The Inflammatory Bowel Disease—Disk Tool for Assessing Disability in Inflammatory Bowel Disease Patients: Validation of the Greek Version
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Design
2.2. Translation and Cross-Cultural Adaptation
2.3. Questionnaire Administration and Data Collection Procedures
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
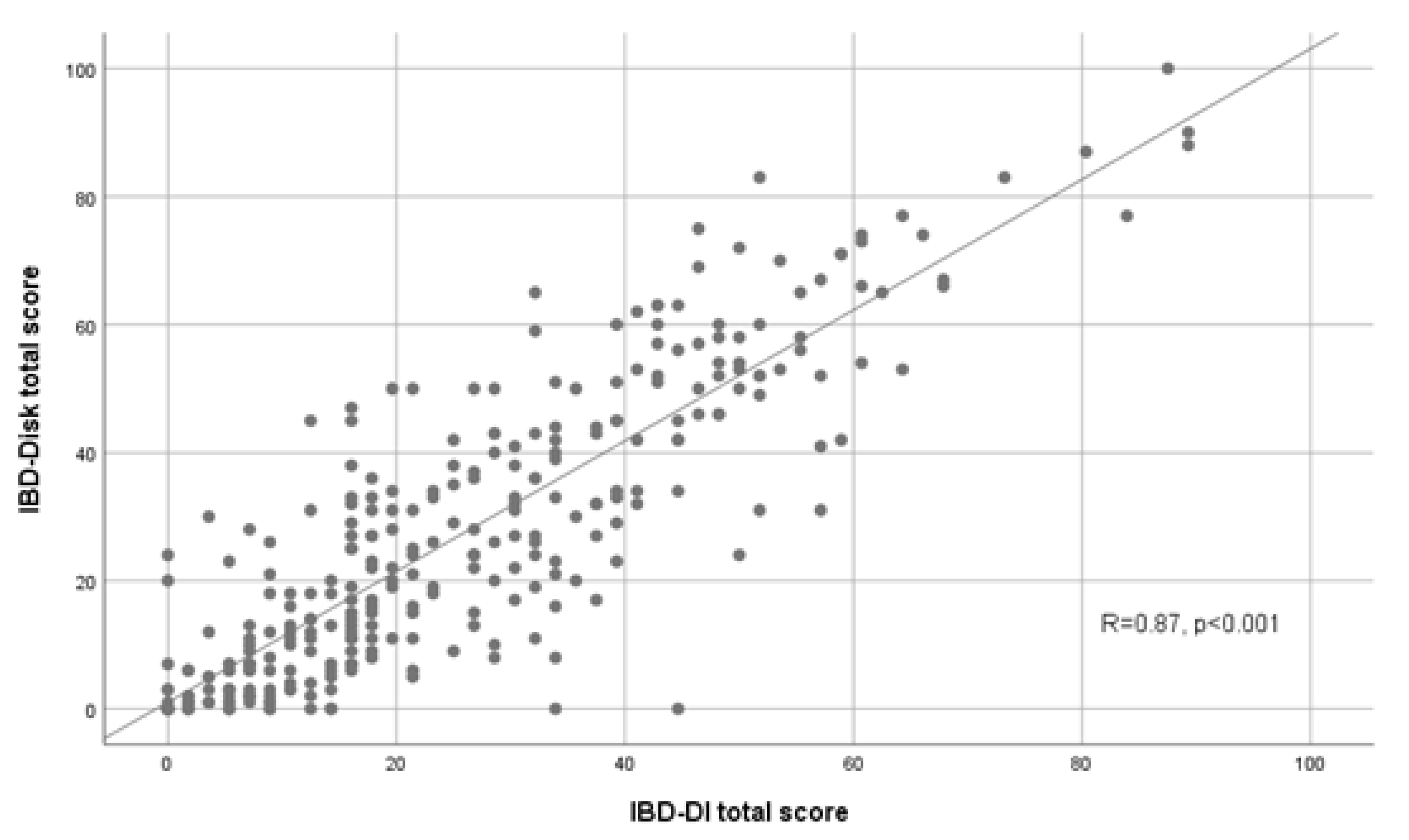
3.2. Validity of the IBD-Disk Score
3.3. Reliability of the IBD-Disk Score
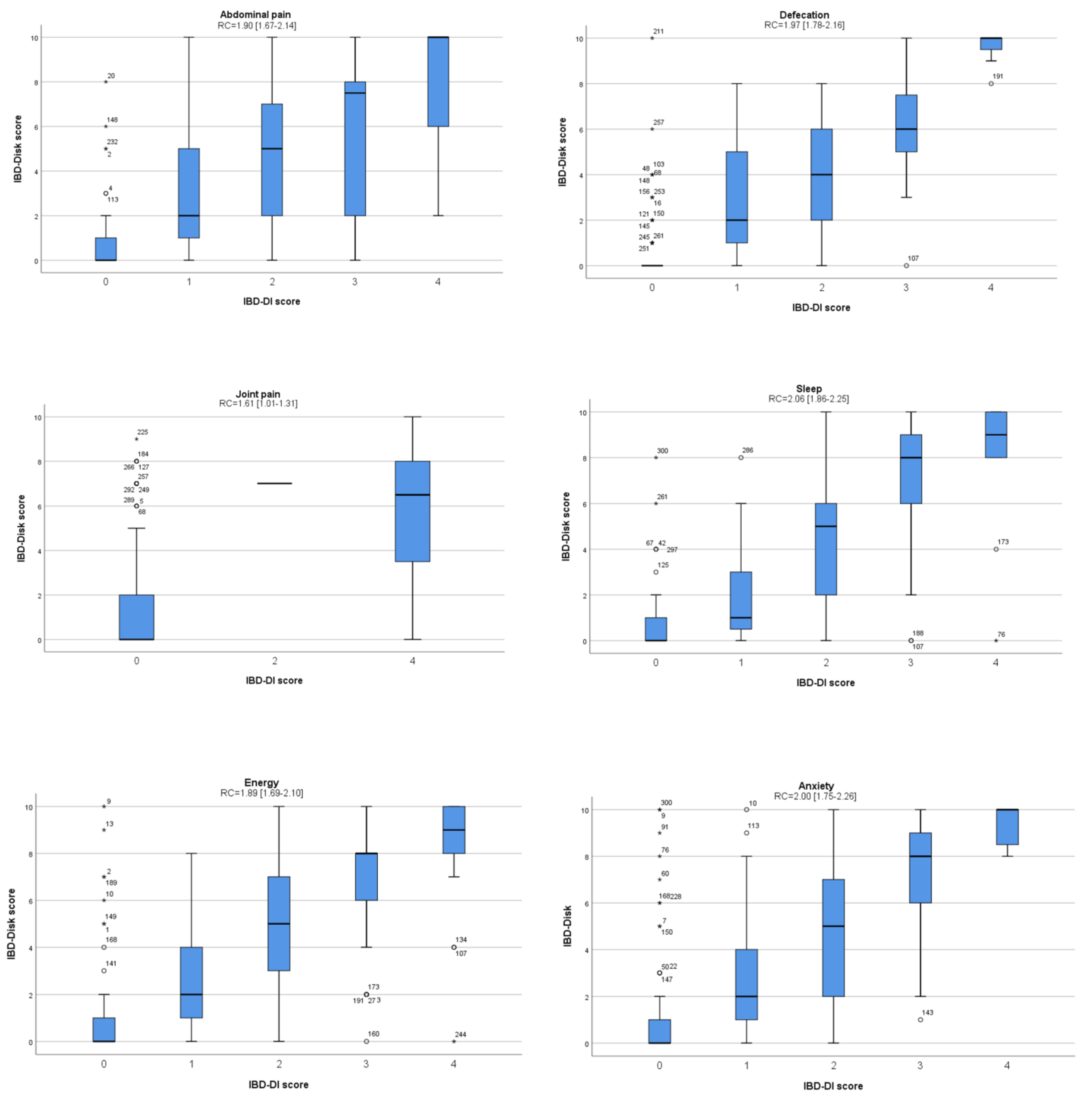
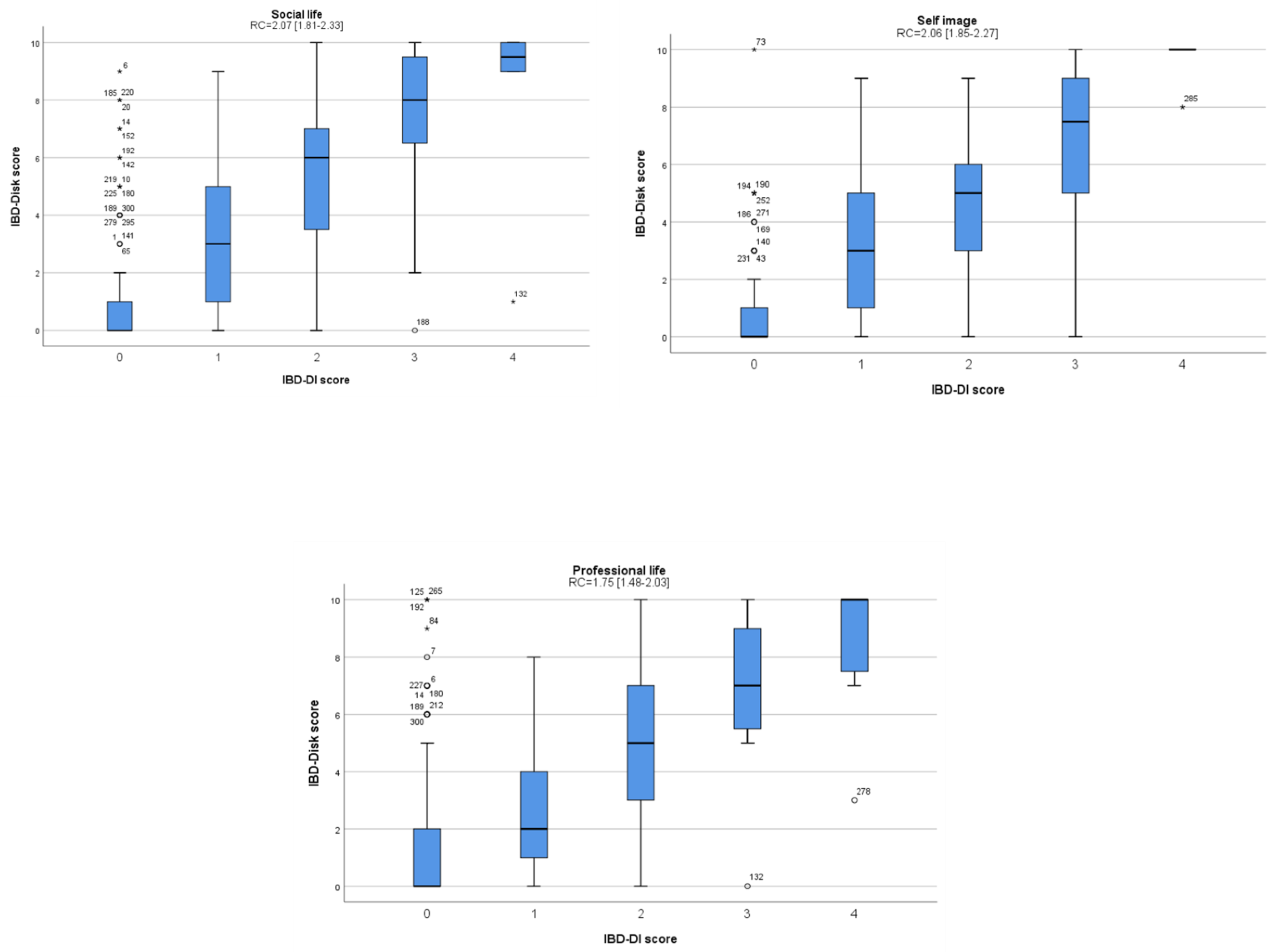
3.4. Analysis of Clinical Factors Associated with the IBD-Disk Score
3.5. IBD-Disk Score Variability between Baseline and Follow-up Visits
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moradkhani, A.; Beckman, L.J.; Tabibian, J.H. Health-related quality of life in inflammatory bowel disease: Psychosocial, clinical, socioeconomic, and demographic predictors. J. Crohn’s Col. 2013, 7, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Shafer, L.A.; Walker, J.R.; Restall, G.; Chhibba, T.; Ivekovic, M.; Singh, H.; Targownik, L.E.; Bernstein, C.N. Association Between IBD Disability and Reduced Work Productivity (Presenteeism): A Population-Based Study in Manitoba, Canada. Inflamm. Bowel Dis. 2019, 25, 352–359. [Google Scholar] [CrossRef]
- Marín, L.; Mañosa, M.; Garcia-Planella, E.; Gordillo, J.; Zabana, Y.; Cabré, E.; Domènech, E. Sexual function and patients’ perceptions in inflammatory bowel disease: A case–control survey. J. Gastroenterol. 2013, 48, 713–720. [Google Scholar] [CrossRef] [PubMed]
- McDermott, E.; Mullen, G.; Moloney, J.; Keegan, D.; Byrne, K.; Doherty, G.A.; Cullen, G.; Malone, K.; Mulcahy, H.E. Body Image Dissatisfaction: Clinical Features, and Psychosocial Disability in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2015, 21, 353–360. [Google Scholar] [CrossRef]
