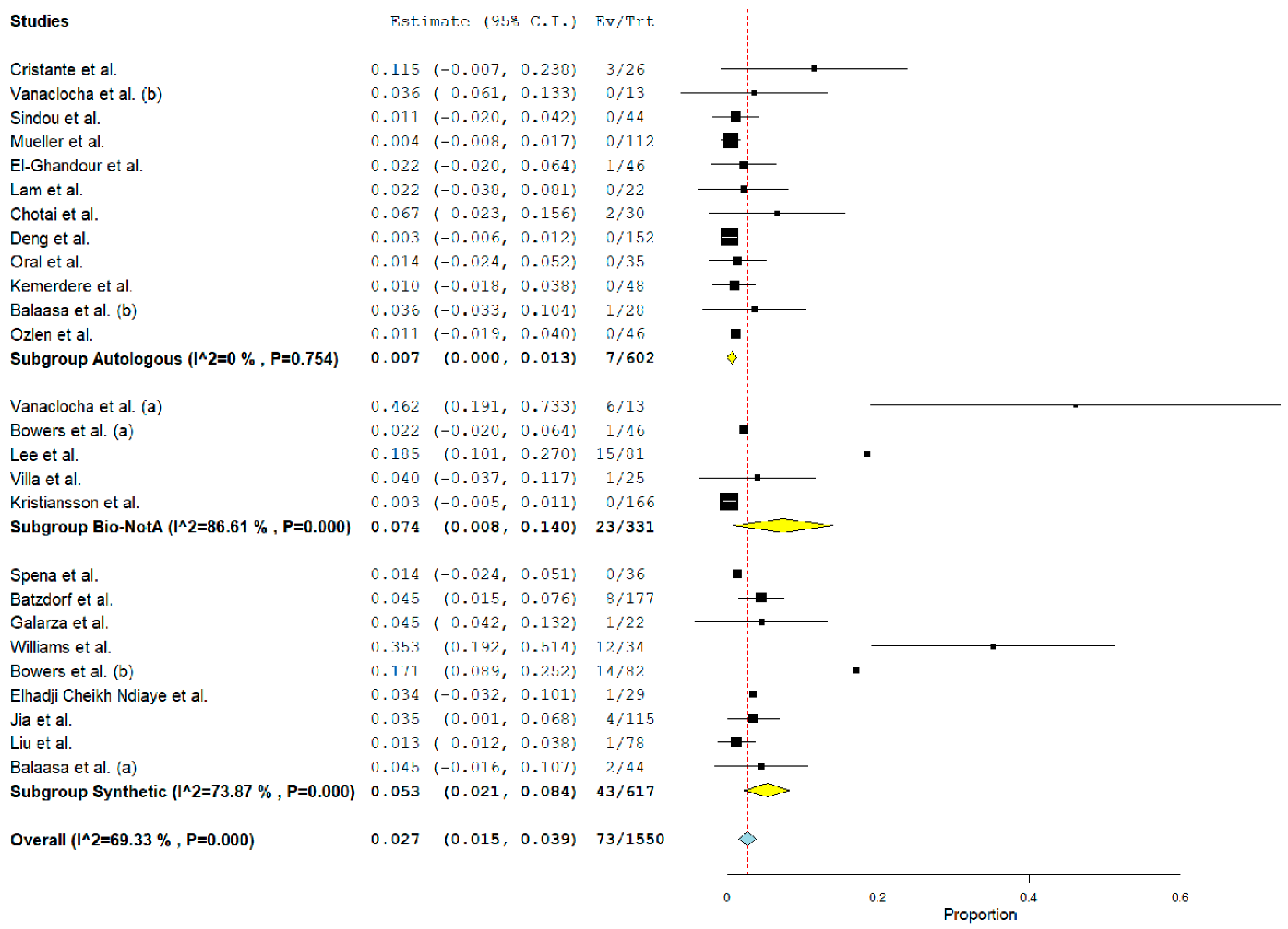
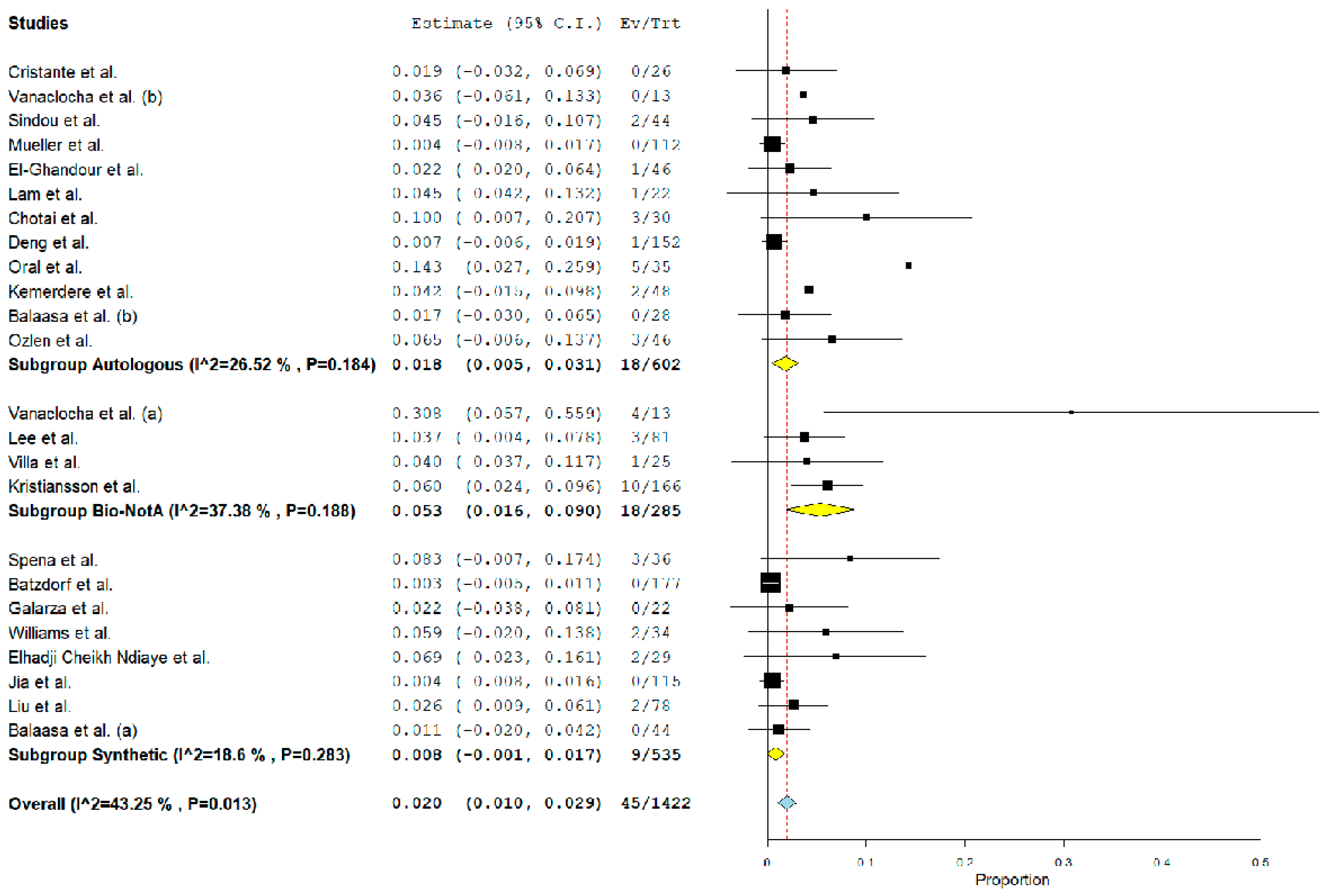
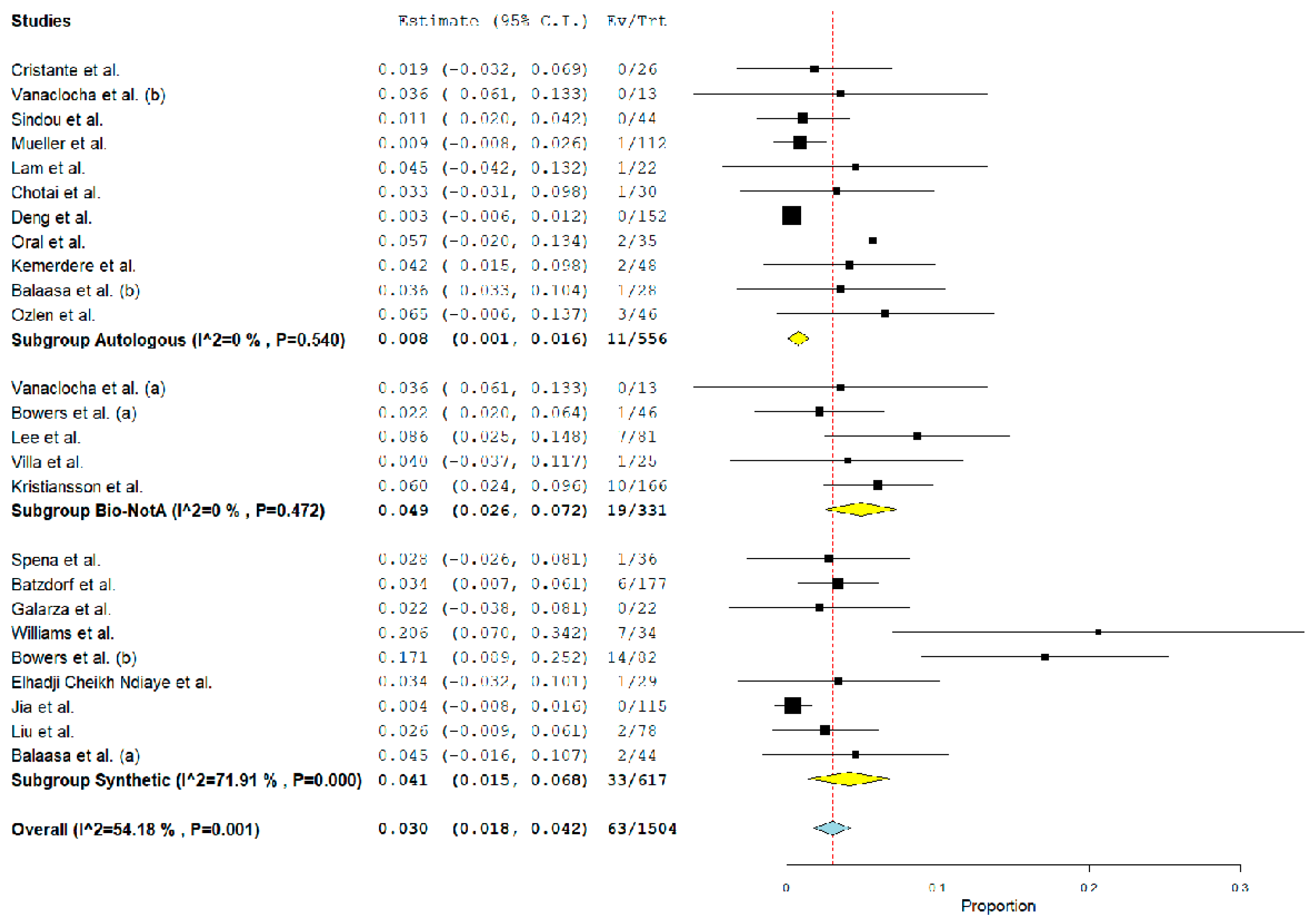
3.4. Post-Operative Complications Related to Graft Material
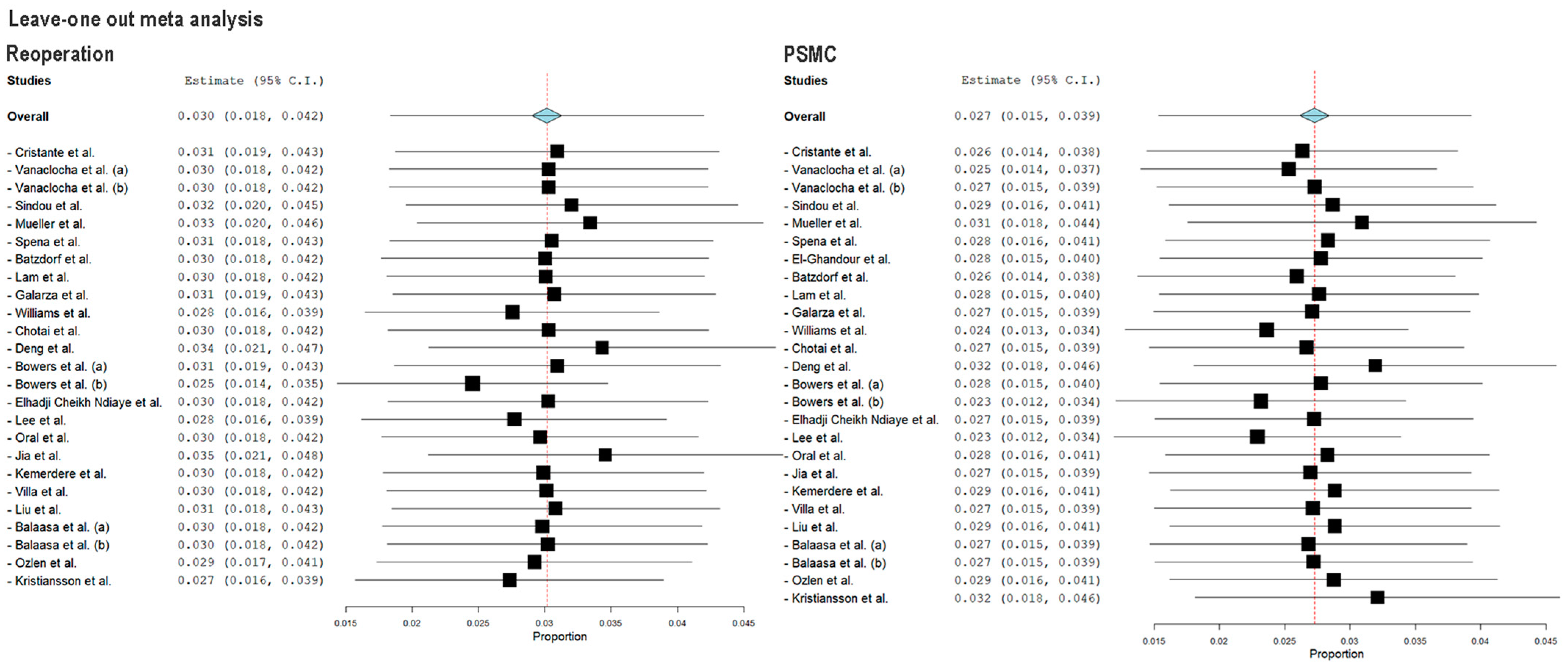
3.5. Study Heterogeneity and Sensitivity Analysis
4. Discussion
Limitation of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ciaramitaro, P.; Massimi, L.; Bertuccio, A.; Solari, A.; Farinotti, M.; Peretta, P.; Saletti, V.; Chiapparini, L.; Barbanera, A.; Garbossa, D.; et al. Diagnosis and Treatment of Chiari Malformation and Syringomyelia in Adults: International Consensus Document. Neurol. Sci. 2022, 43, 1327–1342. [Google Scholar] [CrossRef] [PubMed]
- Förander, P.; Sjåvik, K.; Solheim, O.; Riphagen, I.; Gulati, S.; Salvesen, Ø.; Jakola, A.S. The Case for Duraplasty in Adults Undergoing Posterior Fossa Decompression for Chiari I Malformation: A Systematic Review and Meta-Analysis of Observational Studies. Clin. Neurol. Neurosurg. 2014, 125, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Gurbuz, M.S.; Karaaslan, N.; Caliskan, T.; Unal, E.; Berkman, M.Z. Comparison of the Surgical Results for Foramen Magnum Decompression with and without Duraplasty in Chiari Malformation Type 1. Turk. Neurosurg. 2015, 25, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Perrini, P.; Anania, Y.; Cagnazzo, F.; Benedetto, N.; Morganti, R.; Di Carlo, D.T. Radiological Outcome after Surgical Treatment of Syringomyelia-Chiari I Complex in Adults: A Systematic Review and Meta-Analysis. Neurosurg. Rev. 2021, 44, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Parker, S.R.; Harris, P.; Cummings, T.J.; George, T.; Fuchs, H.; Grant, G. Complications Following Decompression of Chiari Malformation Type I in Children: Dural Graft or Sealant? Clinical Article. J. Neurosurg. Pediatr. 2011, 8, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Perrini, P.; Benedetto, N.; Tenenbaum, R.; Di Lorenzo, N. Extra-Arachnoidal Cranio-Cervical Decompression for Syringomyelia Associated with Chiari I Malformation in Adults: Technique Assessment. Acta Neurochir. 2007, 149, 1015–1022. [Google Scholar] [CrossRef]
