Potential Role of Innate Lymphoid Cells in the Pathogenesis and Treatment of Skin Diseases
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. ILC2s and Cutaneous Inflammation
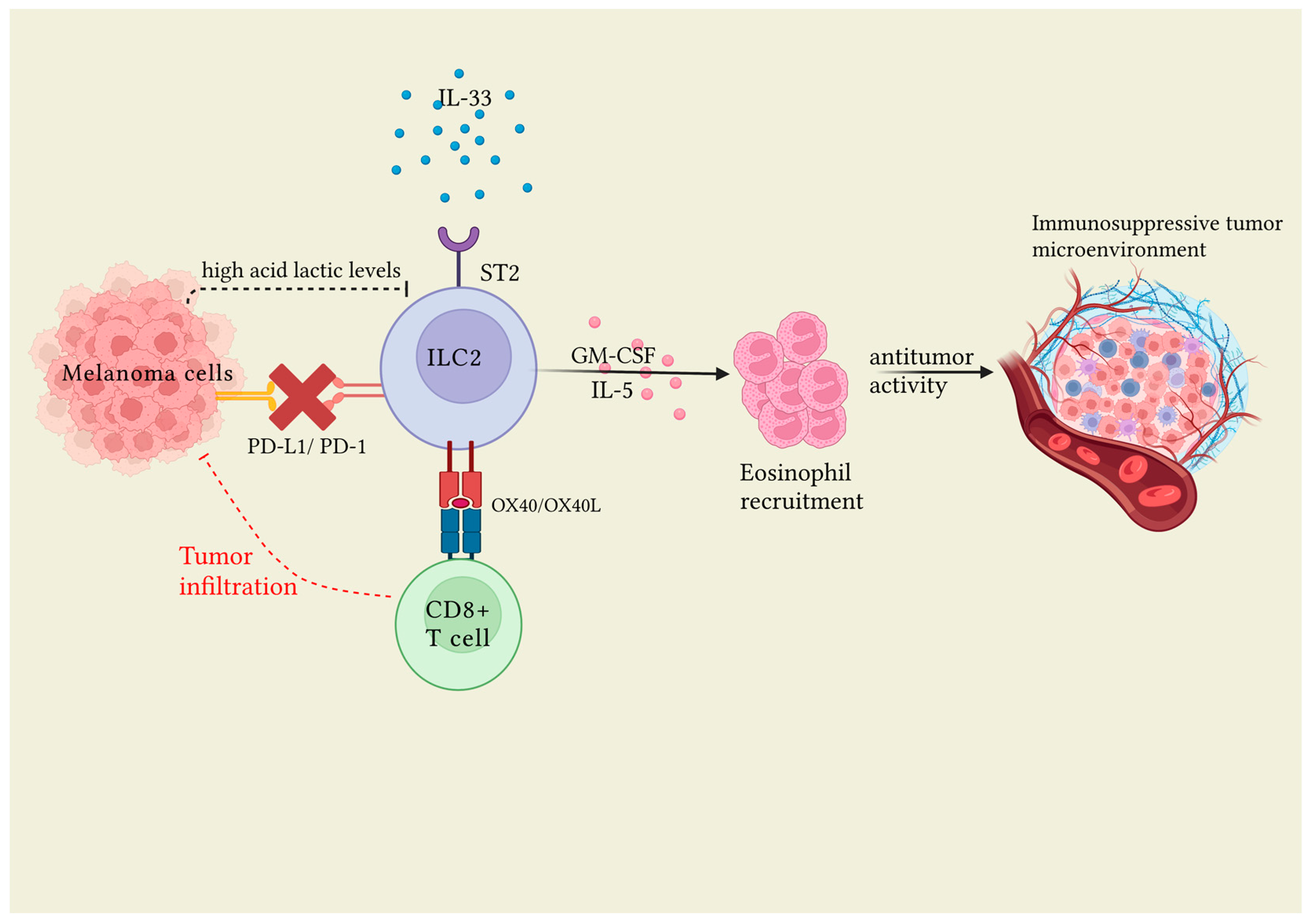
3.1.1. ILC2s and Melanoma
3.1.2. ILC2s and Sistemic Sclerosis
3.1.3. ILC2s and Parasites Infections
3.1.4. ILC2s and Hidradenitis Suppurativa
3.1.5. ILC2s: Skin Wound Healing and Skin Immune Tolerance
3.1.6. ILC2 and Mastocytosis
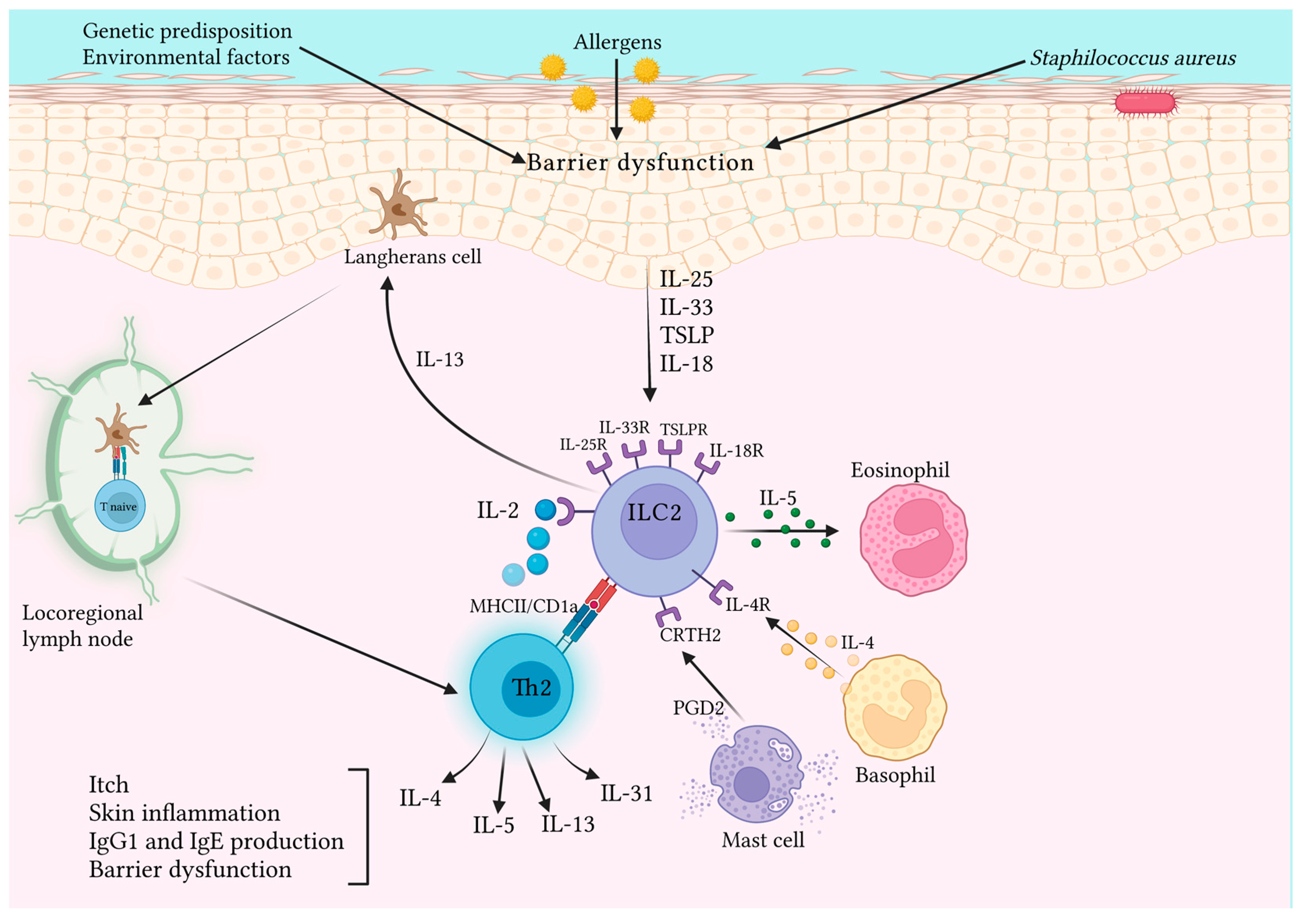
3.2. ILC2s and Allergic Cutaneous Inflammation
Focus on Atopic Dermatitis
3.3. Possible Therapeutic Uses of ILC2s in Inflammatory Pathologies
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xiong, L.; Nutt, S.L.; Seillet, C. Innate Lymphoid Cells: More than Just Immune Cells. Front. Immunol. 2022, 13, 1033904. [Google Scholar] [CrossRef] [PubMed]
- Gasteiger, G.; Fan, X.; Dikiy, S.; Lee, S.Y.; Rudensky, A.Y. Tissue Residency of Innate Lymphoid Cells in Lymphoid and Nonlymphoid Organs. Science 2015, 350, 981–985. [Google Scholar] [CrossRef]
- Weizman, O.-E.; Song, E.; Adams, N.M.; Hildreth, A.D.; Riggan, L.; Krishna, C.; Aguilar, O.A.; Leslie, C.S.; Carlyle, J.R.; Sun, J.C.; et al. Mouse Cytomegalovirus-Experienced ILC1s Acquire a Memory Response Dependent on the Viral Glycoprotein M12. Nat. Immunol. 2019, 20, 1004–1011. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Gonzalez, I.; Mathä, L.; Steer, C.A.; Ghaedi, M.; Poon, G.F.T.; Takei, F. Allergen-Experienced Group 2 Innate Lymphoid Cells Acquire Memory-like Properties and Enhance Allergic Lung Inflammation. Immunity 2016, 45, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Verma, M.; Michalec, L.; Sripada, A.; McKay, J.; Sirohi, K.; Verma, D.; Sheth, D.; Martin, R.; Dyjack, N.; Seibold, M.A.; et al. The Molecular and Epigenetic Mechanisms of Innate Lymphoid Cell (ILC) Memory and Its Relevance for Asthma. J. Exp. Med. 2021, 218, e20201354. [Google Scholar] [CrossRef]
- Serafini, N.; Jarade, A.; Surace, L.; Goncalves, P.; Sismeiro, O.; Varet, H.; Legendre, R.; Coppee, J.-Y.; Disson, O.; Durum, S.K.; et al. Trained ILC3 Responses Promote Intestinal Defense. Science 2022, 375, 859–863. [Google Scholar] [CrossRef]
- Artis, D.; Spits, H. The Biology of Innate Lymphoid Cells. Nature 2015, 517, 293–301. [Google Scholar] [CrossRef]