- Gower-Rousseau, C.; Sarter, H.; Savoye, G.; Tavernier, N.; Fumery, M.; Sandborn, W.J.; Feagan, B.G.; Duhamel, A.; Guillon-Dellac, N.; Colombel, J.F. Validation of the Inflammatory Bowel Disease Disability Index in a population-based cohort. Gut 2017, 66, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Peyrin-Biroulet, L.; Cieza, A.; Sandborn, W.J.; Coenen, M.; Chowers, Y.; Hibi, T.; Kostanjsek, N.; Stucki, G.; Colombel, J.F. Development of the first disability index for inflammatory bowel disease based on the international classification of functioning, disability and health. Gut 2012, 61, 241–247. [Google Scholar] [CrossRef]
- Lo, B.; Prosberg, M.V.; Gluud, L.L.; Chan, W. Systematic review and meta-analysis: Assessment of factors affecting disability in inflammatory bowel disease and the reliability of the inflammatory bowel disease disability index. Alim. Pharmacol. Ther. 2018, 47, 6–15. [Google Scholar] [CrossRef]
- Ghosh, S.; Louis, E.; Beaugerie, L.; Bossuyt, P.; Bouguen, G.; Bourreille, A.; Ferrante, M.; Franchimont, D.; Frost, K.; Hebuterne, X.; et al. Development of the IBD Disk: A Visual Self-administered Tool for Assessing Disability in Inflammatory Bowel Diseases. Inflamm. Bowel Dis. 2017, 23, 333–340. [Google Scholar] [CrossRef]
- Le Berre, C.; Flamant, M.; Bouguen, G.; Siproudhis, L.; Dewitte, M.; Dib, N.; Cesbron-Metivier, E.; Goronflot, T.; Hanf, M.; Gourraud, P.A.; et al. VALIDation of the IBD-Disk Instrument for Assessing Disability in Inflammatory Bowel Diseases in a French Cohort: The VALIDate Study. J. Crohn’s Col. 2020, 14, 1512–1523. [Google Scholar] [CrossRef]
- Mendes, S.S.; Ferreira, P.; Antunes, P.; Gonçalves, M.; Leal, T.; Gonçalves, B.; Rebelo, A.; Arroja, B.; Caetano, A.C.; Gonçalves, R.; et al. Validation of the IBD-Disk in a Portuguese cohort. Eur. J. Gastroenterol. Hepatol. 2021, 33, e961–e969. [Google Scholar] [CrossRef]
