Recent Advances and Potential Multi-Omics Approaches in the Early Phases of Inflammatory Bowel Disease
Abstract
1. Introduction
2. The Concept of Preclinical Disease
2.1. Diagnosis of IBD and Impact of Diagnostic Delay
2.2. Evidence of Symptoms before Diagnosis of IBD
2.3. Defining Preclinical IBD
2.4. Subclinical Bowel Lesions
2.5. Medical Intervention in Early IBD
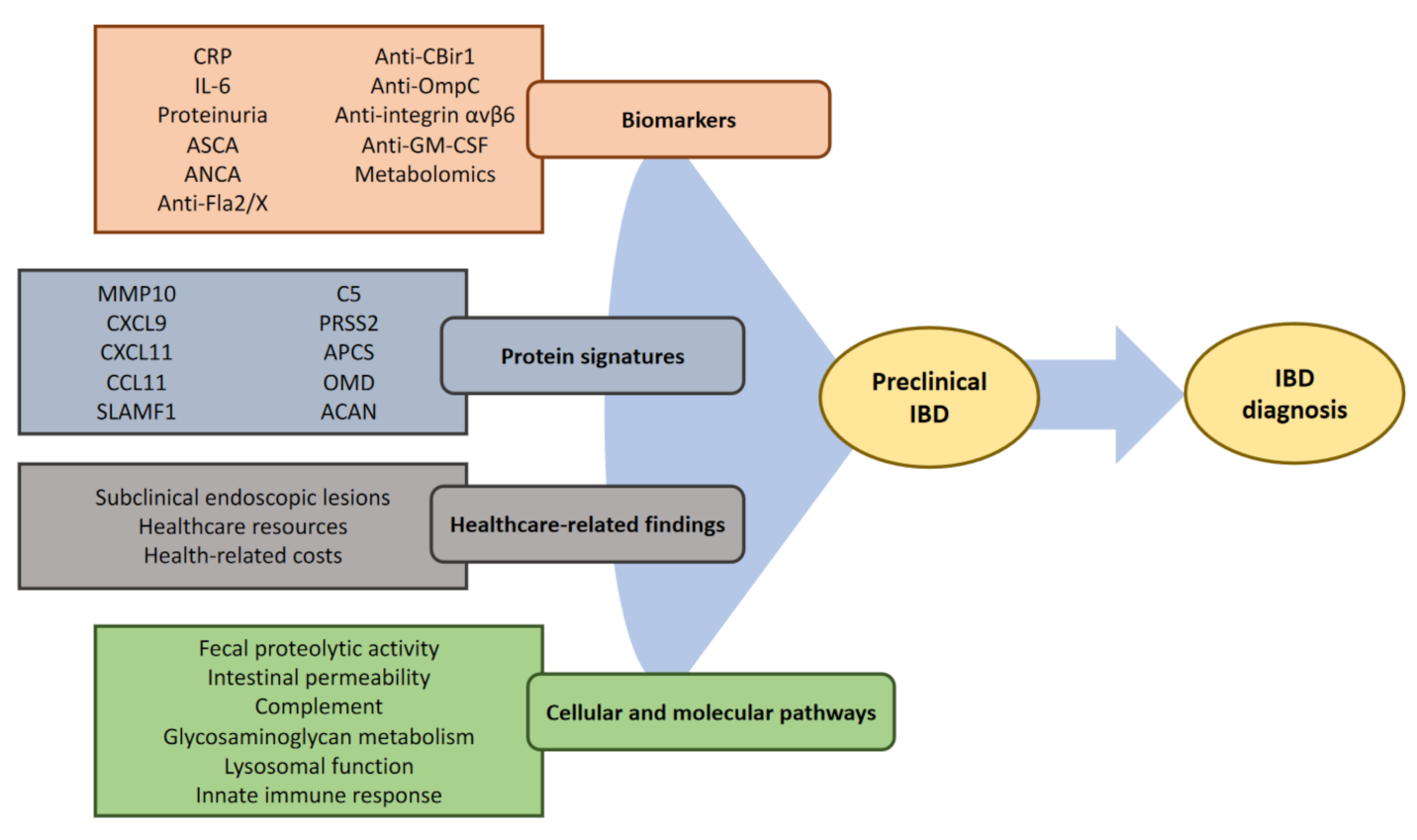
3. Tools for IBD Prediction
3.1. Circulating Biomarkers
3.2. Disease Prediction
4. Integration of Multiple Omics
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| IBD | inflammatory bowel disease |
| CD | Crohn’s disease |
| UC | ulcerative colitis |
| GM-CSF | Granulocyte-Macrophage Colony-Stimulating Factor |
| ASCA | Anti-Saccharomyces cerevisiae antibodies |
| pANCA | perinuclear anti-neutrophil cytoplasmic antibodies |
| ACCA | Antichitobioside antibodies |
| OmpC | Outer membrane proteins |
References
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2018, 390, 2769–2778. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Lago, I.; Zabana, Y.; Barreiro-de Acosta, M. Diagnosis and natural history of preclinical and early inflammatory bowel disease. Ann. Gastroenterol. 2020, 33, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.; Burisch, J.; Riddle, M.; Dubinsky, M.; Colombel, J.F. Preclinical disease and preventive strategies in IBD: Perspectives, challenges and opportunities. Gut 2016, 65, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Jayasooriya, N.; Baillie, S.; Blackwell, J.; Bottle, A.; Petersen, I.; Creese, H.; Saxena, S.; Pollok, R.C.; POP-IBD study group. Systematic review with meta-analysis: Time to diagnosis and the impact of delayed diagnosis on clinical outcomes in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2023, 57, 635–652. [Google Scholar] [CrossRef]
- Torres, J.; Halfvarson, J.; Rodriguez-Lago, I.; Hedin, C.R.H.; Jess, T.; Dubinsky, M.; Croitoru, K.; Colombel, J.F. Results of the Seventh Scientific Workshop of ECCO: Precision Medicine in IBD-Prediction and Prevention of Inflammatory Bowel Disease. J. Crohn’s Colitis 2021, 15, 1443–1454. [Google Scholar] [CrossRef]
- Noor, N.M.; Sousa, P.; Paul, S.; Roblin, X. Early Diagnosis, Early Stratification, and Early Intervention to Deliver Precision Medicine in IBD. Inflamm. Bowel. Dis. 2022, 28, 1254–1264. [Google Scholar] [CrossRef]
- Blackwell, J.; Saxena, S.; Jayasooriya, N.; Bottle, A.; Petersen, I.; Hotopf, M.; Alexakis, C.; Pollok, R.C.; group, P.-I.S. Prevalence and duration of gastrointestinal symptoms before diagnosis of Inflammatory Bowel Disease and predictors of timely specialist review: A population-based study. J. Crohn’s Colitis 2020, 15, 203–211. [Google Scholar] [CrossRef]
- Stapley, S.A.; Rubin, G.P.; Alsina, D.; Shephard, E.A.; Rutter, M.D.; Hamilton, W.T. Clinical features of bowel disease in patients aged <50 years in primary care: A large case-control study. Br. J. Gen. Pract. 2017, 67, e336–e344. [Google Scholar] [CrossRef]
- Turvill, J.; Turnock, D. Audit of the impact of the York faecal calprotectin care pathway on colonoscopy activity. Frontline Gastroenterol. 2020, 11, 285–289. [Google Scholar] [CrossRef]
- Freeman, K.; Ryan, R.; Parsons, N.; Taylor-Phillips, S.; Willis, B.H.; Clarke, A. Faecal calprotectin testing in UK general practice: A retrospective cohort study using The Health Improvement Network database. Br. J. Gen. Pract. 2021, 71, e854–e861. [Google Scholar] [CrossRef]
- Lichtenstein, G.R.; Shahabi, A.; Seabury, S.A.; Lakdawalla, D.N.; Espinosa, O.D.; Green, S.; Brauer, M.; Baldassano, R.N. Increased Lifetime Risk of Intestinal Complications and Extraintestinal Manifestations in Crohn’s Disease and Ulcerative Colitis. Gastroenterol. Hepatol. 2022, 18, 32–43. [Google Scholar]
