Fibro-Stenosing Crohn’s Disease: What Is New and What Is Next?
Abstract
1. Introduction
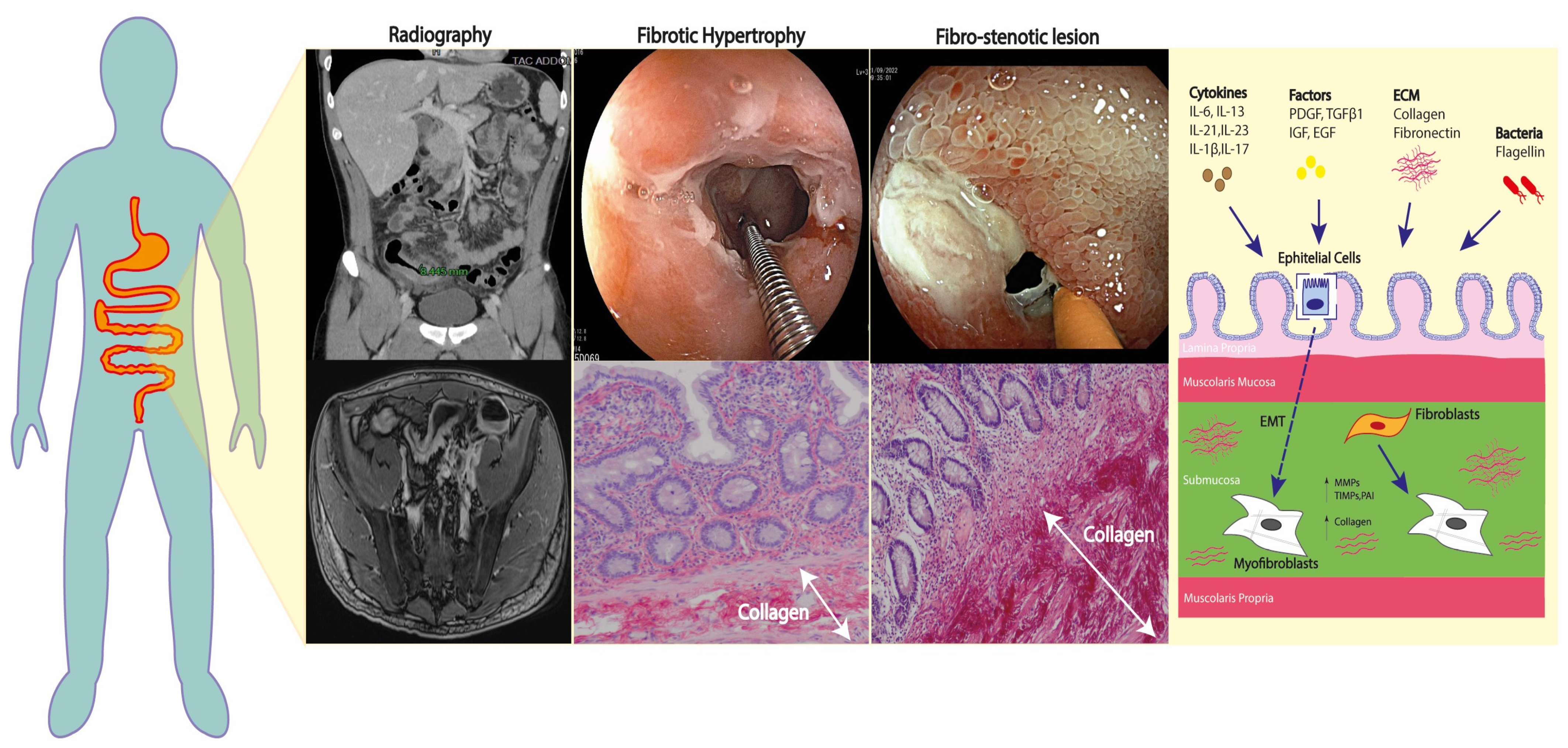
2. Molecular Pathways of Fibrosis in Crohn’s Disease
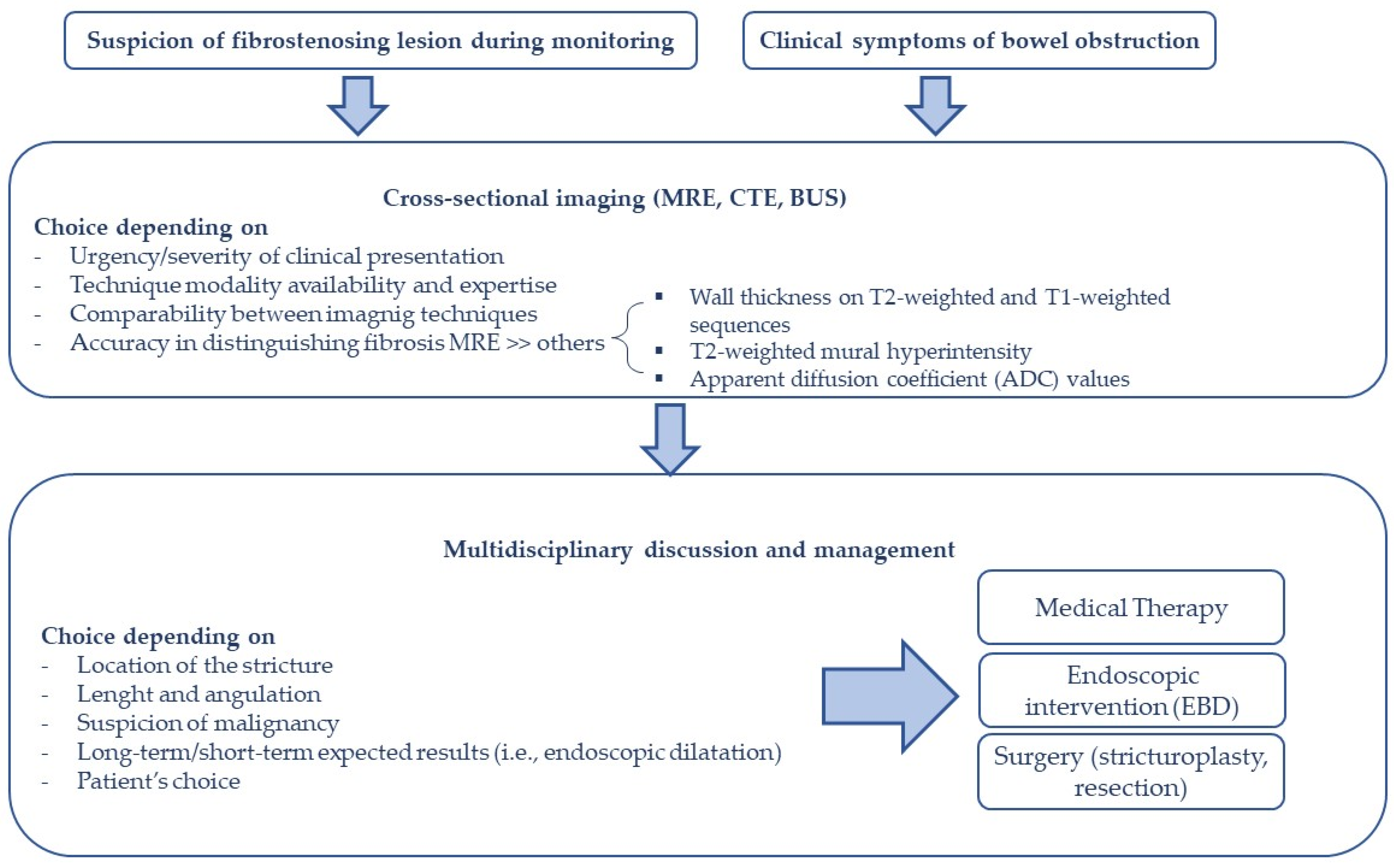
3. Assessment of Fibro-Stenotic Disease in CD
3.1. Bowel Ultrasound and Ultrasound Elastography
3.2. Magnetic Resonance (MR) and MR-Enterography (MRE)
3.3. Computed Tomography (CT)
4. Management of Fibro-Stenosing CD
4.1. Medical Therapy
4.2. Endoscopic Therapy
4.3. Surgical Therapy
5. The Role of Stenosis Therapy and Anti-Fibrotic Research (STAR) Consortium
6. Therapeutic Potential: What Is Next
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rieder, F.; Fiocchi, C.; Rogler, G. Mechanisms, Management, and Treatment of Fibrosis in Patients with Inflammatory Bowel Diseases. Gastroenterology 2017, 152, 340–350.e6. [Google Scholar] [CrossRef]
- Yoo, J.H.; Holubar, S.; Rieder, F. Fibrostenotic Strictures in Crohn’s Disease. Intest. Res. 2020, 18, 379–401. [Google Scholar] [CrossRef]
- Rutgeerts, P.; Geboes, K.; Vantrappen, G.; Beyls, J.; Kerremans, R.; Hiele, M. Predictability of the Postoperative Course of Crohn’s Disease. Gastroenterology 1990, 99, 956–963. [Google Scholar] [CrossRef] [PubMed]
- Rimola, J.; Capozzi, N. Differentiation of Fibrotic and Inflammatory Component of Crohn’s Disease-Associated Strictures. Intest. Res. 2020, 18, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Henderson, N.C.; Rieder, F.; Wynn, T.A. Fibrosis: From Mechanisms to Medicines. Nature 2020, 587, 555–566. [Google Scholar] [CrossRef]
- Rieder, F.; Bettenworth, D.; Ma, C.; Parker, C.E.; Williamson, L.A.; Nelson, S.A.; van Assche, G.; di Sabatino, A.; Bouhnik, Y.; Stidham, R.W.; et al. An Expert Consensus to Standardise Definitions, Diagnosis and Treatment Targets for Anti-Fibrotic Stricture Therapies in Crohn’s Disease. Aliment. Pharmacol. Ther. 2018, 48, 347–357. [Google Scholar] [CrossRef]
- Wang, J.; Lin, S.; Brown, J.M.; van Wagoner, D.; Fiocchi, C.; Rieder, F. Novel Mechanisms and Clinical Trial Endpoints in Intestinal Fibrosis. Immunol. Rev. 2021, 302, 211–227. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Lu, C.; Hirota, C.; Iacucci, M.; Ghosh, S.; Gui, X. Smooth Muscle Hyperplasia/Hypertrophy is the Most Prominent Histological Change in Crohn’s Fibrostenosing Bowel Strictures: A Semiquantitative Analysis by Using a Novel Histological Grading Scheme. J. Crohn’s Colitis 2017, 11, 92–104. [Google Scholar] [CrossRef]
- Welz, L.; Aden, K. Fibrosis and Inflammation in Inflammatory Bowel Disease-More Than 2 Sides of the Same Coin? Gastroenterology 2023, 164, 19–21. [Google Scholar] [CrossRef]
- Zhao, J.F.; Ling, F.M.; Li, J.R.; Chen, Y.D.; Huang, L.; Zhu, L.R. Role of Non-Inflammatory Factors in Intestinal Fibrosis. J. Dig. Dis. 2020, 21, 315–318. [Google Scholar] [CrossRef]
- D’Alessio, S.; Ungaro, F.; Noviello, D.; Lovisa, S.; Peyrin-Biroulet, L.; Danese, S. Revisiting Fibrosis in Inflammatory Bowel Disease: The Gut Thickens. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 169–184. [Google Scholar] [CrossRef]
- Latella, G.; Rogler, G.; Bamias, G.; Breynaert, C.; Florholmen, J.; Pellino, G.; Reif, S.; Speca, S.; Lawrance, I.C. Results of the 4th Scientific Workshop of the ECCO (I): Pathophysiology of Intestinal Fibrosis in IBD. J. Crohn’s Colitis 2014, 8, 1147–1165. [Google Scholar] [CrossRef]
- de Bruyn, J.R.; Meijer, S.L.; Wildenberg, M.E.; Bemelman, W.A.; van den Brink, G.R.; D’Haens, G.R. Development of Fibrosis in Acute and Longstanding Ulcerative Colitis. J. Crohn’s Colitis 2015, 9, 966–972. [Google Scholar] [CrossRef]
- Lawrance, I.C.; Rogler, G.; Bamias, G.; Breynaert, C.; Florholmen, J.; Pellino, G.; Reif, S.; Speca, S.; Latella, G. Cellular and Molecular Mediators of Intestinal Fibrosis. J. Crohn’s Colitis 2017, 11, 1491–1503. [Google Scholar] [CrossRef]