- Klekamp, J. Surgical Treatment of Chiari i Malformation-Analysis of Intraoperative Findings, Complications, and Outcome for 371 Foramen Magnum Decompressions. Neurosurgery 2012, 71, 365–380. [Google Scholar] [CrossRef]
- Yahanda, A.T.; Simon, L.E.; Limbrick, D.D. Outcomes for Various Dural Graft Materials after Posterior Fossa Decompression with Duraplasty for Chiari Malformation Type I: A Systematic Review and Meta-Analysis. J. Neurosurg. 2021, 135, 1356–1369. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. PRISMA 2020 Explanation and Elaboration: Updated Guidance and Exemplars for Reporting Systematic Reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-Analyses. Ottawa Hosp. Res. Inst. 2013, 1–4. [Google Scholar]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring Inconsistency in Meta-Analyses. BMJ Br. Med. J. 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- DerSimonian, R.; Laird, N. Meta-Analysis in Clinical Trials Revisited. Contemp. Clin. Trials 2015, 45, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Cristante, L.; Westphal, M.; Herrmann, H.-D. Cranio-Cervical Decompression for Chiari I Malformation—A Retrospective Evaluation of Functional Outcome with Particular Attention to the Motor Deficits. Acta Neurochir. 1994, 130, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Vanaclocha, V.; Saiz-Sapena, N. Duraplasty with Freeze-Dried Cadaveric Dura versus Occipital Pericranium for Chiari Type I Malformation: Comparative Study. Acta Neurochir. 1997, 139, 112–119. [Google Scholar] [CrossRef]
- Sindou, M.; Chávez-Machuca, J.; Hashish, H.; Bagnall, W.; Sharpe, P.M.; Newham, P.; Tart, J.; Mott, R.A.; Torr, V.R.; Forder, R.A.; et al. Cranio-Cervical Decompression for Chiari Type I-Malformation, Adding Extreme Lateral Foramen Magnum Opening and Expansile Duroplasty with Arachnoid Preservation. Technique and Long-Term Functional Results in 44 Consecutive Adult Cases—Comparison with Li. Acta Neurochir. 2002, 144, 1005–1019. [Google Scholar] [CrossRef]