- Shi, H.Y.; Levy, A.N.; Trivedi, H.D.; Chan, F.; Ng, S.C.; Ananthakrishnan, A.N. Ethnicity influences phenotype and outcomes in inflammatory bowel disease: A systematic review and meta-analysis of population-based studies. Clin. Gastroenterol. Hepatol. 2018, 16, 190–197.e11. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, M.; Cohen-Mekelburg, S.; Kayal, M.; Axelrad, J.; Galati, J.; Tricomi, B.; Kamal, K.; Faye, A.S.; Abrudescu, P.; Scherl, E.; et al. Disability in inflammatory bowel disease patients is associated with race, ethnicity and socio-economic factors. Aliment. Pharmacol. Ther. 2019, 49, 564–571. [Google Scholar] [CrossRef]
- Mokkink, L.B.; Prinsen, C.; Patrick, D.L.; Alonso, J.; Bouter, L.; de Vet, H.C.; Terwee, C.B.; Mokkink, L. COSMIN guideline for systematic reviews of patient-reported outcome measures. Qual. Life Res. 2018, 27, 1147–1157. [Google Scholar] [CrossRef]
- Mokkink, L.B.; De Vet, H.C.; Prinsen, C.A.; Patrick, D.L.; Alonso, J.; Bouter, L.M.; Terwee, C.B. COSMIN Risk of Bias checklist for systematic reviews of Patient-Reported Outcome Measures. Qual. Life Res. Int. J. Qual. Life Aspects Treat. Care Rehab. 2018, 27, 1171–1179. [Google Scholar] [CrossRef] [PubMed]
- Terwee, C.B.; Prinsen, C.A.; Chiarotto, A.; Westerman, M.J.; Patrick, D.L.; Alonso, J.; Bouter, L.M.; De Vet, H.C.; Mokkink, L.B. COSMIN methodology for evaluating the content validity of patient-reported outcome measures: A Delphi study. Qual. Life Res. Int. J. Qual. Life Aspects Treat. Care Rehab. 2018, 27, 1159–1170. [Google Scholar] [CrossRef]
- Harvey, R.F.; Bradshaw, J.M. A simple index of Crohn’s-disease activity. Lancet 1980, 315, 514. [Google Scholar] [CrossRef]
- Lewis, J.D.; Chuai, S.; Nessel, L.; Lichtenstein, G.R.; Aberra, F.N.; Ellenberg, J.H. Use of the noninvasive components of the Mayo score to assess clinical response in ulcerative colitis. Inflamm. Bowel Dis. 2008, 14, 1660–1666. [Google Scholar] [CrossRef] [PubMed]
- Streiner, D.N.G. Health Measurement Scales; Oxford University Press: Oxford, UK, 1989. [Google Scholar]