- Nagasawa, M.; Spits, H.; Ros, X.R. Innate Lymphoid Cells (ILCs): Cytokine Hubs Regulating Immunity and Tissue Homeostasis. Cold Spring Harb. Perspect. Biol. 2018, 10, a030304. [Google Scholar] [CrossRef]
- Nabekura, T.; Shibuya, A. Type 1 Innate Lymphoid Cells: Soldiers at the Front Line of Immunity. Biomed. J. 2021, 44, 115–122. [Google Scholar] [CrossRef]
- Kiniwa, T.; Moro, K. Localization and Site-Specific Cell–Cell Interactions of Group 2 Innate Lymphoid Cells. Int. Immunol. 2021, 33, 251–259. [Google Scholar] [CrossRef]
- Moro, K.; Yamada, T.; Tanabe, M.; Takeuchi, T.; Ikawa, T.; Kawamoto, H.; Furusawa, J.; Ohtani, M.; Fujii, H.; Koyasu, S. Innate Production of TH2 Cytokines by Adipose Tissue-Associated c-Kit+Sca-1+ Lymphoid Cells. Nature 2010, 463, 540–544. [Google Scholar] [CrossRef]
- Neill, D.R.; Wong, S.H.; Bellosi, A.; Flynn, R.J.; Daly, M.; Langford, T.K.A.; Bucks, C.; Kane, C.M.; Fallon, P.G.; Pannell, R.; et al. Nuocytes Represent a New Innate Effector Leukocyte That Mediates Type-2 Immunity. Nature 2010, 464, 1367–1370. [Google Scholar] [CrossRef] [PubMed]
- Pulendran, B.; Artis, D. New Paradigms in Type 2 Immunity. Science 2012, 337, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Klose, C.S.N.; Kiss, E.A.; Schwierzeck, V.; Ebert, K.; Hoyler, T.; d’Hargues, Y.; Göppert, N.; Croxford, A.L.; Waisman, A.; Tanriver, Y.; et al. A T-Bet Gradient Controls the Fate and Function of CCR6−RORγt+ Innate Lymphoid Cells. Nature 2013, 494, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Jarade, A.; Di Santo, J.P.; Serafini, N. Group 3 Innate Lymphoid Cells Mediate Host Defense against Attaching and Effacing Pathogens. Curr. Opin. Microbiol. 2021, 63, 83–91. [Google Scholar] [CrossRef]
- Meininger, I.; Carrasco, A.; Rao, A.; Soini, T.; Kokkinou, E.; Mjösberg, J. Tissue-Specific Features of Innate Lymphoid Cells. Trends Immunol. 2020, 41, 902–917. [Google Scholar] [CrossRef]
- Bielecki, P.; Riesenfeld, S.J.; Hütter, J.-C.; Torlai Triglia, E.; Kowalczyk, M.S.; Ricardo-Gonzalez, R.R.; Lian, M.; Amezcua Vesely, M.C.; Kroehling, L.; Xu, H.; et al. Skin-Resident Innate Lymphoid Cells Converge on a Pathogenic Effector State. Nature 2021, 592, 128–132. [Google Scholar] [CrossRef]