- Mueller, D.; Oro’, J.J. Prospective Analysis of Self-Perceived Quality of Life before and after Posterior Fossa Decompression in 112 Patients with Chiari Malformation with or without Syringomyelia. Neurosurg. Focus 2005, 18, ECP2. [Google Scholar] [CrossRef]
- Spena, G.; Bernucci, C.; Garbossa, D.; Valfrè, W.; Versari, P. Clinical and Radiological Outcome of Craniocervical Osteo-Dural Decompression for Chiari I-Associated Syringomyelia. Neurosurg. Rev. 2010, 33, 297–304. [Google Scholar] [CrossRef]
- El-Ghandour, N.M.F. Long-Term Outcome of Surgical Management of Adult Chiari I Malformation. Neurosurg. Rev. 2012, 35, 537. [Google Scholar] [CrossRef]
- Batzdorf, U.; Mcarthur, D.L.; Bentson, J.R. Surgical Treatment of Chiari Malformation with and without Syringomyelia: Experience with 177 Adult Patients; Clinical Article. J. Neurosurg. 2013, 118, 232–242. [Google Scholar] [CrossRef]
- Lam, F.C.; Penumaka, A.; Chen, C.C.; Fischer, E.G.; Kasper, E.M. Fibrin Sealant Augmentation with Autologous Pericranium for Duraplasty after Suboccipital Decompression in Chiari 1 Patients: A Case Series. Surg. Neurol. Int. 2013, 4, 6. [Google Scholar] [CrossRef]
- Galarza, M.; Gazzeri, R.; Alfieri, A.; Martínez-Lage, J.F. “Triple R” Tonsillar Technique for the Management of Adult Chiari I Malformation: Surgical Note. Acta Neurochir. 2013, 155, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.E.; Vannemreddy, P.S.; Watson, K.S.; Slavin, K. V The Need in Dural Graft Suturing in Chiari I Malformation Decompression: A Prospective, Single-Blind, Randomized Trial Comparing Sutured and Sutureless Duraplasty Materials. Surg. Neurol. Int. 2013, 4, 26. [Google Scholar] [CrossRef]
- Chotai, S.; Kshettry, V.R.; Lamki, T.; Ammirati, M. Surgical Outcomes Using Wide Suboccipital Decompression for Adult Chiari I Malformation with and without Syringomyelia. Clin. Neurol. Neurosurg. 2014, 120, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Yang, C.; Gan, J.; Wu, L.; Yang, T.; Yang, J.; Xu, Y. Long-Term Outcomes After Small-Bone-Window Posterior Fossa Decompression and Duraplasty in Adults with Chiari Malformation Type I. World Neurosurg. 2015, 84, 998–1004. [Google Scholar] [CrossRef]