- Leeb, S.N.; Vogl, D.; Grossmann, J.; Falk, W.; Schölmerich, J.; Rogler, G.; Gelbmann, C.M. Autocrine Fibronectin-Induced Migration of Human Colonic Fibroblasts. Am. J. Gastroenterol. 2004, 99, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Burke, J.P.; Cunningham, M.F.; Sweeney, C.; Docherty, N.G.; O’Connell, P.R. N-Cadherin Is Overexpressed in Crohn’s Stricture Fibroblasts and Promotes Intestinal Fibroblast Migration. Inflamm. Bowel Dis. 2011, 17, 1665–1673. [Google Scholar] [CrossRef] [PubMed]
- Jun, Y.K.; Kwon, S.H.; Yoon, H.T.; Park, H.; Soh, H.; Lee, H.J.; Im, J.P.; Kim, J.S.; Kim, J.W.; Koh, S.J. Toll-Like Receptor 4 Regulates Intestinal Fibrosis via Cytokine Expression and Epithelial-Mesenchymal Transition. Sci. Rep. 2020, 10, 19867. [Google Scholar] [CrossRef] [PubMed]
- Sadler, T.; Bhasin, J.M.; Xu, Y.; Barnholz-Sloan, J.; Chen, Y.; Ting, A.H.; Stylianou, E. Genome-Wide Analysis of DNA Methylation and Gene Expression Defines Molecular Characteristics of Crohn’s Disease-Associated Fibrosis. Clin. Epigenet. 2016, 8, 30. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, W.; Yu, T.; Yu, Y.; Cui, X.; Zhou, Z.; Yang, H.; Yu, Y.; Bilotta, A.J.; Yao, S.; et al. Th17 Cell-Derived Amphiregulin Promotes Colitis-Associated Intestinal Fibrosis Through Activation of MTOR and MEK in Intestinal Myofibroblasts. Gastroenterology 2023, 164, 89–102. [Google Scholar] [CrossRef]
- Lovisa, S.; Genovese, G.; Danese, S. Role of Epithelial-to-Mesenchymal Transition in Inflammatory Bowel Disease. J. Crohn’s Colitis 2019, 13, 659–668. [Google Scholar] [CrossRef]
- Yang, J.; Zhou, C.Z.; Zhu, R.; Fan, H.; Liu, X.X.; Duan, X.Y.; Tang, Q.; Shou, Z.X.; Zuo, D.M. MiR-200b-Containing Microvesicles Attenuate Experimental Colitis Associated Intestinal Fibrosis by Inhibiting Epithelial-Mesenchymal Transition. J. Gastroenterol. Hepatol. 2017, 32, 1966–1974. [Google Scholar] [CrossRef] [PubMed]
- Rieder, F.; Kessler, S.P.; West, G.A.; Bhilocha, S.; de La Motte, C.; Sadler, T.M.; Gopalan, B.; Stylianou, E.; Fiocchi, C. Inflammation-Induced Endothelial-to-Mesenchymal Transition: A Novel Mechanism of Intestinal Fibrosis. Am. J. Pathol. 2011, 179, 2660–2673. [Google Scholar] [CrossRef]
- Scharl, M.; Huber, N.; Lang, S.; Fürst, A.; Jehle, E.; Rogler, G. Hallmarks of Epithelial to Mesenchymal Transition Are Detectable in Crohn’s Disease Associated Intestinal Fibrosis. Clin. Transl. Med. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Rieder, F.; Kessler, S.; Sans, M.; Fiocchi, C. Animal Models of Intestinal Fibrosis: New Tools for the Understanding of Pathogenesis and Therapy of Human Disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G786–G801. [Google Scholar] [CrossRef] [PubMed]
- Rieder, F. The Gut Microbiome in Intestinal Fibrosis: Environmental Protector or Provocateur? Sci. Transl. Med. 2013, 5, 190ps10. [Google Scholar] [CrossRef]
- Kuroda, N.; Masuya, M.; Tawara, I.; Tsuboi, J.; Yoneda, M.; Nishikawa, K.; Kageyama, Y.; Hachiya, K.; Ohishi, K.; Miwa, H.; et al. Infiltrating CCR2+ Monocytes and Their Progenies, Fibrocytes, Contribute to Colon Fibrosis by Inhibiting Collagen Degradation through the Production of TIMP-1. Sci. Rep. 2019, 9, 8568. [Google Scholar] [CrossRef]
- Imai, J.; Yahata, T.; Ichikawa, H.; Ibrahim, A.A.; Yazawa, M.; Sumiyoshi, H.; Inagaki, Y.; Matsushima, M.; Suzuki, T.; Mine, T.; et al. Inhibition of Plasminogen Activator Inhibitor-1 Attenuates against Intestinal Fibrosis in Mice. Intest. Res. 2020, 18, 219–228. [Google Scholar] [CrossRef]
- Warnaar, N.; Hofker, H.S.; Maathuis, M.H.J.; Niesing, J.; Bruggink, A.H.; Dijkstra, G.; Ploeg, R.J.; Schuurs, T.A. Matrix Metalloproteinases as Profibrotic Factors in Terminal Ileum in Crohn’s Disease. Inflamm. Bowel Dis. 2006, 12, 863–869. [Google Scholar] [CrossRef]
- Bettenworth, D.; Bokemeyer, A.; Baker, M.; Mao, R.; Parker, C.E.; Nguyen, T.; Ma, C.; Panés, J.; Rimola, J.; Fletcher, J.G.; et al. Assessment of Crohn’s Disease-Associated Small Bowel Strictures and Fibrosis on Cross-Sectional Imaging: A Systematic Review. Gut 2019, 68, 1115–1126. [Google Scholar] [CrossRef] [PubMed]