- Gronke, K.; Kofoed-Nielsen, M.; Diefenbach, A. Innate Lymphoid Cells, Precursors and Plasticity. Immunol. Lett. 2016, 179, 9–18. [Google Scholar] [CrossRef]
- Carrega, P.; Campana, S.; Bonaccorsi, I.; Ferlazzo, G. The Yin and Yang of Innate Lymphoid Cells in Cancer. Immunol. Lett. 2016, 179, 29–35. [Google Scholar] [CrossRef]
- Clottu, A.S.; Humbel, M.; Fluder, N.; Karampetsou, M.P.; Comte, D. Innate Lymphoid Cells in Autoimmune Diseases. Front. Immunol. 2022, 12, 789788. [Google Scholar] [CrossRef]
- Sabbagh, F.; Kim, B.S. Microneedles for Transdermal Drug Delivery Using Clay-Based Composites. Expert Opin. Drug Deliv. 2022, 19, 1099–1113. [Google Scholar] [CrossRef] [PubMed]
- Sabbagh, F.; Kim, B.S. Recent Advances in Polymeric Transdermal Drug Delivery Systems. J. Control. Release 2022, 341, 132–146. [Google Scholar] [CrossRef] [PubMed]
- Salimi, M.; Wang, R.; Yao, X.; Li, X.; Wang, X.; Hu, Y.; Chang, X.; Fan, P.; Dong, T.; Ogg, G. Activated Innate Lymphoid Cell Populations Accumulate in Human Tumour Tissues. BMC Cancer 2018, 18, 341. [Google Scholar] [CrossRef] [PubMed]
- Bie, Q.; Zhang, P.; Su, Z.; Zheng, D.; Ying, X.; Wu, Y.; Yang, H.; Chen, D.; Wang, S.; Xu, H. Polarization of ILC2s in Peripheral Blood Might Contribute to Immunosuppressive Microenvironment in Patients with Gastric Cancer. J. Immunol. Res. 2014, 2014, 923135. [Google Scholar] [CrossRef]
- Trabanelli, S.; Chevalier, M.F.; Martinez-Usatorre, A.; Gomez-Cadena, A.; Salomé, B.; Lecciso, M.; Salvestrini, V.; Verdeil, G.; Racle, J.; Papayannidis, C.; et al. Tumour-Derived PGD2 and NKp30-B7H6 Engagement Drives an Immunosuppressive ILC2-MDSC Axis. Nat. Commun. 2017, 8, 593. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Y.; Wang, Y.; Ye, P.; Li, J.; Li, H.; Ding, Q.; Xia, J. Amphiregulin Confers Regulatory T Cell Suppressive Function and Tumor Invasion via the EGFR/GSK-3β/Foxp3 Axis. J. Biol. Chem. 2016, 291, 21085–21095. [Google Scholar] [CrossRef]
- Busser, B.; Sancey, L.; Brambilla, E.; Coll, J.-L.; Hurbin, A. The Multiple Roles of Amphiregulin in Human Cancer. Biochim. Et Biophys. Acta (BBA) Rev. Cancer 2011, 1816, 119–131. [Google Scholar] [CrossRef]