- Bowers, C.A.; Brimley, C.; Cole, C.; Gluf, W.; Schmidt, R.H. AlloDerm for Duraplasty in Chiari Malformation: Superior Outcomes. Acta Neurochir. 2015, 157, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Elhadji Cheikh Ndiaye, S.Y.; Troude, L.; Al-Falasi, M.; Faye, M.; Melot, A.; Roche, P.-H. Chiari Malformations in Adults: A Single Center Surgical Experience with Special Emphasis on the Kinetics of Clinical Improvement. Neurochirurgie 2019, 65, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.K.; Mokhtari, T.; Connolly, I.D.; Li, G.; Shuer, L.M.; Chang, S.D.; Steinberg, G.K.; Hayden Gephart, M. Comparison of Porcine and Bovine Collagen Dural Substitutes in Posterior Fossa Decompression for Chiari I Malformation in Adults. World Neurosurg. 2017, 108, 33–40. [Google Scholar] [CrossRef]
- Oral, S.; Yilmaz, A.; Kucuk, A.; Tumturk, A.; Menku, A. Comparison of Dural Splitting and Duraplasty in Patients with Chiari Type I Malformation: Relationship between Tonsillo-Dural Distance and Syrinx Cavity. Turk. Neurosurg. 2019, 29, 229–236. [Google Scholar] [CrossRef]
- Jia, C.; Li, H.; Wu, J.; Gao, K.; Zhao, C.B.; Li, M.; Sun, X.; Yang, B. Comparison Decompression by Duraplasty or Cerebellar Tonsillectomy for Chiari Malformation-I Complicated with Syringomyelia. Clin. Neurol. Neurosurg. 2019, 176, 1–7. [Google Scholar] [CrossRef]
- Kemerdere, R.; Akgun, M.Y.; Cetintas, S.C.; Kacira, T.; Tanriverdi, T. Clinical and Radiological Outcomes of Arachnoid-Preseving Suboccipital Decompression for Adult Chiari I Malformation with and without Syringomyelia. Clin. Neurol. Neurosurg. 2020, 188, 105598. [Google Scholar] [CrossRef]
- Villa, A.; Imperato, A.; Maugeri, R.; Visocchi, M.; Iacopino, D.G.; Francaviglia, N. Surgical Treatment in Symptomatic Chiari Malformation Type I: A Series of 25 Adult Patients Treated with Cerebellar Tonsil Shrinkage. Acta Neurochir. Suppl. 2019, 125, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Wang, Y.; Liu, S.; Zhang, Y.; Lu, D.; Chen, L.; Zheng, T.; Zhao, T.; Zhao, L.; Sankey, E.W.; et al. Tonsillectomy with Modified Reconstruction of the Cisterna Magna with and without Craniectomy for the Treatment of Adult Chiari Malformation Type I with Syringomyelia. Acta Neurochir. 2020, 162, 1585–1595. [Google Scholar] [CrossRef]