- Sleiman, J.; Chirra, P.; Gandhi, N.S.; Baker, M.E.; Lu, C.; Gordon, I.O.; Viswanath, S.E.; Rieder, F. Crohn’s Disease Related Strictures in Cross-Sectional Imaging: More than Meets the Eye? United Eur. Gastroenterol. J. 2022, 10, 1167–1178. [Google Scholar] [CrossRef]
- Bruining, D.H.; Zimmermann, E.M.; Loftus, E.V., Jr.; Sandborn, W.J.; Sauer, C.G.; Strong, S.A.; Fletcher, J.G. Consensus Recommendations for Evaluation, Interpretation, and Utilization of Computed Tomography and Magnetic Resonance Enterography in Patients With Small Bowel Crohn’s Disease. Radiology 2018, 286, 776–799. [Google Scholar] [CrossRef]
- Xu, C.; Jiang, W.; Wang, L.; Mao, X.; Ye, Z.; Zhang, H. Intestinal Ultrasound for Differentiating Fibrotic or Inflammatory Stenosis in Crohn’s Disease: A Systematic Review and Meta-Analysis. J. Crohn’s Colitis 2022, 16, 1493–1504. [Google Scholar] [CrossRef]
- Maconi, G.; Carsana, L.; Fociani, P.; Sampietro, G.M.; Ardizzone, S.; Cristaldi, M.; Parente, F.; Vago, G.L.; Taschieri, A.M.; Bianchi Porro, G. Small Bowel Stenosis in Crohn’s Disease: Clinical, Biochemical and Ultrasonographic Evaluation of Histological Features. Aliment. Pharmacol. Ther. 2003, 18, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Pallotta, N.; Vincoli, G.; Montesani, C.; Chirletti, P.; Pronio, A.; Caronna, R.; Ciccantelli, B.; Romeo, E.; Marcheggiano, A.; Corazziari, E. Small Intestine Contrast Ultrasonography (SICUS) for the Detection of Small Bowel Complications in Crohn’s Disease: A Prospective Comparative Study versus Intraoperative Findings. Inflamm. Bowel Dis. 2012, 18, 74–84. [Google Scholar] [CrossRef]
- Quaia, E.; Gennari, A.G.; Cova, M.A.; van Beek, E.J.R. Differentiation of Inflammatory From Fibrotic Ileal Strictures among Patients with Crohn’s Disease Based on Visual Analysis: Feasibility Study Combining Conventional B-Mode Ultrasound, Contrast-Enhanced Ultrasound and Strain Elastography. Ultrasound Med. Biol. 2018, 44, 762–770. [Google Scholar] [CrossRef] [PubMed]
- Dal Buono, A.; Faita, F.; Peyrin-Biroulet, L.; Danese, S.; Allocca, M. Ultrasound Elastography in Inflammatory Bowel Diseases: A Systematic Review of Accuracy Compared with Histopathological Assessment. J. Crohn’s Colitis 2022, 16, 1637–1646. [Google Scholar] [CrossRef]
- Sinha, R.; Murphy, P.; Sanders, S.; Ramachandran, I.; Hawker, P.; Rawat, S.; Roberts, S. Diagnostic Accuracy of High-Resolution MR Enterography in Crohn’s Disease: Comparison with Surgical and Pathological Specimen. Clin. Radiol. 2013, 68, 917–927. [Google Scholar] [CrossRef]
- Zappa, M.; Stefanescu, C.; Cazals-Hatem, D.; Bretagnol, F.; Deschamps, L.; Attar, A.; Larroque, B.; Tréton, X.; Panis, Y.; Vilgrain, V.; et al. Which Magnetic Resonance Imaging Findings Accurately Evaluate Inflammation in Small Bowel Crohn’s Disease? A Retrospective Comparison with Surgical Pathologic Analysis. Inflamm. Bowel Dis. 2011, 17, 984–993. [Google Scholar] [CrossRef]
- Tielbeek, J.A.W.; Ziech, M.L.W.; Li, Z.; Lavini, C.; Bipat, S.; Bemelman, W.A.; Roelofs, J.J.T.H.; Ponsioen, C.Y.; Vos, F.M.; Stoker, J. Evaluation of Conventional, Dynamic Contrast Enhanced and Diffusion Weighted MRI for Quantitative Crohn’s Disease Assessment with Histopathology of Surgical Specimens. Eur. Radiol. 2014, 24, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Rimola, J.; Planell, N.; Rodríguez, S.; Delgado, S.; Ordás, I.; Ramírez-Morros, A.; Ayuso, C.; Aceituno, M.; Ricart, E.; Jauregui-Amezaga, A.; et al. Characterization of Inflammation and Fibrosis in Crohn’s Disease Lesions by Magnetic Resonance Imaging. Am. J. Gastroenterol. 2015, 110, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Pouillon, L.; Laurent, V.; Pouillon, M.; Bossuyt, P.; Bonifacio, C.; Danese, S.; Deepak, P.; Loftus, E.V., Jr.; Bruining, D.H.; Peyrin-Biroulet, L. Diffusion-Weighted MRI in Inflammatory Bowel Disease. Lancet Gastroenterol. Hepatol. 2018, 3, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Rimola, J.; Ordás, I.; Rodriguez, S.; García-Bosch, O.; Aceituno, M.; Llach, J.; Ayuso, C.; Ricart, E.; Panés, J. Magnetic Resonance Imaging for Evaluation of Crohn’s Disease: Validation of Parameters of Severity and Quantitative Index of Activity. Inflamm. Bowel Dis. 2011, 17, 1759–1768. [Google Scholar] [CrossRef]