- Blackwell, J.; Saxena, S.; Pollok, R.C. Role of thiopurines as disease-modifying agents in Crohn’s disease. Gut 2018, 67, 2229–2230. [Google Scholar] [CrossRef] [PubMed]
- Berg, D.R.; Colombel, J.F.; Ungaro, R. The Role of Early Biologic Therapy in Inflammatory Bowel Disease. Inflamm. Bowel. Dis. 2019, 25, 1896–1905. [Google Scholar] [CrossRef]
- Bezzio, C.; Festa, S.; Saibeni, S.; Papi, C. Chemoprevention of colorectal cancer in ulcerative colitis: Digging deep in current evidence. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Safroneeva, E.; Vavricka, S.R.; Fournier, N.; Pittet, V.; Peyrin-Biroulet, L.; Straumann, A.; Rogler, G.; Schoepfer, A.M.; Bauerfeind, P.; Swiss IBD Cohort Study Group. Impact of the early use of immunomodulators or TNF antagonists on bowel damage and surgery in Crohn’s disease. Aliment. Pharmacol. Ther. 2015, 42, 977–989. [Google Scholar] [CrossRef]
- Chaparro, M.; Garre, A.; Nunez Ortiz, A.; Diz-Lois Palomares, M.T.; Rodriguez, C.; Riestra, S.; Vela, M.; Benitez, J.M.; Fernandez Salgado, E.; Sanchez Rodriguez, E.; et al. Incidence, Clinical Characteristics and Management of Inflammatory Bowel Disease in Spain: Large-Scale Epidemiological Study. J. Clin. Med. 2021, 10, 2885. [Google Scholar] [CrossRef] [PubMed]
- Vavricka, S.R.; Rogler, G.; Gantenbein, C.; Spoerri, M.; Prinz Vavricka, M.; Navarini, A.A.; French, L.E.; Safroneeva, E.; Fournier, N.; Straumann, A.; et al. Chronological Order of Appearance of Extraintestinal Manifestations Relative to the Time of IBD Diagnosis in the Swiss Inflammatory Bowel Disease Cohort. Inflamm. Bowel. Dis. 2015, 21, 1794–1800. [Google Scholar] [CrossRef] [PubMed]
- Louis-Auguste, J.; Cohen, P.; Martin, J.; Smith, G. New diagnoses of inflammatory bowel disease during bowel cancer screening colonoscopy. Gut 2011, 60, A217. [Google Scholar] [CrossRef]
- Rodriguez-Lago, I.; Merino, O.; Azagra, I.; Maiz, A.; Zapata, E.; Higuera, R.; Montalvo, I.; Fernandez-Calderon, M.; Arreba, P.; Carrascosa, J.; et al. Characteristics and Progression of Preclinical Inflammatory Bowel Disease. Clin. Gastroenterol. Hepatol. 2018, 16, 1459–1466. [Google Scholar] [CrossRef]
- Vadstrup, K.; Alulis, S.; Borsi, A.; Gustafsson, N.; Nielsen, A.; Wennerstrom, E.C.M.; Jorgensen, T.R.; Qvist, N.; Munkholm, P. Cost Burden of Crohn’s Disease and Ulcerative Colitis in the 10-Year Period Before Diagnosis-A Danish Register-Based Study From 2003–2015. Inflamm. Bowel. Dis. 2020, 26, 1377–1382. [Google Scholar] [CrossRef]
- Rodriguez-Lago, I.; Agirre, U.; Intxaurza, N.; Cantero, D.; Cabriada, J.L.; Barreiro-de Acosta, M. Increased use of healthcare resources during the preclinical period of inflammatory bowel disease. Dig. Liver Dis. 2021, 53, 927–930. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Lago, I.; Aguirre, U.; Ramirez de la Piscina, P.; Munagorri, A.; Zapata, E.; Higuera, R.; Montalvo, I.; Iriarte, A.; Fernandez-Calderon, M.; Arreba, P.; et al. Subclinical bowel inflammation increases healthcare resources utilization and steroid use before diagnosis of inflammatory bowel disease. United Eur. Gastroenterol. J. 2023, 11, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Card, T.R.; Siffledeen, J.; Fleming, K.M. Are IBD patients more likely to have a prior diagnosis of irritable bowel syndrome? Report of a case-control study in the General Practice Research Database. United Eur. Gastroenterol. J. 2014, 2, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Fang, F.; Olen, O.; Song, M.; Halfvarson, J.; Roelstraete, B.; Khalili, H.; Ludvigsson, J.F. Long-term risk of inflammatory bowel disease after endoscopic biopsy with normal mucosa: A population-based, sibling-controlled cohort study in Sweden. PLoS Med. 2023, 20, e1004185. [Google Scholar] [CrossRef]