- Balasa, A.; Kunert, P.; Dziedzic, T.; Bielecki, M.; Kujawski, S.; Marchel, A. Comparison of Dural Grafts and Methods of Graft Fixation in Chiari Malformation Type I Decompression Surgery. Sci. Rep. 2021, 11, 14801. [Google Scholar] [CrossRef] [PubMed]
- Özlen, F.; Kucukyuruk, B.; Alizada, O.; Guler, H.; Akgun, M.Y.; Kafadar, A.M.; Tuzgen, S.; Sanus, G.Z.; Hanci, M. Comparison of Two Surgical Techniques in Chiari Malformation Type 1 Patients: Duraplasty Alone vs Duraplasty with Arachnoid Dissection. Clin. Neurol. Neurosurg. 2021, 206, 106686. [Google Scholar] [CrossRef]
- Kristiansson, H.; Fletcher-Sandersjöö, A.; Cesarini, K.; Fransson, M.; Vlachogiannis, P.; Burström, G.; Hessington, A.; Bartek, J.J.; Edström, E.; Holmgren, R.T.; et al. Dura Management Strategies in the Surgical Treatment of Adult Chiari Type I Malformation: A Retrospective, Multicenter, Population-Based Parallel Cohort Case Series. Oper. Neurosurg. 2022, 23, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Perrini, P.; Rawlinson, A.; Cowie, R.A.; King, A.T. Acute External Hydrocephalus Complicating Craniocervical Decompression for Syringomyelia-Chiari I Complex: Case Report and Review of the Literature. Neurosurg. Rev. 2008, 31, 331–335. [Google Scholar] [CrossRef]
- Paré, L.S.; Batzdorf, U. Syringomyelia Persistence after Chiari Decompression as a Result of Pseudomeningocele Formation: Implications for Syrinx Pathogenesis: Report of Three Cases. Neurosurgery 1998, 43, 945–948. [Google Scholar] [CrossRef]
- Yahanda, A.T.; Adelson, P.D.; Akbari, S.H.A.; Albert, G.W.; Aldana, P.R.; Alden, T.D.; Anderson, R.C.E.; Bauer, D.F.; Bethel-Anderson, T.; Brockmeyer, D.L.; et al. Dural Augmentation Approaches and Complication Rates after Posterior Fossa Decompression for Chiari I Malformation and Syringomyelia: A Park-Reeves Syringomyelia Research Consortium Study. J. Neurosurg. Pediatr. 2021, 27, 459–468. [Google Scholar] [CrossRef]
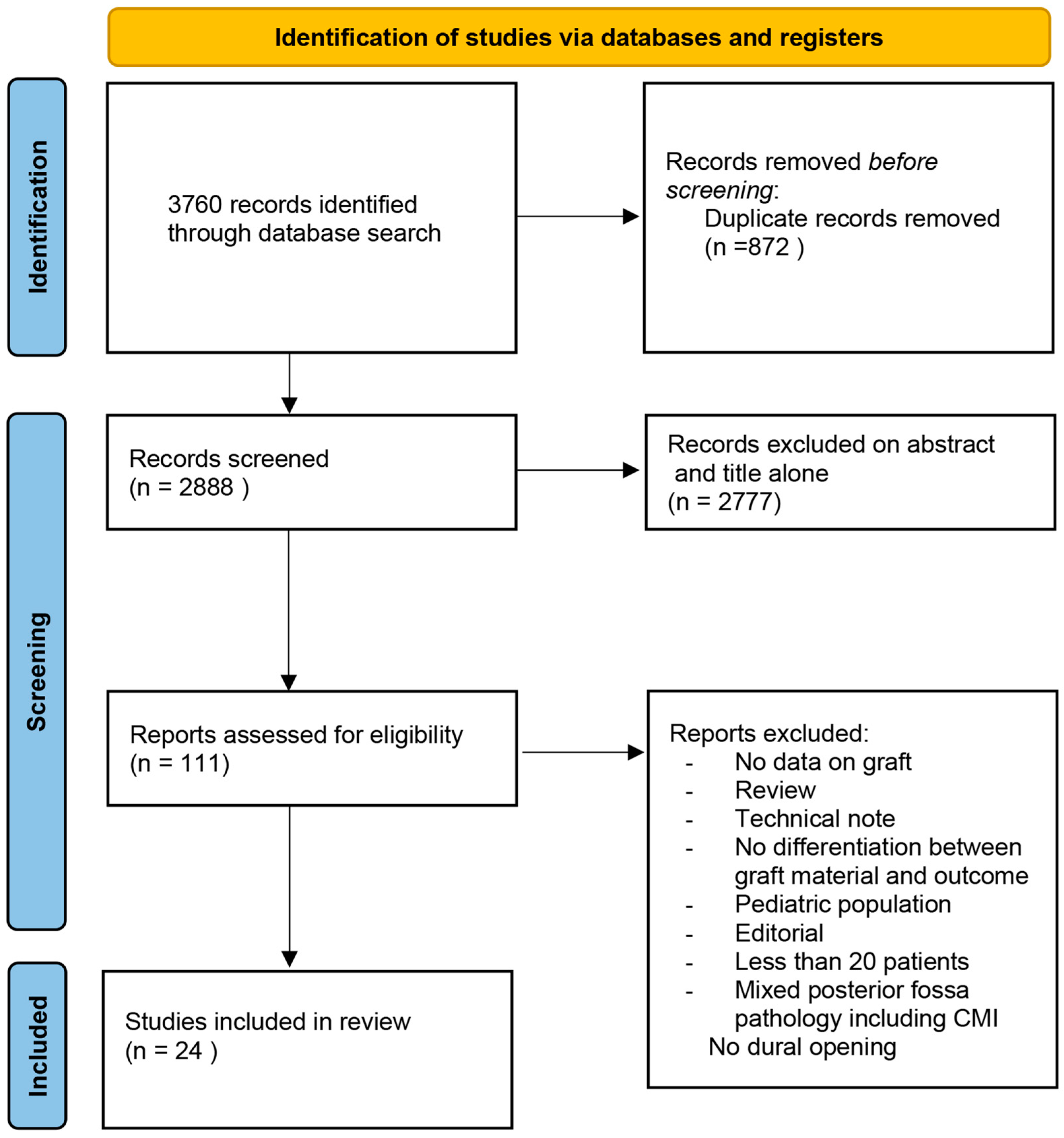