- Shrout, P.E.; Fleiss, J.L. Intraclass correlations: Uses in assessing rater reliability. Psychol. Bull. 1979, 86, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Bolarinwa, O.A. Principles and methods of validity and reliability testing of questionnaires used in social and health science researches. Niger. Postgrad. Med. J. 2015, 22, 195–201. [Google Scholar] [CrossRef]
- Allen, P.B.; Gower-Rousseau, C.; Danese, S.; Peyrin-Biroulet, L. Preventing disability in inflammatory bowel disease. Ther. Adv. Gastroenterol. 2017, 10, 865–876. [Google Scholar] [CrossRef]
- Nurmi, E.; Haapamäki, J.; Paavilainen, E.; Rantanen, A.; Hillilä, M.; Arkkila, P. The burden of inflammatory bowel disease on health care utilization and quality of life. Scand. J. Gastroenterol. 2013, 48, 51–57. [Google Scholar] [CrossRef]
- Greuter, T.; Manser, C.; Pittet, V.; Vavricka, S.R.; Biedermann, L. Gender Differences in Inflammatory Bowel Disease. Digestion 2020, 101, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Rustgi, S.D.; Kayal, M. Sex-based differences in inflammatory bowel diseases: A review. Ther. Adv. Gastroenterol. 2020, 13, 1756284820915043. [Google Scholar] [CrossRef]
- Tannoury, J.; Nachury, M.; Martins, C.; Serrero, M.; Filippi, J.; Roblin, X.; Bourrier, A.; Bouguen, G.; Franchimont, D.; Savoye, G.; et al. Determinants of IBD-related disability: A cross-sectional survey from the GETAID. Alim. Pharmacol. Ther. 2021, 53, 1098. [Google Scholar]
- Lo, B.; Julsgaard, M.; Vester-Andersen, M.K.; Vind, I.; Burisch, J. Disease activity, steroid use and extraintestinal manifestation are associated with increased disability in patients with inflammatory bowel disease using the inflammatory bowel disease disability index: A cross-sectional multicentre cohort study. Eur. J. Gastroenterol. Hepatol. 2018, 30, 1130–1136. [Google Scholar] [CrossRef]