- Chevalier, M.F.; Trabanelli, S.; Racle, J.; Salomé, B.; Cesson, V.; Gharbi, D.; Bohner, P.; Domingos-Pereira, S.; Dartiguenave, F.; Fritschi, A.-S.; et al. ILC2-Modulated T Cell–to-MDSC Balance Is Associated with Bladder Cancer Recurrence. J. Clin. Investig. 2017, 127, 2916–2929. [Google Scholar] [CrossRef]
- Long, A.; Dominguez, D.; Qin, L.; Chen, S.; Fan, J.; Zhang, M.; Fang, D.; Zhang, Y.; Kuzel, T.M.; Zhang, B. Type 2 Innate Lymphoid Cells Impede IL-33–Mediated Tumor Suppression. J. Immunol. 2018, 201, 3456–3464. [Google Scholar] [CrossRef]
- Schuijs, M.J.; Png, S.; Richard, A.C.; Tsyben, A.; Hamm, G.; Stockis, J.; Garcia, C.; Pinaud, S.; Nicholls, A.; Ros, X.R.; et al. ILC2-Driven Innate Immune Checkpoint Mechanism Antagonizes NK Cell Antimetastatic Function in the Lung. Nat. Immunol. 2020, 21, 998–1009. [Google Scholar] [CrossRef]
- Heinrich, B.; Gertz, E.M.; Schäffer, A.A.; Craig, A.; Ruf, B.; Subramanyam, V.; McVey, J.C.; Diggs, L.P.; Heinrich, S.; Rosato, U.; et al. The Tumour Microenvironment Shapes Innate Lymphoid Cells in Patients with Hepatocellular Carcinoma. Gut 2022, 71, 1161–1175. [Google Scholar] [CrossRef] [PubMed]
- Moral, J.A.; Leung, J.; Rojas, L.A.; Ruan, J.; Zhao, J.; Sethna, Z.; Ramnarain, A.; Gasmi, B.; Gururajan, M.; Redmond, D.; et al. ILC2s Amplify PD-1 Blockade by Activating Tissue-Specific Cancer Immunity. Nature 2020, 579, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Li Pomi, F.; Borgia, F.; Custurone, P.; Vaccaro, M.; Pioggia, G.; Gangemi, S. Role of HMGB1 in Cutaneous Melanoma: State of the Art. Int. J. Mol. Sci. 2022, 23, 9327. [Google Scholar] [CrossRef] [PubMed]
- Incorvaia, L.; Badalamenti, G.; Rinaldi, G.; Iovanna, J.L.; Olive, D.; Swayden, M.; Terruso, L.; Vincenzi, B.; Fulfaro, F.; Bazan, V.; et al. Can the Plasma PD-1 Levels Predict the Presence and Efficiency of Tumor-Infiltrating Lymphocytes in Patients with Metastatic Melanoma? Ther. Adv. Med. Oncol. 2019, 11, 175883591984887. [Google Scholar] [CrossRef] [PubMed]
- Howard, E.; Hurrell, B.P.; Helou, D.G.; Quach, C.; Painter, J.D.; Shafiei-Jahani, P.; Fung, M.; Gill, P.S.; Soroosh, P.; Sharpe, A.H.; et al. PD-1 Blockade on Tumor Microenvironment-Resident ILC2s Promotes TNF-α Production and Restricts Progression of Metastatic Melanoma. Front. Immunol. 2021, 12, 733136. [Google Scholar] [CrossRef]