- Turner, D.; Ricciuto, A.; Lewis, A.; D’Amico, F.; Dhaliwal, J.; Griffiths, A.M.; Bettenworth, D.; Sandborn, W.J.; Sands, B.E.; Reinisch, W.; et al. STRIDE-II: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): Determining Therapeutic Goals for Treat-to-Target strategies in IBD. Gastroenterology 2021, 160, 1570–1583. [Google Scholar] [CrossRef]
- Le Berre, C.; Peyrin-Biroulet, L.; group, S.-I.S. Selecting End Points for Disease-Modification Trials in Inflammatory Bowel Disease: The SPIRIT Consensus From the IOIBD. Gastroenterology 2021, 160, 1452–1460.e1421. [Google Scholar] [CrossRef]
- Gisbert, J.P.; Chaparro, M. Primary Failure to an Anti-TNF Agent in Inflammatory Bowel Disease: Switch (to a Second Anti-TNF Agent) or Swap (for Another Mechanism of Action)? J. Clin. Med. 2021, 10, 5318. [Google Scholar] [CrossRef]
- Barreiro-de Acosta, M.; Fernandez-Clotet, A.; Mesonero, F.; Garcia-Alonso, F.J.; Casanova, M.J.; Fernandez-de la Varga, M.; Canete, F.; de Castro, L.; Gutierrez, A.; Sicilia, B.; et al. Long-Term Outcomes of Biological Therapy in Crohn’s Disease Complicated with Internal Fistulizing Disease: BIOSCOPE Study from GETECCU. Am. J. Gastroenterol. 2023, 10–14309. [Google Scholar] [CrossRef]
- Rodriguez-Lago, I.; Hoyo, J.D.; Perez-Girbes, A.; Garrido-Marin, A.; Casanova, M.J.; Chaparro, M.; Fernandez-Clotet, A.; Castro-Poceiro, J.; Garcia, M.J.; Sanchez, S.; et al. Early treatment with anti-tumor necrosis factor agents improves long-term effectiveness in symptomatic stricturing Crohn’s disease. United Eur. Gastroenterol. J. 2020, 8, 2050640620947579. [Google Scholar] [CrossRef]
- Lauriot Dit Prevost, C.; Azahaf, M.; Nachury, M.; Branche, J.; Gerard, R.; Wils, P.; Lambin, T.; Desreumaux, P.; Ernst, O.; Pariente, B. Bowel damage and disability in Crohn’s disease: A prospective study in a tertiary referral centre of the Lemann Index and Inflammatory Bowel Disease Disability Index. Aliment Pharmacol. Ther. 2020, 51, 889–898. [Google Scholar] [CrossRef]
- Bodini, G.; Giannini, E.G.; De Maria, C.; Dulbecco, P.; Furnari, M.; Marabotto, E.; Savarino, V.; Savarino, E. Anti-TNF therapy is able to stabilize bowel damage progression in patients with Crohn’s disease. A study performed using the Lemann Index. Dig. Liver Dis. 2017, 49, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Sudhakar, P.; Alsoud, D.; Wellens, J.; Verstockt, S.; Arnauts, K.; Verstockt, B.; Vermeire, S. Tailoring Multi-omics to Inflammatory Bowel Diseases: All for One and One for All. J. Crohn’s Colitis 2022, 16, 1306–1320. [Google Scholar] [CrossRef] [PubMed]
- Verstockt, B.; Bressler, B.; Martinez-Lozano, H.; McGovern, D.; Silverberg, M.S. Time to Revisit Disease Classification in Inflammatory Bowel Disease: Is the Current Classification of Inflammatory Bowel Disease Good Enough for Optimal Clinical Management? Gastroenterology 2022, 162, 1370–1382. [Google Scholar] [CrossRef] [PubMed]
- Choung, R.S.; Princen, F.; Stockfisch, T.P.; Torres, J.; Maue, A.C.; Porter, C.K.; Leon, F.; De Vroey, B.; Singh, S.; Riddle, M.S.; et al. Serologic microbial associated markers can predict Crohn’s disease behaviour years before disease diagnosis. Aliment Pharmacol. Ther. 2016, 43, 1300–1310. [Google Scholar] [CrossRef]
- Choung, R.S.; Petralia, F.; Torres, J.; Ungaro, R.C.; Porter, C.; Sato, T.; Telesco, S.; Strauss, R.S.; Plevy, S.; Princen, F.; et al. Preclinical Serological Signatures are Associated With Complicated Crohn’s Disease Phenotype at Diagnosis. Clin. Gastroenterol. Hepatol. 2023, in press. [CrossRef]
- Lee, S.H.; Turpin, W.; Espin-Garcia, O.; Raygoza Garay, J.A.; Smith, M.I.; Leibovitzh, H.; Goethel, A.; Turner, D.; Mack, D.; Deslandres, C.; et al. Anti-Microbial Antibody Response is Associated With Future Onset of Crohn’s Disease Independent of Biomarkers of Altered Gut Barrier Function, Subclinical Inflammation, and Genetic Risk. Gastroenterology 2021, 161, 1540–1551. [Google Scholar] [CrossRef]