- Marinelli, C.; Savarino, E. Factors Influencing Disability and Quality of Life during Treatment: A Cross-Sectional Study on IBD Patients. Gastroenterol. Res. Pract. 2019, 2019, 5354320. [Google Scholar] [CrossRef]
- Yoon, J.Y.; Shin, J.E.; Park, S.H.; Park, D.I.; Cha, J.M. Disability due to Inflammatory Bowel Disease Is Correlated with Drug Compliance, Disease Activity, and Quality of Life. Gut Liver 2017, 11, 370–376. [Google Scholar] [CrossRef] [PubMed]
| Total (n = 300) | |
|---|---|
| Gender, n (%) | |
| Male | 161 (53.7) |
| Female | 139 (46.3) |
| Age (years), mean (SD) | 41.3 (13.7) |
| Disease type, n (%) | |
| Crohn’s disease | 199 (66.3) |
| Ulcerative colitis | 101 (33.7) |
| Disease duration (years), n (%) | |
| <2 | 24 (8) |
| 2–8 | 139 (46.3) |
| >8 | 137 (45.7) |
| Age at diagnosis (years, CD patients), n (%) | |
| A1 | 34 (17.1) |
| A2 | 123 (61.8) |
| A3 | 42 (21.1) |
| Disease behavior (CD patients), n (%) | |
| B1 | 114 (57.3) |
| B2 | 51 (25.6) |
| B3 | 34 (17.1) |
| Perianal involvement, n (%) | 44 (22.1) |
| Disease location in CD, n (%) | |
| Ileal [L1] | 76 (38.2) |
| Colonic [L2] | 24 (12.1) |
| Ileocolonic [L3] | 99 (49.7) |
| Upper GI involvement [L4] | 13 (6.5) |
| Disease location in UC, n (%) | |
| Proctitis [E1] | 9 (8.9) |
| Left-sided colitis [E2] | 40 (39.6) |
| Pancolitis [E3] | 52 (51.5) |
| Extraintestinal manifestations, n (%) | 120 (40.0) |
| Biologics/small molecules, n (%) | |
| None | 63 (21.0) |
| Infliximab | 127 (42.3) |
| Adalimumab | 30 (10.0) |
| Ustekinumab | 25 (8.3) |
| Vedolizumab | 46 (15.3) |
| Golimumab | 6 (2.0) |
| Tofacitinib | 2 (0.7) |
| Cyclosporin | 1 (0.3) |
| AZA, n (%) | 72 (24.0) |
| MTX, n (%) | 13 (4.3) |
| 5-ASA, n (%) | 96 (32.0) |
| Steroids, n (%) | 39 (13.0) |
| Smoking, n (%) | 79 (26.3) |
| IBD-related surgery, n (%) | 36 (12.0) |
| Hospitalization, n (%) | 40 (13.3) |
| pMayo, n (%) | |
| pMayo < 2 | 73 (72.2) |
| pMayo 2–5 | 25 (24.8) |
| pMayo > 5 | 3 (3.0) |
| HBI, n (%) | |
| HBI < 4 | 138 (69.3) |
| HBI 4–12 | 57 (28.6) |