- Jacquelot, N.; Seillet, C.; Wang, M.; Pizzolla, A.; Liao, Y.; Hediyeh-zadeh, S.; Grisaru-Tal, S.; Louis, C.; Huang, Q.; Schreuder, J.; et al. Blockade of the Co-Inhibitory Molecule PD-1 Unleashes ILC2-Dependent Antitumor Immunity in Melanoma. Nat. Immunol. 2021, 22, 851–864. [Google Scholar] [CrossRef]
- Wagner, M.; Ealey, K.N.; Tetsu, H.; Kiniwa, T.; Motomura, Y.; Moro, K.; Koyasu, S. Tumor-Derived Lactic Acid Contributes to the Paucity of Intratumoral ILC2s. Cell Rep. 2020, 30, 2743–2757.e5. [Google Scholar] [CrossRef]
- Okuyama, Y.; Okajima, A.; Sakamoto, N.; Hashimoto, A.; Tanabe, R.; Kawajiri, A.; Kawabe, T.; Ishii, N. IL-33-ILC2 Axis Promotes Anti-Tumor CD8+ T Cell Responses via OX40 Signaling. Biochem. Biophys. Res. Commun. 2022, 637, 9–16. [Google Scholar] [CrossRef]
- Wohlfahrt, T.; Usherenko, S.; Englbrecht, M.; Dees, C.; Weber, S.; Beyer, C.; Gelse, K.; Distler, O.; Schett, G.; Distler, J.H.W.; et al. Type 2 Innate Lymphoid Cell Counts Are Increased in Patients with Systemic Sclerosis and Correlate with the Extent of Fibrosis. Ann. Rheum. Dis. 2016, 75, 623–626. [Google Scholar] [CrossRef]
- Bagnato, G.L.; Irrera, N.; Pizzino, G.; Santoro, D.; Roberts, W.N.; Bagnato, G.; Pallio, G.; Vaccaro, M.; Squadrito, F.; Saitta, A.; et al. Dual Avβ3 and Avβ5 Blockade Attenuates Fibrotic and Vascular Alterations in a Murine Model of Systemic Sclerosis. Clin Sci. 2018, 132, 231–242. [Google Scholar] [CrossRef]
- Laurent, P.; Allard, B.; Manicki, P.; Jolivel, V.; Levionnois, E.; Jeljeli, M.; Henrot, P.; Izotte, J.; Leleu, D.; Groppi, A.; et al. TGFβ Promotes Low IL10-Producing ILC2 with Profibrotic Ability Involved in Skin Fibrosis in Systemic Sclerosis. Ann. Rheum. Dis. 2021, 80, 1594–1603. [Google Scholar] [CrossRef] [PubMed]
- Guggino, G.; Lo Pizzo, M.; Di Liberto, D.; Rizzo, A.; Cipriani, P.; Ruscitti, P.; Candore, G.; Gambino, C.M.; Sireci, G.; Dieli, F.; et al. Interleukin-9 over-Expression and T Helper 9 Polarization in Systemic Sclerosis Patients. Clin. Exp. Immunol. 2017, 190, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, C.; Harrison, O.J.; Schmitt, V.; Pelletier, M.; Spencer, S.P.; Urban, J.F.; Ploch, M.; Ramalingam, T.R.; Siegel, R.M.; Belkaid, Y. Critical Role of Fatty Acid Metabolism in ILC2-Mediated Barrier Protection during Malnutrition and Helminth Infection. J. Exp. Med. 2016, 213, 1409–1418. [Google Scholar] [CrossRef] [PubMed]