- Mortha, A.; Remark, R.; Del Valle, D.M.; Chuang, L.S.; Chai, Z.; Alves, I.; Azevedo, C.; Gaifem, J.; Martin, J.; Petralia, F.; et al. Neutralizing Anti-Granulocyte Macrophage-Colony Stimulating Factor Autoantibodies Recognize Post-Translational Glycosylations on Granulocyte Macrophage-Colony Stimulating Factor Years Before Diagnosis and Predict Complicated Crohn’s Disease. Gastroenterology 2022, 163, 659–670. [Google Scholar] [CrossRef]
- Livanos, A.E.; Dunn, A.; Fischer, J.; Ungaro, R.C.; Turpin, W.; Lee, S.H.; Rui, S.; Del Valle, D.M.; Jougon, J.J.; Martinez-Delgado, G.; et al. Anti-Integrin alphavbeta6 Autoantibodies Are a Novel Biomarker That Antedate Ulcerative Colitis. Gastroenterology 2023, 164, 619–629. [Google Scholar] [CrossRef]
- Torres, J.; Petralia, F.; Sato, T.; Wang, P.; Telesco, S.E.; Choung, R.S.; Strauss, R.; Li, X.J.; Laird, R.M.; Gutierrez, R.L.; et al. Serum Biomarkers Identify Patients Who Will Develop Inflammatory Bowel Diseases Up to 5 Years Before Diagnosis. Gastroenterology 2020, 159, 96–104. [Google Scholar] [CrossRef]
- Leibovitzh, H.; Lee, S.H.; Raygoza Garay, J.A.; Espin-Garcia, O.; Xue, M.; Neustaeter, A.; Goethel, A.; Huynh, H.Q.; Griffiths, A.M.; Turner, D.; et al. Immune response and barrier dysfunction-related proteomic signatures in preclinical phase of Crohn’s disease highlight earliest events of pathogenesis. Gut 2023. [Google Scholar] [CrossRef]
- Bergemalm, D.; Andersson, E.; Hultdin, J.; Eriksson, C.; Rush, S.T.; Kalla, R.; Adams, A.T.; Keita, A.V.; D’Amato, M.; Gomollon, F.; et al. Systemic Inflammation in Preclinical Ulcerative Colitis. Gastroenterology 2021, 161, 1526–1539.e1529. [Google Scholar] [CrossRef] [PubMed]
- Hua, X.; Ungaro, R.C.; Petrick, L.M.; Chan, A.T.; Porter, C.K.; Khalili, H.; Nurses’ Health, S.; Collaborators, P. Inflammatory Bowel Disease Is Associated With Prediagnostic Perturbances in Metabolic Pathways. Gastroenterology 2023, 164, 147–150.e142. [Google Scholar] [CrossRef] [PubMed]
- Brand, E.C.; Klaassen, M.A.Y.; Gacesa, R.; Vich Vila, A.; Ghosh, H.; de Zoete, M.R.; Boomsma, D.I.; Hoentjen, F.; Horjus Talabur Horje, C.S.; van de Meeberg, P.C.; et al. Healthy Cotwins Share Gut Microbiome Signatures With Their Inflammatory Bowel Disease Twins and Unrelated Patients. Gastroenterology 2021, 160, 1970–1985. [Google Scholar] [CrossRef] [PubMed]
- Turpin, W.; Lee, S.H.; Raygoza Garay, J.A.; Madsen, K.L.; Meddings, J.B.; Bedrani, L.; Power, N.; Espin-Garcia, O.; Xu, W.; Smith, M.I.; et al. Increased Intestinal Permeability Is Associated With Later Development of Crohn’s Disease. Gastroenterology 2020, 159, 2092–2100.e2095. [Google Scholar] [CrossRef]
- Leibovitzh, H.; Lee, S.H.; Xue, M.; Raygoza Garay, J.A.; Hernandez-Rocha, C.; Madsen, K.L.; Meddings, J.B.; Guttman, D.S.; Espin-Garcia, O.; Smith, M.I.; et al. Altered Gut Microbiome Composition and Function Are Associated With Gut Barrier Dysfunction in Healthy Relatives of Patients With Crohn’s Disease. Gastroenterology 2022, 163, 1364–1376.e1310. [Google Scholar] [CrossRef]
- Galipeau, H.J.; Caminero, A.; Turpin, W.; Bermudez-Brito, M.; Santiago, A.; Libertucci, J.; Constante, M.; Raygoza Garay, J.A.; Rueda, G.; Armstrong, S.; et al. Novel Fecal Biomarkers That Precede Clinical Diagnosis of Ulcerative Colitis. Gastroenterology 2021, 160, 1532–1545. [Google Scholar] [CrossRef]
- Turpin, W.; Bedrani, L.; Espin-Garcia, O.; Xu, W.; Silverberg, M.S.; Smith, M.I.; Garay, J.A.R.; Lee, S.H.; Guttman, D.S.; Griffiths, A.; et al. Associations of NOD2 polymorphisms with Erysipelotrichaceae in stool of in healthy first degree relatives of Crohn’s disease subjects. BMC Med. Genet. 2020, 21, 204. [Google Scholar] [CrossRef]