| PubMed Search Accessed on December 2022 (1801 Articles) | SCOPUS Search Accessed on December 2022 (1942 Articles) | Cochrane Library Search Accessed on December 2022 (17 Articles) |
|---|---|---|
| (((chiari[Title/Abstract]) AND (I[Title/Abstract])) AND (malformation[Title/Abstract]) AND (2000/1/1:2022/11/30[pdat])) AND ((“1992/01/01”[Date–Publication]: “3000”[Date–Publication])) | (TITLE-ABS-KEY (chiari) AND TITLE-ABS-KEY (i) AND TITLE-ABS-KEY (malformation)) AND PUBYEAR > 1991 AND (LIMIT-TO (PUBSTAGE, “final”)) AND (LIMIT-TO (DOCTYPE, “ar”)) | (chiari):ti,ab,kw AND (I):ti,ab,kw AND (malformation):ti,ab,kw” (Word variations have been searched) |
| Inclusion criteria | Exclusion criteria | |
| (1) series detailing surgical treatment and post-operative complications after FMDD for CMI; (2) series with dural graft distinction; (3) series with more than 20 patients. | (1) case reports; (2) review articles; (3) conference abstract; (4) technical notes; (5) studies published in languages other than English with no available English translations; (6) studies with overlapping patient population; (7) pediatric populations; (8) series with outcome not accounted for graft material | |
| Study | Year | N° Patients | Syrinx (%) | Age (Mean) | Age (Range) | M | F | FU Mean a | FU Range a |
|---|---|---|---|---|---|---|---|---|---|
| Cristante et al. [13] | 1994 | 26 | 21 (80) | 50.4 | 35–65 | 13 | 13 | 5–74 | |
| Vanaclocha et al. [14] | 1997 | 26 | 19–38 | 12 | 14 | 27 | 6–58 | ||
| Sindou et al. [15] | 2002 | 44 | 29 (66) | 40 | 13 | 31 | 50 | 13–120 | |
| Mueller et al. [16] | 2005 | 112 | 22 (25) | 40 | 17–70 | 8 | 104 | ||
| Spena et al. [17] | 2010 | 36 | 36 (100) | 40.4 | 18–68 | 17 | 19 | 40 | 16–72 |
| El-Ghandour et al. [18] | 2011 | 46 | 32 (70) | 37.4 | 19–56 | 21 | 25 | 88 | |
| Batzdorf et al. [19] | 2013 | 177 | 97 (55) | 37.9 | 15–78 | 45 | 132 | 22.125 | 2–155 |
| Lam et al. [20] | 2013 | 22 | 37.3 | 21–61 | 4 | 18 | 3 | 3 | |
| Galarza et al. [21] | 2013 | 22 | 7 (32) | 38.95 | 18–65 | 10 | 12 | 18 | 12–32 |
| Williams et al. [22] | 2013 | 34 | 38.7 | 6 | 28 | 3 | 3 | ||
| Chotai et al. [23] | 2014 | 30 | 7 (23) | 37 | 20–67 | 2 | 28 | 27.5 | 5–72 |