- Ricardo-Gonzalez, R.R.; Kotas, M.E.; O’Leary, C.E.; Singh, K.; Damsky, W.; Liao, C.; Arouge, E.; Tenvooren, I.; Marquez, D.M.; Schroeder, A.W.; et al. Innate Type 2 Immunity Controls Hair Follicle Commensalism by Demodex Mites. Immunity 2022, 55, 1891–1908.e12. [Google Scholar] [CrossRef]
- Li Pomi, F.; Macca, L.; Motolese, A.; Ingrasciotta, Y.; Berretta, M.; Guarneri, C. Neoplastic Implications in Patients Suffering from Hidradenitis Suppurativa under Systemic Treatments. Biomedicines 2021, 9, 1594. [Google Scholar] [CrossRef]
- Petrasca, A.; Hambly, R.; Molloy, O.; Kearns, S.; Moran, B.; Smith, C.M.; Hughes, R.; O’Donnell, M.; Zaborowski, A.; Winter, D.; et al. Innate Lymphoid Cell (ILC) Subsets Are Enriched in the Skin of Patients with Hidradenitis Suppurativa. PLoS ONE 2023, 18, e0281688. [Google Scholar] [CrossRef]
- Rak, G.D.; Osborne, L.C.; Siracusa, M.C.; Kim, B.S.; Wang, K.; Bayat, A.; Artis, D.; Volk, S.W. IL-33-Dependent Group 2 Innate Lymphoid Cells Promote Cutaneous Wound Healing. J. Investig. Dermatol. 2016, 136, 487–496. [Google Scholar] [CrossRef]
- Li, Y.; Lin, S.; Xiong, S.; Xie, Q. Recombinant Expression of Human IL-33 Protein and Its Effect on Skin Wound Healing in Diabetic Mice. Bioengineering 2022, 9, 734. [Google Scholar] [CrossRef]
- Borgia, F.; Custurone, P.; Li Pomi, F.; Vaccaro, M.; Alessandrello, C.; Gangemi, S. IL-33 and IL-37: A Possible Axis in Skin and Allergic Diseases. Int. J. Mol. Sci. 2022, 24, 372. [Google Scholar] [CrossRef]
- Walton, K.; Walker, K.; Riddle, M.; Koehn, B.H.; Reff, J.; Sagatys, E.M.; Linden, M.A.; Pidala, J.; Kim, J.; Lee, M.C.; et al. Dual JAK2/Aurora Kinase A Inhibition Prevents Human Skin Graft Rejection by Allo-Inactivation and ILC2-Mediated Tissue Repair. Am. J. Transplant. 2022, 22, 717–730. [Google Scholar] [CrossRef]
- Munneke, J.M.; Björklund, A.T.; Mjösberg, J.M.; Garming-Legert, K.; Bernink, J.H.; Blom, B.; Huisman, C.; van Oers, M.H.J.; Spits, H.; Malmberg, K.-J.; et al. Activated Innate Lymphoid Cells Are Associated with a Reduced Susceptibility to Graft-versus-Host Disease. Blood 2014, 124, 812–821. [Google Scholar] [CrossRef] [PubMed]