- Spencer, E.A.; Helmus, D.; Telesco, S.; Colombel, J.F.; Dubinsky, M.C.; Road to Prevention Study, G. Inflammatory Bowel Disease Clusters Within Affected Sibships in Ashkenazi Jewish Multiplex Families. Gastroenterology 2020, 159, 381–382. [Google Scholar] [CrossRef]
- Rodriguez-Lago, I.; Ramirez, C.; Merino, O.; Azagra, I.; Maiz, A.; Zapata, E.; Higuera, R.; Montalvo, I.; Fernandez-Calderon, M.; Arreba, P.; et al. Early microscopic findings in preclinical inflammatory bowel disease. Dig. Liver Dis. 2020, 52, 1467–1472. [Google Scholar] [CrossRef]
- Yang, H.; Ge, Z.; Dai, J.; Li, X.; Gao, Y. Effectiveness of the immunofecal occult blood test for colorectal cancer screening in a large population. Dig. Dis. Sci. 2011, 56, 203–207. [Google Scholar] [CrossRef]
- Mayberry, J.F.; Ballantyne, K.C.; Hardcastle, J.D.; Mangham, C.; Pye, G. Epidemiological study of asymptomatic inflammatory bowel disease: The identification of cases during a screening programme for colorectal cancer. Gut 1989, 30, 481–483. [Google Scholar] [CrossRef] [PubMed]
- Sakata, T.; Niwa, Y.; Goto, H.; Hirooka, Y.; Hayakawa, T.; Ohmiya, N.; Kobayashi, S. Asymptomatic inflammatory bowel disease with special reference to ulcerative colitis in apparently healthy persons. Am. J. Gastroenterol. 2001, 96, 735–739. [Google Scholar] [CrossRef] [PubMed]
- Park, S.K.; Ye, B.D.; Yang, S.K.; Kim, S.O.; Kim, J.; Kim, J.W.; Park, S.H.; Yang, D.H.; Jung, K.W.; Kim, K.J.; et al. Clinical features and course of ulcerative colitis diagnosed in asymptomatic subjects. J. Crohn’s Colitis 2014, 8, 1254–1260. [Google Scholar] [CrossRef] [PubMed]
- Howarth, G.F.; Robinson, M.H.E.; Jenkins, D.; Hardcastle, J.D.; Logan, R.F.A. High prevalence of undetected inflammatory bowel disease (IBD): Data from the Nottingham faecal occult blood (FOB) screening trial. Am. J. Gastroenterol. 2002, 97, 690–694. [Google Scholar] [CrossRef]
- Katicic, M.; Antoljak, N.; Kujundzic, M.; Stamenic, V.; Skoko Poljak, D.; Kramaric, D.; Stimac, D.; Strnad Pesikan, M.; Samija, M.; Ebling, Z. Results of National Colorectal Cancer Screening Program in Croatia (2007–2011). World J. Gastroenterol. 2012, 18, 4300–4307. [Google Scholar] [CrossRef]
- Logan, R.F.; Patnick, J.; Nickerson, C.; Coleman, L.; Rutter, M.D.; von Wagner, C.; English Bowel Cancer Screening Evaluation, C. Outcomes of the Bowel Cancer Screening Programme (BCSP) in England after the first 1 million tests. Gut 2012, 61, 1439–1446. [Google Scholar] [CrossRef]
- Butcher, R.O.; Mehta, S.J.; Ahmad, O.F.; Boyd, C.A.; Anand, R.L.; Stein, J.; Abbasi, A.M.; George, R.; Prudham, R.C.; Vega, R.; et al. Mo1302 Incidental Diagnosis of Inflammatory Bowel Disease in a British Bowel Cancer Screening Cohort: A Multi-Centre Study. Gastroenterology 2013, 144, S-630–S-631. [Google Scholar] [CrossRef]
- Jayasooriya, N.; Saxena, S.; Blackwell, J.; Petersen, I.; Bottle, A.; Creese, H.; Pollok, R. PMO-6 Impact of consultation frequency and time to diagnosis on subsequent Inflammatory Bowel Disease outcomes. Gut 2021, 70, A78. [Google Scholar] [CrossRef]
- Magro, F.; Rodrigues-Pinto, E.; Coelho, R.; Andrade, P.; Santos-Antunes, J.; Lopes, S.; Camila-Dias, C.; Macedo, G. Is it possible to change phenotype progression in Crohn’s disease in the era of immunomodulators? Predictive factors of phenotype progression. Am. J. Gastroenterol. 2014, 109, 1026–1036. [Google Scholar] [CrossRef]
- Ramadas, A.V.; Gunesh, S.; Thomas, G.A.; Williams, G.T.; Hawthorne, A.B. Natural history of Crohn’s disease in a population-based cohort from Cardiff (1986–2003): A study of changes in medical treatment and surgical resection rates. Gut 2010, 59, 1200–1206. [Google Scholar] [CrossRef]