| HBI > 12 | 4 (2.1) |
| ICC [95%CI] | |
|---|---|
| Abdominal pain | 0.78 [0.73–0.83] |
| Defecation | 0.76 [0.70–0.81] |
| Social life | 0.85 [0.81–0.88] |
| Professional life | 0.79 [0.74–0.84] |
| Sleep | 0.79 [0.74–0.83] |
| Energy | 0.82 [0.78–0.86] |
| Anxiety | 0.79 [0.74–0.84] |
| Self-image | 0.88 [0.85–0.91] |
| Sexual function | 0.82 [0.77–0.85] |
| Joint pain | 0.87 [0.83–0.89] |
| Total score | 0.89 [0.86–0.91] |
| Abdominal Pain | Defecation | Social Life | Professional Life | Sleep | Energy | Anxiety | Self-Image | Sexual Function | Joint Pain | |
|---|---|---|---|---|---|---|---|---|---|---|
| Abdominal pain | - | |||||||||
| Defecation | 0.75 | - | ||||||||
| Social life | 0.65 | 0.77 | - | |||||||
| Professional life | 0.68 | 0.72 | 0.89 | - | ||||||
| Sleep | 0.55 | 0.53 | 0.66 | 0.72 | - | |||||
| Energy | 0.71 | 0.63 | 0.70 | 0.78 | 0.76 | - | ||||
| Anxiety | 0.61 | 0.55 | 0.77 | 0.74 | 0.69 | 0.80 | - | |||
| Self-image | 0.40 | 0.31 | 0.48 | 0.52 | 0.56 | 0.56 | 0.52 | - | ||
| Sexual function | 0.54 | 0.53 | 0.78 | 0.70 | 0.61 | 0.65 | 0.72 | 0.61 | - | |
| Joint pain | 0.64 | 0.62 | 0.58 | 0.66 | 0.63 | 0.68 | 0.59 | 0.55 | 0.61 | - |
| IBD-Disk Total Score, Median (IQR) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Inactive at Both Visits a | Active Becoming Inactive a | Active at Both Visits a | Inactive Becoming Active a | ||||||||||||
| B | FU | p-Value | B | FU | p-Value | B | FU | p-Value | B | FU | p-Value | ||||
| Total (n = 157) | 13 (3, 30.5) | 16 (4, 29.5) | 0.364 | Total (n = 39) | 32 (13, 52) | 21 (9, 43) | 0.003 * | Total (n = 43) | 53 (31, 67) | 46 (33, 59) | 0.260 | Total (n = 30) | 25.5 (11, 37.5) | 39 (12.5, 45.2) | 0.025 * |
| CD (n = 107) | 13 (3, 31) | 17 (4, 30) | 0.364 | CD (n = 27) | 34 (19, 59) | 23 (11, 43) | 0.005 * | CD (n = 28) | 54 (33.5, 72.2) | 49.5 (36, 62.2) | 0.062 | CD (n = 15) | 24 (13, 42) | 40 (13, 53) | 0.096 |