- Li, X.H.; Mao, R.; Huang, S.Y.; Sun, C.H.; Cao, Q.H.; Fang, Z.N.; Zhang, Z.W.; Huang, L.; Lin, J.J.; Chen, Y.J.; et al. Characterization of Degree of Intestinal Fibrosis in Patients with Crohn Disease by Using Magnetization Transfer MR Imaging. Radiology 2018, 287, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lu, B.; Lin, J.; He, S.; Huang, L.; Wang, Y.; Meng, J.; Li, Z.; Feng, S.T.; Lin, S.; et al. A Type I Collagen-Targeted MR Imaging Probe for Staging Fibrosis in Crohn’s Disease. Front. Mol. Biosci. 2021, 8, 1080. [Google Scholar] [CrossRef] [PubMed]
- Chiorean, M.V.; Sandrasegaran, K.; Saxena, R.; Maglinte, D.D.; Nakeeb, A.; Johnson, C.S. Correlation of CT Enteroclysis with Surgical Pathology in Crohn’s Disease. Am. J. Gastroenterol. 2007, 102, 2541–2550. [Google Scholar] [CrossRef] [PubMed]
- Vogel, J.; da Luz Moreira, A.; Baker, M.; Hammel, J.; Einstein, D.; Stocchi, L.; Fazio, V. CT Enterography for Crohn’s Disease: Accurate Preoperative Diagnostic Imaging. Dis. Colon. Rectum 2007, 50, 1761–1769. [Google Scholar] [CrossRef]
- Adler, J.; Punglia, D.R.; Dillman, J.R.; Polydorides, A.D.; Dave, M.; Al-Hawary, M.M.; Platt, J.F.; McKenna, B.J.; Zimmermann, E.M. Computed Tomography Enterography Findings Correlate with Tissue Inflammation, Not Fibrosis in Resected Small Bowel Crohn’s Disease. Inflamm. Bowel Dis. 2012, 18, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Rieder, F.; Latella, G.; Magro, F.; Yuksel, E.S.; Higgins, P.D.; Di Sabatino, A.; de Bruyn, J.R.; Rimola, J.; Brito, J.; Bettenworth, D.; et al. European Crohn’s and Colitis Organisation Topical Review on Prediction, Diagnosis and Management of Fibrostenosing Crohn’s Disease. J. Crohn’s Colitis 2016, 10, 873–885. [Google Scholar] [CrossRef]
- Ma, C.; Jairath, V.; Click, B.; Hirota, S.A.; Lu, C.; Parker, C.E.; Rieder, F.; Stenosis Therapy and Anti-Fibrotic Research (STAR) Consortium. Targeting Anti-Fibrotic Pathways in Crohn’s Disease—The Final Frontier? Best. Pract. Res. Clin. Gastroenterol. 2019, 38–39, 101603. [Google Scholar] [CrossRef]
- El Ouali, S.; Click, B.; Holubar, S.D.; Rieder, F. Natural History, Diagnosis and Treatment Approach to Fibrostenosing Crohn’s Disease. United Eur. Gastroenterol. J. 2020, 8, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Yaffe, B.H.; Korelitz, B.I. Prognosis for Nonoperative Management of Small-Bowel Obstruction in Crohn’s Disease. J. Clin. Gastroenterol. 1983, 5, 211–216. [Google Scholar] [CrossRef]
- Kurahara, L.H.; Hiraishi, K.; Hu, Y.; Koga, K.; Onitsuka, M.; Doi, M.; Aoyagi, K.; Takedatsu, H.; Kojima, D.; Fujihara, Y.; et al. Activation of Myofibroblast TRPA1 by Steroids and Pirfenidone Ameliorates Fibrosis in Experimental Crohn’s Disease. Cell. Mol. Gastroenterol. Hepatol. 2017, 5, 299–318. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, A.L.; Kalisazan, B.; Wienckiewicz, J.; Bouarioua, N.; SoulÉ, J.C. Infliximab Treatment for Symptomatic Crohn’s Disease Strictures. Aliment. Pharmacol. Ther. 2009, 29, 279–285. [Google Scholar] [CrossRef]
- Lu, C.; Baraty, B.; Lee Robertson, H.; Filyk, A.; Shen, H.; Fung, T.; Novak, K.; Ma, C.; Panaccione, R.; Achkar, J.P.; et al. Systematic Review: Medical Therapy for Fibrostenosing Crohn’s Disease. Aliment. Pharmacol. Ther. 2020, 51, 1233–1246. [Google Scholar] [CrossRef] [PubMed]
- Pallotta, N.; Barberani, F.; Hassan, N.A.; Guagnozzi, D.; Vincoli, G.; Corazziari, E. Effect of Infliximab on Small Bowel Stenoses in Patients with Crohn’s Disease. World J. Gastroenterol. 2008, 14, 1885–1890. [Google Scholar] [CrossRef] [PubMed]