- Bruce, D.W.; Kolupaev, O.; Laurie, S.J.; Bommiasamy, H.; Stefanski, H.; Blazar, B.R.; Coghill, J.M.; Serody, J.S. Third-Party Type 2 Innate Lymphoid Cells Prevent and Treat GI Tract GvHD. Blood Adv. 2021, 5, 4578–4589. [Google Scholar] [CrossRef] [PubMed]
- van der Ploeg, E.K.; Hermans, M.A.W.; van der Velden, V.H.J.; Dik, W.A.; van Daele, P.L.A.; Stadhouders, R. Increased Group 2 Innate Lymphoid Cells in Peripheral Blood of Adults with Mastocytosis. J. Allergy Clin. Immunol. 2021, 147, 1490–1496.e2. [Google Scholar] [CrossRef] [PubMed]
- Roediger, B.; Kyle, R.; Yip, K.H.; Sumaria, N.; Guy, T.V.; Kim, B.S.; Mitchell, A.J.; Tay, S.S.; Jain, R.; Forbes-Blom, E.; et al. Cutaneous Immunosurveillance and Regulation of Inflammation by Group 2 Innate Lymphoid Cells. Nat. Immunol. 2013, 14, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Nakatani-Kusakabe, M.; Yasuda, K.; Tomura, M.; Nagai, M.; Yamanishi, K.; Kuroda, E.; Kanazawa, N.; Imai, Y. Monitoring Cellular Movement with Photoconvertible Fluorescent Protein and Single-Cell RNA Sequencing Reveals Cutaneous Group 2 Innate Lymphoid Cell Subtypes, Circulating ILC2 and Skin-Resident ILC2. JID Innov. 2021, 1, 100035. [Google Scholar] [CrossRef] [PubMed]
- Leyva-Castillo, J.M.; Galand, C.; Mashiko, S.; Bissonnette, R.; McGurk, A.; Ziegler, S.F.; Dong, C.; McKenzie, A.N.J.; Sarfati, M.; Geha, R.S. ILC2 Activation by Keratinocyte-Derived IL-25 Drives IL-13 Production at Sites of Allergic Skin Inflammation. J. Allergy Clin. Immunol. 2020, 145, 1606–1614.e4. [Google Scholar] [CrossRef]
- Ricardo-Gonzalez, R.R.; Van Dyken, S.J.; Schneider, C.; Lee, J.; Nussbaum, J.C.; Liang, H.-E.; Vaka, D.; Eckalbar, W.L.; Molofsky, A.B.; Erle, D.J.; et al. Tissue Signals Imprint ILC2 Identity with Anticipatory Function. Nat. Immunol. 2018, 19, 1093–1099. [Google Scholar] [CrossRef]
- Kim, B.S.; Wang, K.; Siracusa, M.C.; Saenz, S.A.; Brestoff, J.R.; Monticelli, L.A.; Noti, M.; Tait Wojno, E.D.; Fung, T.C.; Kubo, M.; et al. Basophils Promote Innate Lymphoid Cell Responses in Inflamed Skin. J. Immunol. 2014, 193, 3717–3725. [Google Scholar] [CrossRef]
- Hsu, C.-L.; Chhiba, K.D.; Krier-Burris, R.; Hosakoppal, S.; Berdnikovs, S.; Miller, M.L.; Bryce, P.J. Allergic Inflammation Is Initiated by IL-33–Dependent Crosstalk between Mast Cells and Basophils. PLoS ONE 2020, 15, e0226701. [Google Scholar] [CrossRef]