| Deng et al. [24] | 2015 | 152 | 112 (74) | 39.2 | 18–60 | 52 | 100 | 72 | |
| Bowers et al. [25] | 2015 | 119 | 13 (11) | 33.98 | 23 | 96 | 22.7 | ||
| Elhadji Cheikh Ndiaye et al. [26] | 2017 | 29 | 39 | 19–66 | 12 | 17 | 20 | 3–96 | |
| Lee et al. [27] | 2017 | 81 | 38.56 | 18–74 | 10 | 71 | 10.7 | ||
| Oral et al. [28] | 2018 | 35 | 17 (49) | 22–56 | 21 | 14 | 12 | 12 | |
| Jia et al. [29] | 2019 | 115 | 115 (100) | 43.4 | 35 | 80 | 6 | 6 | |
| Kemerdere et al. [30] | 2019 | 48 | 21 (44) | 38.8 | 18–69 | 14 | 33.984 | 30.24 | 6–108 |
| Villa et al. [31] | 2019 | 25 | 12 (28) | 39.2 | 19–66 | 10 | 15 | 33 | 3–60 |
| Liu et al. [32] | 2020 | 78 | 78 (100) | 40.6 | 36 | 42 | 20.3 | 18–60 | |
| Balasa et al. [33] | 2021 | 72 | 54 (75) | 41.9 | 18–66 | 16 | 54 | 67.3 | 3–187 |
| Ozlen et al. [34] | 2021 | 46 | 24 (52) | 37.5 | 11 | 35 | 83.5 | ||
| Kristiansson et al. [35] | 2022 | 166 | 70 (42) | 31 | 21–44 | 37 | 129 | 4.9 | 4–6.6 |
| Raw Data | Rate (95% CI) | N° of Articles | |
|---|---|---|---|
| Demographic data | |||
| N patients included in the analysis | 1541 | 23 | |
| Female patients | 1111/1541 | 72.1% (69.6–74.3%) | 23 |
| Mean age (range) | 31–50.4 | – | 21 |
| Associated syringomyelia | 767/1349 | 56.9% (54.2–59.4%) | 18 |
| Graft Material | |||
| Autologous | 602/1550 b | 38.4% (36.4–41.3%) | 12 |
| Biological nonautologous a | 331/1550 b | 21.4% (19.4–23.5%) | 5 |
| Synthetic | 617/1550 b | 39.8% (37.4–42.3%) | 9 |
| Raw Data | Rate (95% CI), p-Value | Heterogeneity | Meta-Regression (p-Value) | |
|---|---|---|---|---|
| Pseudomeningocele | ||||
| Autologous | 7/602 | 0.7% (0–1.3%). 0.04 | 0% | – |
| Biological a | 23/331 | 7.4% (0.8–14%). 0.03 | 86.6% | 0.9 |
| Synthetic | 43/617 | 5.3% (2.1–8.4%). <0.01 | 73.9% | <0.01 |
| CSF leak | ||||
| Autologous | 18/602 | 1.8% (0.5–3.1%). <0.01 | 26% | – |
| Biological a | 18/285 | 5.3% (1.6–9%). <0.01 | 37% | <0.01 |
| Synthetic | 9/535 | 0.8% (0–1.7%). 0.08 | 18% | 0.3 |
| Reoperation | ||||
| Autologous | 12/556 | 0.8% (0.1–1.6%). 0.03 | 0% | – |
| Biological a | 19/331 | 4.9% (2.6–7.2%). <0.01 | 0% | <0.01 |