- Hanauer, S.B.; Feagan, B.G.; Lichtenstein, G.R.; Mayer, L.F.; Schreiber, S.; Colombel, J.F.; Rachmilewitz, D.; Wolf, D.C.; Olson, A.; Bao, W.; et al. Maintenance Infliximab for Crohn’s Disease: The ACCENT I Randomised Trial. Lancet 2002, 359, 1541–1549. [Google Scholar] [CrossRef]
- Bouhnik, Y.; Carbonnel, F.; Laharie, D.; Stefanescu, C.; Hébuterne, X.; Abitbol, V.; Nachury, M.; Brixi, H.; Bourreille, A.; Picon, L.; et al. Efficacy of Adalimumab in Patients with Crohn’s Disease and Symptomatic Small Bowel Stricture: A Multicentre, Prospective, Observational Cohort (CREOLE) Study. Gut 2018, 67, 53–60. [Google Scholar] [CrossRef] [PubMed]
- di Sabatino, A.; Ciccocioppo, R.; Benazzato, L.; Sturniolo, G.C.; Corazza, G.R. Infliximab Downregulates Basic Fibroblast Growth Factor and Vascular Endothelial Growth Factor in Crohn’s Disease Patients. Aliment. Pharmacol. Ther. 2004, 19, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
- Schulberg, J.D.; Wright, E.K.; Holt, B.A.; Hamilton, A.L.; Sutherland, T.R.; Ross, A.L.; Vogrin, S.; Miller, A.M.; Connell, W.C.; Lust, M.; et al. Intensive Drug Therapy versus Standard Drug Therapy for Symptomatic Intestinal Crohn’s Disease Strictures (STRIDENT): An Open-Label, Single-Centre, Randomised Controlled Trial. Lancet Gastroenterol. Hepatol. 2022, 7, 318–331. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Lago, I.; Gisbert, J.P. The Role of Immunomodulators and Biologics in the Medical Management of Stricturing Crohn’s Disease. J. Crohn’s Colitis 2020, 14, 557–566. [Google Scholar] [CrossRef]
- Adamina, M.; Bonovas, S.; Raine, T.; Spinelli, A.; Warusavitarne, J.; Armuzzi, A.; Bachmann, O.; Bager, P.; Biancone, L.; Bokemeyer, B.; et al. ECCO Guidelines on Therapeutics in Crohn’s Disease: Surgical Treatment. J. Crohn’s Colitis 2020, 14, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.E.; Lim, F.; Faye, A.S.; Shen, B.; Hur, C. Endoscopic Balloon Dilation Is Cost-Effective for Crohn’s Disease Strictures. Dig. Dis. Sci. 2022, 67, 5462–5471. [Google Scholar] [CrossRef]
- Sivasailam, B.; Lane, B.F.; Cross, R.K. Endoscopic Balloon Dilation of Strictures: Techniques, Short- and Long-Term Outcomes, and Complications. Gastrointest. Endosc. Clin. 2022, 32, 675–686. [Google Scholar] [CrossRef]
- Yamamoto, H.; Yano, T.; Araki, A.; Esaki, M.; Ohtsuka, K.; Ohmiya, N.; Oka, S.; Nakase, H.; Bamba, S.; Hirai, F.; et al. Guidelines for Endoscopic Balloon Dilation in Treating Crohn’s Disease-Associated Small Intestinal Strictures (Supplement to the Clinical Practice Guidelines for Enteroscopy). Dig. Endosc. 2022, 34, 1278–1296. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Kochhar, G.; Navaneethan, U.; Farraye, F.A.; Schwartz, D.A.; Iacucci, M.; Bernstein, C.N.; Dryden, G.; Cross, R.; Bruining, D.H.; et al. Practical Guidelines on Endoscopic Treatment for Crohn’s Disease Strictures: A Consensus Statement from the Global Interventional Inflammatory Bowel Disease Group. Lancet Gastroenterol. Hepatol. 2020, 5, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Reutemann, B.A.; Turkeltaub, J.A.; Al-Hawary, M.; Waljee, A.K.; Higgins, P.D.R.; Stidham, R.W. Endoscopic Balloon Dilation Size and Avoidance of Surgery in Stricturing Crohn’s Disease. Inflamm. Bowel Dis. 2017, 23, 1803–1809. [Google Scholar] [CrossRef] [PubMed]
- Bettenworth, D.; Gustavsson, A.; Atreja, A.; Lopez, R.; Tysk, C.; Van Assche, G.; Rieder, F. A Pooled Analysis of Efficacy, Safety, and Long-Term Outcome of Endoscopic Balloon Dilation Therapy for Patients with Stricturing Crohn’s Disease. Inflamm. Bowel Dis. 2017, 23, 133–142. [Google Scholar] [CrossRef]
- Fumery, M.; de Chambrun, G.P.; Stefanescu, C.; Buisson, A.; Bressenot, A.; Beaugerie, L.; Amiot, A.; Altwegg, R.; Savoye, G.; Abitbol, V.; et al. Detection of Dysplasia or Cancer in 3.5% of Patients With Inflammatory Bowel Disease and Colonic Strictures. Clin. Gastroenterol. Hepatol. 2015, 13, 1770–1775. [Google Scholar] [CrossRef]