- Markowitz, J.; Grancher, K.; Kohn, N.; Lesser, M.; Daum, F. A multicenter trial of 6-mercaptopurine and prednisone in children with newly diagnosed Crohn’s disease. Gastroenterology 2000, 119, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Chhaya, V.; Pollok, R.C.; Cecil, E.; Subramanian, V.; Curcin, V.; Majeed, A.; Saxena, S. Impact of early thiopurines on surgery in 2770 children and young people diagnosed with inflammatory bowel disease: A national population-based study. Aliment Pharmacol. Ther. 2015, 42, 990–999. [Google Scholar] [CrossRef] [PubMed]
- Cosnes, J.; Bourrier, A.; Laharie, D.; Nahon, S.; Bouhnik, Y.; Carbonnel, F.; Allez, M.; Dupas, J.L.; Reimund, J.M.; Savoye, G.; et al. Early administration of azathioprine vs conventional management of Crohn’s Disease: A randomized controlled trial. Gastroenterology 2013, 145, 758–765.e752. [Google Scholar] [CrossRef]
- Schreiber, S.; Reinisch, W.; Colombel, J.F.; Sandborn, W.J.; Hommes, D.W.; Robinson, A.M.; Huang, B.; Lomax, K.G.; Pollack, P.F. Subgroup analysis of the placebo-controlled CHARM trial: Increased remission rates through 3 years for adalimumab-treated patients with early Crohn’s disease. J. Crohn’s Colitis 2013, 7, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Rubin, D.T.; Uluscu, O.; Sederman, R. Response to biologic therapy in Crohn’s disease is improved with early treatment: An analysis of health claims data. Inflamm. Bowel. Dis. 2012, 18, 2225–2231. [Google Scholar] [CrossRef]
- Ma, C.; Beilman, C.L.; Huang, V.W.; Fedorak, D.K.; Kroeker, K.I.; Dieleman, L.A.; Halloran, B.P.; Fedorak, R.N. Anti-TNF Therapy Within 2 Years of Crohn’s Disease Diagnosis Improves Patient Outcomes: A Retrospective Cohort Study. Inflamm. Bowel. Dis. 2016, 22, 870–879. [Google Scholar] [CrossRef]
- Choe, Y.J.; Han, K.; Shim, J.O. Treatment patterns of anti-tumour necrosis factor-alpha and prognosis of paediatric and adult-onset inflammatory bowel disease in Korea: A nationwide population-based study. Aliment Pharmacol. Ther. 2022, 56, 980–988. [Google Scholar] [CrossRef]
- Ben-Horin, S.; Novack, L.; Mao, R.; Guo, J.; Zhao, Y.; Sergienko, R.; Zhang, J.; Kobayashi, T.; Hibi, T.; Chowers, Y.; et al. Efficacy of Biologic Drugs in Short-Duration Versus Long-Duration Inflammatory Bowel Disease: A Systematic Review and an Individual-Patient Data Meta-Analysis of Randomized Controlled Trials. Gastroenterology 2022, 162, 482–494. [Google Scholar] [CrossRef]
- Focht, G.; Lujan, R.; Atia, O.; Greenfeld, S.; Kariv, R.; Loewenberg Weisband, Y.; Lederman, N.; Matz, E.; Dotan, I.; Turner, D. OP12 Does early initiation of biologics change the natural history of IBD? a nationwide study from the epi-IIRN. J. Crohn’s Colitis 2023, 17, i17–i18. [Google Scholar] [CrossRef]
- Krugliak Cleveland, N.; Torres, J.; Rubin, D.T. What Does Disease Progression Look Like in Ulcerative Colitis, and How Might It Be Prevented? Gastroenterology 2022, 162, 1396–1408. [Google Scholar] [CrossRef]
- Beaugerie, L.; Seksik, P.; Nion-Larmurier, I.; Gendre, J.P.; Cosnes, J. Predictors of Crohn’s disease. Gastroenterology 2006, 130, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Billiet, T.; Ferrante, M.; Van Assche, G. The use of prognostic factors in inflammatory bowel diseases. Curr. Gastroenterol. Rep. 2014, 16, 416. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Lyons, P.A.; McKinney, E.F.; Sowerby, J.M.; Carr, E.J.; Bredin, F.; Rickman, H.M.; Ratlamwala, H.; Hatton, A.; Rayner, T.F.; et al. Gene expression profiling of CD8+ T cells predicts prognosis in patients with Crohn disease and ulcerative colitis. J. Clin. Investig. 2011, 121, 4170–4179. [Google Scholar] [CrossRef] [PubMed]
- Michielan, A.; Basso, D.; Martinato, M.; Pathak, S.; Banerjee, A.; Oliva, L.; Plebani, M.; Sturniolo, G.C.; D’Inca, R. Increased antibody response to microbial antigens in patients with Crohn’s disease and their unaffected first-degree relatives. Dig. Liver Dis. 2013, 45, 894–898. [Google Scholar] [CrossRef] [PubMed]