| Synthetic | 33/617 | 4.1% (1.5–6.8%). <0.01 | 71.9% | 0.3 |
| Aseptic meningitis | ||||
| Autologous | 3/624 | 0.8% (0–1.6%). 0.04 | 0% | – |
| Biological a | 12/285 | 4.9% (0–10.8%). 0.1 | 77% | 0.8 |
| Synthetic | 6/535 | 0.5% (0–1.2%). <0.01 | 1% | 0.8 |
| Bacterial Meningitis | ||||
| Autologous | 10/602 | 1.2% (0.3–2%). <0.01 | 0% | – |
| Biological a | 6/285 | 2.1% (0.4–3.7%). 0.01 | 0% | 0.3 |
| Synthetic | 5/535 | 0.05% (0.1.1%) <0.01 | 0% | 0.2 |
| Wound Infections | ||||
| Autologous | 10/602 | 0.9% (10–1.6%). <0.01 | 0% | – |
| Biological a | 1/285 | 0.4% (0–1.1%). 0.01 | 0% | 0.4 |
| Synthetic | 6/535 | 0.6% (0–1%). <0.01 | 0% | 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perrini, P.; Lorenzini, D.; Vercelli, A.; Perrone, A.; Di Carlo, D.T. Post-Operative Complications after Foramen Magnum Decompression with Duraplasty Using Different Graft Materials in Adults Patients with Chiari I Malformation: A Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 3382. https://doi.org/10.3390/jcm12103382
Perrini P, Lorenzini D, Vercelli A, Perrone A, Di Carlo DT. Post-Operative Complications after Foramen Magnum Decompression with Duraplasty Using Different Graft Materials in Adults Patients with Chiari I Malformation: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2023; 12(10):3382. https://doi.org/10.3390/jcm12103382
Chicago/Turabian StylePerrini, Paolo, Daniele Lorenzini, Alberto Vercelli, Alessandra Perrone, and Davide Tiziano Di Carlo. 2023. "Post-Operative Complications after Foramen Magnum Decompression with Duraplasty Using Different Graft Materials in Adults Patients with Chiari I Malformation: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 12, no. 10: 3382. https://doi.org/10.3390/jcm12103382
APA StylePerrini, P., Lorenzini, D., Vercelli, A., Perrone, A., & Di Carlo, D. T. (2023). Post-Operative Complications after Foramen Magnum Decompression with Duraplasty Using Different Graft Materials in Adults Patients with Chiari I Malformation: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 12(10), 3382. https://doi.org/10.3390/jcm12103382