- Navaneethan, U.; Lourdusamy, V.; Njei, B.; Shen, B. Endoscopic Balloon Dilation in the Management of Strictures in Crohn’s Disease: A Systematic Review and Meta-Analysis of Non-Randomized Trials. Surg. Endosc. 2016, 30, 5434–5443. [Google Scholar] [CrossRef] [PubMed]
- Winder, O.; Fliss-Isakov, N.; Winder, G.; Scapa, E.; Yanai, H.; Barnes, S.; Dekel, R.; Dotan, I.; Maharshak, N. Clinical Outcomes of Endoscopic Balloon Dilatation of Intestinal Strictures in Patients with Crohn’s Disease. Medicine 2019, 98, e16864. [Google Scholar] [CrossRef]
- Fousekis, F.S.; Mitselos, I.V.; Tepelenis, K.; Pappas-Gogos, G.; Katsanos, K.H.; Lianos, G.D.; Frattini, F.; Vlachos, K.; Christodoulou, D.K. Medical, Endoscopic and Surgical Management of Stricturing Crohn’s Disease: Current Clinical Practice. J. Clin. Med. 2022, 11, 2366. [Google Scholar] [CrossRef] [PubMed]
- Navaneethan, U.; Lourdusamy, D. Endoscopic Stricturotomy and Strictureplasty. Gastrointest. Endosc. Clin. 2022, 32, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Lan, N.; Shen, B. Endoscopic Stricturotomy with Needle Knife in the Treatment of Strictures from Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2017, 23, 502–513. [Google Scholar] [CrossRef]
- Lan, N.; Shen, B. Endoscopic Stricturotomy Versus Balloon Dilation in the Treatment of Anastomotic Strictures in Crohn’s Disease. Inflamm. Bowel Dis. 2018, 24, 897–907. [Google Scholar] [CrossRef]
- Das, R.; Singh, R.; Din, S.; Lund, J.; Krishnamoorthy, R.; Hearing, S.; Norton, B.; Williams, J.; Fraser, C.; Goddard, A.; et al. Therapeutic Resolution of Focal, Predominantly Anastomotic Crohn’s Disease Strictures Using Removable Stents: Outcomes from a Single-Center Case Series in the United Kingdom. Gastrointest. Endosc. 2020, 92, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Attar, A.; Branche, J.; Coron, E.; Privat, J.; Caillo, L.; Chevaux, J.B.; Vuitton, L.; Amiot, A.; Belkhodja, H.; Dray, X.; et al. An Anti-Migration Self-Expandable and Removable Metal Stent for Crohn’s Disease Strictures: A Nationwide Study From GETAID and SFED. J. Crohn’s Colitis 2021, 15, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Giri, S.; Gopan, A.; Sundaram, S.; Kale, A. Efficacy and Safety of Endoscopic Stenting for Crohn’s Disease Related Strictures: A Systematic Review and Meta-Analysis. Korean J. Gastroenterol. 2022, 80, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Loras, C.; Andújar, X.; Gornals, J.B.; Sanchiz, V.; Brullet, E.; Sicilia, B.; Martín-Arranz, M.D.; Naranjo, A.; Barrio, J.; Dueñas, C.; et al. (GETECCU) Self-Expandable Metal Stents versus Endoscopic Balloon Dilation for the Treatment of Strictures in Crohn’s Disease (ProtDilat Study): An Open-Label, Multicentre, Randomised Trial. Lancet Gastroenterol. Hepatol. 2022, 7, 332–341. [Google Scholar] [CrossRef]
- Lightner, A.L.M.; Vogel, J.D.M.; Carmichael, J.C.M.; Keller, D.S.M.; Shah, S.A.M.; Mahadevan, U.M.; Kane, S.V.M.; Paquette, I.M.M.; Steele, S.R.M.; Feingold, D.L.M. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Surgical Management of Crohn’s Disease. Dis. Colon. Rectum 2020, 63, 1028–1052. [Google Scholar] [CrossRef]
- Aratari, A.; Papi, C.; Leandro, G.; Viscido, A.; Capurso, L.; Caprilli, R. Early versus Late Surgery for Ileo-Caecal Crohn’s Disease. Aliment. Pharmacol. Ther. 2007, 26, 1303–1312. [Google Scholar] [CrossRef]
- Latella, G.; Cocco, A.; Angelucci, E.; Viscido, A.; Bacci, S.; Necozione, S.; Caprilli, R. Clinical Course of Crohn’s Disease First Diagnosed at Surgery for Acute Abdomen. Dig. Liver Dis. 2009, 41, 269–276. [Google Scholar] [CrossRef]