- Standaert-Vitse, A.; Sendid, B.; Joossens, M.; Francois, N.; Vandewalle-El Khoury, P.; Branche, J.; Van Kruiningen, H.; Jouault, T.; Rutgeerts, P.; Gower-Rousseau, C.; et al. Candida albicans colonization and ASCA in familial Crohn’s disease. Am. J. Gastroenterol 2009, 104, 1745–1753. [Google Scholar] [CrossRef] [PubMed]
- Torok, H.P.; Glas, J.; Hollay, H.C.; Gruber, R.; Osthoff, M.; Tonenchi, L.; Bruckl, C.; Mussack, T.; Folwaczny, M.; Folwaczny, C. Serum antibodies in first-degree relatives of patients with IBD: A marker of disease susceptibility? A follow-up pilot-study after 7 years. Digestion 2005, 72, 119–123. [Google Scholar] [CrossRef]
- Lochhead, P.; Khalili, H.; Ananthakrishnan, A.N.; Richter, J.M.; Chan, A.T. Association Between Circulating Levels of C-Reactive Protein and Interleukin-6 and Risk of Inflammatory Bowel Disease. Clin. Gastroenterol. Hepatol. 2016, 14, 818–824.e816. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Lago, I.; Marigorta, U.M.; Barreiro-de Acosta, M. Preclinical Inflammatory Bowel Disease: Back to the Future. Gastroenterology 2021, 160, 475–476. [Google Scholar] [CrossRef]
- Ferrante, M.; Henckaerts, L.; Joossens, M.; Pierik, M.; Joossens, S.; Dotan, N.; Norman, G.L.; Altstock, R.T.; Van Steen, K.; Rutgeerts, P.; et al. New serological markers in inflammatory bowel disease are associated with complicated disease behaviour. Gut 2007, 56, 1394–1403. [Google Scholar] [CrossRef]
- Lee, J.C.; Biasci, D.; Roberts, R.; Gearry, R.B.; Mansfield, J.C.; Ahmad, T.; Prescott, N.J.; Satsangi, J.; Wilson, D.C.; Jostins, L.; et al. Genome-wide association study identifies distinct genetic contributions to prognosis and susceptibility in Crohn’s disease. Nat. Genet. 2017, 49, 262–268. [Google Scholar] [CrossRef]
- Haritunians, T.; Taylor, K.D.; Targan, S.R.; Dubinsky, M.; Ippoliti, A.; Kwon, S.; Guo, X.; Melmed, G.Y.; Berel, D.; Mengesha, E.; et al. Genetic predictors of medically refractory ulcerative colitis. Inflamm Bowel Dis 2010, 16, 1830–1840. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, A.N. IBD risk prediction using multi-ethnic polygenic risk scores. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 217–218. [Google Scholar] [CrossRef] [PubMed]
- Constantine-Cooke, N.; Monterrubio-Gómez, K.; Plevris, N.; Derikx, L.A.A.P.; Gros, B.; Jones, G.-R.; Marioni, R.E.; Lees, C.W.; Vallejos, C.A. Longitudinal Faecal Calprotectin Profiles Characterise Disease Course Heterogeneity in Crohn’s Disease. Clin. Gastroenterol Hepatol. 2023. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Ferru-Clement, R.; Boucher, G.; Forest, A.; Bouchard, B.; Bitton, A.; Lesage, S.; Schumm, P.; Lazarev, M.; Brant, S.; Duerr, R.H.; et al. Serum Lipidomic Screen Identifies Key Metabolites, Pathways, and Disease Classifiers in Crohn’s Disease. Inflamm. Bowel. Dis. 2023, izac281. [Google Scholar] [CrossRef] [PubMed]
- Kugathasan, S.; Denson, L.A.; Walters, T.D.; Kim, M.O.; Marigorta, U.M.; Schirmer, M.; Mondal, K.; Liu, C.; Griffiths, A.; Noe, J.D.; et al. Prediction of complicated disease course for children newly diagnosed with Crohn’s disease: A multicentre inception cohort study. Lancet 2017, 389, 1710–1718. [Google Scholar] [CrossRef]
- Biasci, D.; Lee, J.C.; Noor, N.M.; Pombal, D.R.; Hou, M.; Lewis, N.; Ahmad, T.; Hart, A.; Parkes, M.; McKinney, E.F.; et al. A blood-based prognostic biomarker in IBD. Gut 2019, 68, 1386–1395. [Google Scholar] [CrossRef]
- Parkes, M.; Noor, N.M.; Dowling, F.; Leung, H.; Bond, S.; Whitehead, L.; Upponi, S.; Kinnon, P.; Sandham, A.P.; Lyons, P.A.; et al. PRedicting Outcomes For Crohn’s dIsease using a moLecular biomarkEr (PROFILE): Protocol for a multicentre, randomised, biomarker-stratified trial. BMJ Open 2018, 8, e026767. [Google Scholar] [CrossRef]
- GEM Project. The Crohn’s and Colitis Canada GEM Project. Available online: https://www.gemproject.ca/ (accessed on 15 March 2023).