| UC (n = 50) | 15.5 (4.5, 28.2) | 12.5 (4, 28.2) | 0.779 | UC (n = 12) | 23.5 (7, 43.5) | 11 (7.3, 41) | 0.286 | UC (n = 15) | 42 (12, 60) | 46 (19, 54) | 0.209 | UC (n = 15) | 31 (3, 36) | 33 (10, 42) | 0.140 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katsoula, A.; Axiaris, G.; Mpitouli, A.; Palatianou, M.; Christidou, A.; Dimitriadis, N.; Nakos, A.; Pastras, P.; Kourkoulis, P.; Karatzas, P.; et al. The Inflammatory Bowel Disease—Disk Tool for Assessing Disability in Inflammatory Bowel Disease Patients: Validation of the Greek Version. J. Clin. Med. 2023, 12, 3023. https://doi.org/10.3390/jcm12083023
Katsoula A, Axiaris G, Mpitouli A, Palatianou M, Christidou A, Dimitriadis N, Nakos A, Pastras P, Kourkoulis P, Karatzas P, et al. The Inflammatory Bowel Disease—Disk Tool for Assessing Disability in Inflammatory Bowel Disease Patients: Validation of the Greek Version. Journal of Clinical Medicine. 2023; 12(8):3023. https://doi.org/10.3390/jcm12083023
Chicago/Turabian StyleKatsoula, Anastasia, Georgios Axiaris, Afroditi Mpitouli, Maria Palatianou, Angeliki Christidou, Nikolaos Dimitriadis, Andreas Nakos, Ploutarchos Pastras, Panagiotis Kourkoulis, Pantelis Karatzas, and et al. 2023. "The Inflammatory Bowel Disease—Disk Tool for Assessing Disability in Inflammatory Bowel Disease Patients: Validation of the Greek Version" Journal of Clinical Medicine 12, no. 8: 3023. https://doi.org/10.3390/jcm12083023
APA StyleKatsoula, A., Axiaris, G., Mpitouli, A., Palatianou, M., Christidou, A., Dimitriadis, N., Nakos, A., Pastras, P., Kourkoulis, P., Karatzas, P., Moutzoukis, M., Zlatinoudis, C., Philippidis, A., Kourikou, A., Kokkotis, G., Gklavas, A., Machaira, A., Mantaka, A., Talimtzi, P., ... Giouleme, O. (2023). The Inflammatory Bowel Disease—Disk Tool for Assessing Disability in Inflammatory Bowel Disease Patients: Validation of the Greek Version. Journal of Clinical Medicine, 12(8), 3023. https://doi.org/10.3390/jcm12083023