- Golovics, P.A.; Lakatos, L.; Nagy, A.; Pandur, T.; Szita, I.; Balogh, M.; Molnar, C.; Komaromi, E.; Lovasz, B.D.; Mandel, M.; et al. Is Early Limited Surgery Associated with a More Benign Disease Course in Crohn’s Disease? World J. Gastroenterol. 2013, 19, 7701–7710. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Stocchi, L.; Shen, B.; Liu, X.; Remzi, F.H. Salvage Surgery after Failure of Endoscopic Balloon Dilatation versus Surgery First for Ileocolonic Anastomotic Stricture Due to Recurrent Crohn’s Disease. Br. J. Surg. 2015, 102, 1418–1425. [Google Scholar] [CrossRef] [PubMed]
- Lan, N.; Stocchi, L.; Ashburn, J.H.; Hull, T.L.; Steele, S.R.; Delaney, C.P.; Shen, B. Outcomes of Endoscopic Balloon Dilation vs Surgical Resection for Primary Ileocolic Strictures in Patients With Crohn’s Disease. Clin. Gastroenterol. Hepatol. 2018, 16, 1260–1267. [Google Scholar] [CrossRef]
- Ozuner, G.; Fazio, V.W.; Lavery, I.C.; Milsom, J.W.; Strong, S.A. Reoperative Rates for Crohn’s Disease Following Strictureplasty. Long-Term Analysis. Dis. Colon. Rectum 1996, 39, 1199–1203. [Google Scholar] [CrossRef] [PubMed]
- Bellolio, F.; Cohen, Z.; Macrae, H.M.; O’connor, B.I.; Victor, J.C.; Huang, H.; McLeod, R.S. Strictureplasty in Selected Crohn’s Disease Patients Results in Acceptable Long-Term Outcome. Dis. Colon. Rectum 2012, 55, 864–869. [Google Scholar] [CrossRef] [PubMed]
- Strong, S.A. Strictureplasty in complex Crohn’s disease: Beyond the basics. Clin. Colon Rectal Surg. 2019, 32, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Broering, D.; Eisenberger, C.; Koch, A.; Bloechle, C.; Knoefel, W.; Dürig, M.; Raedler, A.; Izbicki, J. Strictureplasty for Large Bowel Stenosis in Crohn’s Disease: Quality of Life after Surgical Therapy. Int. J. Color. Dis. 2001, 16, 81–87. [Google Scholar] [CrossRef]
- Yamamoto, T.; Fazio, V.W.; Tekkis, P.P. Safety and Efficacy of Strictureplasty for Crohn’s Disease: A Systematic Review and Meta-Analysis. Dis. Colon. Rectum 2007, 50, 1968–1986. [Google Scholar] [CrossRef]
- Campbell, L.; Ambe, R.; Weaver, J.; Marcus, S.M.; Cagir, B. Comparison of Conventional and Nonconventional Strictureplasties in Crohn’s Disease: A Systematic Review and Meta-Analysis. Dis. Colon. Rectum 2012, 55, 714–726. [Google Scholar] [CrossRef]
- Dietz, D.W.; Laureti, S.; A Strong, S.; Hull, T.L.; Church, J.; Remzi, F.H.; Lavery, I.C.; Fazio, V.W. Safety and Longterm Efficacy of Strictureplasty in 314 Patients with Obstructing Small Bowel Crohn’s Disease. J. Am. Coll. Surg. 2001, 192, 330–337. [Google Scholar] [CrossRef]
- Geltzeiler, C.B.; Hart, K.D.; Lu, K.C.; DeVeney, K.E.; Herzig, D.O.; Tsikitis, V.L. Trends in the Surgical Management of Crohn’s Disease. J. Gastrointest. Surg. 2015, 19, 1862–1868. [Google Scholar] [CrossRef]
- Higgins, P.D. Measurement of Fibrosis in Crohn’s Disease Strictures with Imaging and Blood Biomarkers to Inform Clinical Decisions. Dig. Dis. 2017, 35, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Gordon, I.O.; Bettenworth, D.; Bokemeyer, A.; Srivastava, A.; Rosty, C.; de Hertogh, G.; Robert, M.E.; Valasek, M.A.; Mao, R.; Kurada, S.; et al. Histopathology Scoring Systems of Stenosis Associated With Small Bowel Crohn’s Disease: A Systematic Review. Gastroenterology 2020, 158, 137–150.e1. [Google Scholar] [CrossRef]
- Gordon, I.O.; Bettenworth, D.; Bokemeyer, A.; Srivastava, A.; Rosty, C.; de Hertogh, G.; Robert, M.E.; Valasek, M.A.; Mao, R.; Li, J.; et al. International Consensus to Standardise Histopathological Scoring for Small Bowel Strictures in Crohn’s Disease. Gut 2022, 71, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Friedman, S.L.; Bansal, M.B. Reversal of Hepatic Fibrosis-Fact or Fantasy? Hepatology 2006, 43 (Suppl. S1), S82–S88. [Google Scholar] [CrossRef] [PubMed]
- Rockey, D.C.; Bell, P.D.; Hill, J.A. Fibrosis—A Common Pathway to Organ Injury and Failure. N. Engl. J. Med. 2015, 372, 1138–1149. [Google Scholar] [CrossRef]
- Noble, P.W.; Albera, C.; Bradford, W.Z.; Costabel, U.; Glassberg, M.K.; Kardatzke, D.; King, T.E.; Lancaster, L.; Sahn, S.A.; Szwarcberg, J.; et al. Pirfenidone in Patients with Idiopathic Pulmonary Fibrosis (CAPACITY): Two Randomised Trials. Lancet 2011, 377, 1760–1769. [Google Scholar] [CrossRef] [PubMed]