- Nowak, J.K.; Adams, A.T.; Kalla, R.; Lindstrom, J.C.; Vatn, S.; Bergemalm, D.; Keita, A.V.; Gomollon, F.; Jahnsen, J.; Vatn, M.H.; et al. Characterisation of the Circulating Transcriptomic Landscape in Inflammatory Bowel Disease Provides Evidence for Dysregulation of Multiple Transcription Factors Including NFE2, SPI1, CEBPB, and IRF2. J. Crohn’s Colitis 2022, 16, 1255–1268. [Google Scholar] [CrossRef]
- Kalla, R.; Adams, A.T.; Nowak, J.K.; Bergemalm, D.; Vatn, S.; Ventham, N.T.; Kennedy, N.A.; Ricanek, P.; Lindstrom, J.; Consortium, I.B.-C.; et al. Analysis of Systemic Epigenetic Alterations in Inflammatory Bowel Disease: Defining Geographical, Genetic and Immune-Inflammatory influences on the Circulating Methylome. J. Crohn’s Colitis 2023, 17, 170–184. [Google Scholar] [CrossRef]
- Nair, N.; Austin, C.; Curtin, P.; Gouveia, C.; Arora, M.; Torres, J.; Mount Sinai Road to Prevention, G. Association between Early-life Exposures and Inflammatory Bowel Diseases, Based on Analyses of Deciduous Teeth. Gastroenterology 2020, 159, 383–385. [Google Scholar] [CrossRef]
- Taylor, K.M.; Hanscombe, K.B.; Prescott, N.J.; Iniesta, R.; Traylor, M.; Taylor, N.S.; Fong, S.; Powell, N.; Irving, P.M.; Anderson, S.H.; et al. Genetic and Inflammatory Biomarkers Classify Small Intestine Inflammation in Asymptomatic First-degree Relatives of Patients With Crohn’s Disease. Clin. Gastroenterol. Hepatol. 2020, 18, 908–916.e913. [Google Scholar] [CrossRef]
- Kevans, D.; Turpin, W.; Madsen, K.; Meddings, J.; Shestopaloff, K.; Xu, W.; Moreno-Hagelsieb, G.; Griffiths, A.; Silverberg, M.S.; Paterson, A.; et al. Determinants of intestinal permeability in healthy first-degree relatives of individuals with Crohn’s disease. Inflamm. Bowel. Dis. 2015, 21, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Imhann, F.; Van der Velde, K.J.; Barbieri, R.; Alberts, R.; Voskuil, M.D.; Vich Vila, A.; Collij, V.; Spekhorst, L.M.; Van der Sloot, K.W.J.; Peters, V.; et al. The 1000IBD project: Multi-omics data of 1000 inflammatory bowel disease patients; data release 1. BMC Gastroenterol. 2019, 19, 5. [Google Scholar] [CrossRef]
- Marigorta, U.M.; Denson, L.A.; Hyams, J.S.; Mondal, K.; Prince, J.; Walters, T.D.; Griffiths, A.; Noe, J.D.; Crandall, W.V.; Rosh, J.R.; et al. Transcriptional risk scores link GWAS to eQTLs and predict complications in Crohn’s disease. Nat. Genet. 2017, 49, 1517–1521. [Google Scholar] [CrossRef] [PubMed]
- Porcu, E.; Sadler, M.C.; Lepik, K.; Auwerx, C.; Wood, A.R.; Weihs, A.; Sleiman, M.S.B.; Ribeiro, D.M.; Bandinelli, S.; Tanaka, T.; et al. Differentially expressed genes reflect disease-induced rather than disease-causing changes in the transcriptome. Nat. Commun. 2021, 12, 5647. [Google Scholar] [CrossRef] [PubMed]

| Study/Cohort | Type and Number of Individuals | Type of IBD | Type of Samples | Omics | Main Findings |
|---|---|---|---|---|---|
| Malmö Diet and Cancer cohort | 72 UC 140 healthy controls 37 healthy twins | UC | Blood, dried blood spots | Proteomics | Proteomic profile found to be upregulated years before UC diagnosis [41] |
| Swedish Newborn Dry Blood Spots cohort | |||||
| Swedish IBD Twin cohort | |||||
| Northern Sweden Health and Disease Study | |||||
| The Crohn’s and Colitis Canada Genetics, Environmental, Microbial (GEM) Project | 5122 first-degree relatives | UC CD | Blood, stool, urine | Multiomics | |
| Multiplex families, Road to Prevention cohort | 38 multiplex families | UC CD | Blood, stool, teeth, hair, saliva | Multiomics | Affected siblings are likely consecutively distributed [48] |
| TWIN cohort | 124 | UC CD | Blood, urine, stool, oral, rectal biopsies | Multiomics | Microbiome signatures [43] |
| PREDICTS cohort | 1000 UC 1000 CD 500 Healthy controls | UC CD | Blood | Proteomics Serology | |
| Nurses’ Health Study | 104 IBD | UC CD | Urine | Metabolome |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Lago, I.; Blackwell, J.; Mateos, B.; Marigorta, U.M.; Barreiro-de Acosta, M.; Pollok, R. Recent Advances and Potential Multi-Omics Approaches in the Early Phases of Inflammatory Bowel Disease. J. Clin. Med. 2023, 12, 3418. https://doi.org/10.3390/jcm12103418
Rodríguez-Lago I, Blackwell J, Mateos B, Marigorta UM, Barreiro-de Acosta M, Pollok R. Recent Advances and Potential Multi-Omics Approaches in the Early Phases of Inflammatory Bowel Disease. Journal of Clinical Medicine. 2023; 12(10):3418. https://doi.org/10.3390/jcm12103418
Chicago/Turabian StyleRodríguez-Lago, Iago, Jonathan Blackwell, Beatriz Mateos, Urko M. Marigorta, Manuel Barreiro-de Acosta, and Richard Pollok. 2023. "Recent Advances and Potential Multi-Omics Approaches in the Early Phases of Inflammatory Bowel Disease" Journal of Clinical Medicine 12, no. 10: 3418. https://doi.org/10.3390/jcm12103418
APA StyleRodríguez-Lago, I., Blackwell, J., Mateos, B., Marigorta, U. M., Barreiro-de Acosta, M., & Pollok, R. (2023). Recent Advances and Potential Multi-Omics Approaches in the Early Phases of Inflammatory Bowel Disease. Journal of Clinical Medicine, 12(10), 3418. https://doi.org/10.3390/jcm12103418