- Richeldi, L.; du Bois, R.M.; Raghu, G.; Azuma, A.; Brown, K.K.; Costabel, U.; Cottin, V.; Flaherty, K.R.; Hansell, D.M.; Inoue, Y.; et al. Efficacy and Safety of Nintedanib in Idiopathic Pulmonary Fibrosis. N. Engl. J. Med. 2014, 370, 2071–2082. [Google Scholar] [CrossRef]
- Holvoet, T.; Devriese, S.; Castermans, K.; Boland, S.; Leysen, D.; Vandewynckel, Y.P.; Devisscher, L.; van den Bossche, L.; van Welden, S.; Dullaers, M.; et al. Treatment of Intestinal Fibrosis in Experimental Inflammatory Bowel Disease by the Pleiotropic Actions of a Local Rho Kinase Inhibitor. Gastroenterology 2017, 153, 1054–1067. [Google Scholar] [CrossRef]
- Elias, M.; Zhao, S.; Le, H.T.; Wang, J.; Neurath, M.F.; Neufert, C.; Fiocchi, C.; Rieder, F. IL-36 in Chronic Inflammation and Fibrosis—Bridging the Gap? J. Clin. Investig. 2021, 131, e144336. [Google Scholar] [CrossRef]
- Scheibe, K.; Kersten, C.; Schmied, A.; Vieth, M.; Primbs, T.; Carlé, B.; Knieling, F.; Claussen, J.; Klimowicz, A.C.; Zheng, J.; et al. Inhibiting Interleukin 36 Receptor Signaling Reduces Fibrosis in Mice With Chronic Intestinal Inflammation. Gastroenterology 2019, 156, 1082–1097.e11. [Google Scholar] [CrossRef] [PubMed]
- Bachelez, H.; Choon, S.-E.; Marrakchi, S.; Burden, A.D.; Tsai, T.-F.; Morita, A.; Navarini, A.A.; Zheng, M.; Xu, J.; Turki, H.; et al. Trial of Spesolimab for Generalized Pustular Psoriasis. N. Engl. J. Med. 2021, 385, 2431–2440. [Google Scholar] [CrossRef]
- Zhou, L.Y.; Lin, S.N.; Rieder, F.; Chen, M.H.; Zhang, S.H.; Mao, R. Noncoding RNAs as Promising Diagnostic Biomarkers and Therapeutic Targets in Intestinal Fibrosis of Crohn’s Disease: The Path From Bench to Bedside. Inflamm. Bowel Dis. 2021, 27, 971–982. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Zhao, Q. Lnc-ITSN1-2, Derived From RNA Sequencing, Correlates with Increased Disease Risk, Activity and Promotes CD4+ T Cell Activation, Proliferation and Th1/Th17 Cell Differentiation by Serving as a CeRNA for IL-23R via Sponging MiR-125a in Inflammatory Bowel Disease. Front. Immunol. 2020, 11, 852. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.; Mehta, S.; Hanna, L.N.; Rogalski, L.A.; Jeffery, R.; Nijhuis, A.; Kumagai, T.; Biancheri, P.; Bundy, J.G.; Bishop, C.L.; et al. Low Serum Levels of MicroRNA-19 Are Associated with a Stricturing Crohn’s Disease Phenotype. Inflamm. Bowel Dis. 2015, 21, 1926–1934. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Huang, S.; Wang, H.; Chao, K.; Ding, L.; Feng, R.; Qiu, Y.; Feng, T.; Zhou, G.; Hu, J.F.; et al. Cytokine IL9 Triggers the Pathogenesis of Inflammatory Bowel Disease Through the MiR21-CLDN8 Pathway. Inflamm. Bowel Dis. 2018, 24, 2211–2223. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solitano, V.; Dal Buono, A.; Gabbiadini, R.; Wozny, M.; Repici, A.; Spinelli, A.; Vetrano, S.; Armuzzi, A. Fibro-Stenosing Crohn’s Disease: What Is New and What Is Next? J. Clin. Med. 2023, 12, 3052. https://doi.org/10.3390/jcm12093052
Solitano V, Dal Buono A, Gabbiadini R, Wozny M, Repici A, Spinelli A, Vetrano S, Armuzzi A. Fibro-Stenosing Crohn’s Disease: What Is New and What Is Next? Journal of Clinical Medicine. 2023; 12(9):3052. https://doi.org/10.3390/jcm12093052
Chicago/Turabian StyleSolitano, Virginia, Arianna Dal Buono, Roberto Gabbiadini, Marek Wozny, Alessandro Repici, Antonino Spinelli, Stefania Vetrano, and Alessandro Armuzzi. 2023. "Fibro-Stenosing Crohn’s Disease: What Is New and What Is Next?" Journal of Clinical Medicine 12, no. 9: 3052. https://doi.org/10.3390/jcm12093052
APA StyleSolitano, V., Dal Buono, A., Gabbiadini, R., Wozny, M., Repici, A., Spinelli, A., Vetrano, S., & Armuzzi, A. (2023). Fibro-Stenosing Crohn’s Disease: What Is New and What Is Next? Journal of Clinical Medicine, 12(9), 3052. https://doi.org/10.3390/jcm12093052