- Malhotra, N.; Leyva-Castillo, J.M.; Jadhav, U.; Barreiro, O.; Kam, C.; O’Neill, N.K.; Meylan, F.; Chambon, P.; von Andrian, U.H.; Siegel, R.M.; et al. RORα-Expressing T Regulatory Cells Restrain Allergic Skin Inflammation. Sci. Immunol. 2018, 3, eaao6923. [Google Scholar] [CrossRef]
- Rafei-Shamsabadi, D.A.; van de Poel, S.; Dorn, B.; Kunz, S.; Martin, S.F.; Klose, C.S.N.; Arnold, S.J.; Tanriver, Y.; Ebert, K.; Diefenbach, A.; et al. Lack of Type 2 Innate Lymphoid Cells Promotes a Type I-Driven Enhanced Immune Response in Contact Hypersensitivity. J. Investig. Dermatol. 2018, 138, 1962–1972. [Google Scholar] [CrossRef] [PubMed]
- Shane, H.L.; Lukomska, E.; Kashon, M.L.; Anderson, S.E. Topical Application of the Quaternary Ammonium Compound Didecyldimethylammonium Chloride Activates Type 2 Innate Lymphoid Cells and Initiates a Mixed-Type Allergic Response. Toxicol. Sci. 2019, 168, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.-G.; Liou, J.-H.; Hung, S.-I.; Chen, C.-B.; Chiu, T.-M.; Wang, C.-W.; Chung, W.-H. Increased Type 2 Innate Lymphoid Cells in Patients with Drug Reaction with Eosinophilia and Systemic Symptoms Syndrome. J. Investig. Dermatol. 2019, 139, 1722–1731. [Google Scholar] [CrossRef] [PubMed]
- Numazaki, M.; Abe, M.; Hanaoka, K.; Imamura, E.; Maeda, M.; Kimura, A.; Miyanohara, J.; Saito, T.; Arai, K.; Suzuki, H.; et al. ASP7266, a Novel Antibody against Human Thymic Stromal Lymphopoietin Receptor for the Treatment of Allergic Diseases. J. Pharmacol. Exp. Ther. 2022, 380, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Borgia, F.; Custurone, P.; Li Pomi, F.; Cordiano, R.; Alessandrello, C.; Gangemi, S. IL-31: State of the Art for an Inflammation-Oriented Interleukin. Int. J. Mol. Sci. 2022, 23, 6507. [Google Scholar] [CrossRef]
- Früh, S.P.; Saikia, M.; Eule, J.; Mazulis, C.A.; Miller, J.E.; Cowulich, J.M.; Oyesola, O.O.; Webb, L.M.; Peng, S.A.; Cubitt, R.L.; et al. Elevated Circulating Th2 but Not Group 2 Innate Lymphoid Cell Responses Characterize Canine Atopic Dermatitis. Vet. Immunol. Immunopathol. 2020, 221, 110015. [Google Scholar] [CrossRef]
- Nishikawa, Y.; Fukaya, T.; Fukui, T.; Uto, T.; Takagi, H.; Nasu, J.; Miyanaga, N.; Riethmacher, D.; Choijookhuu, N.; Hishikawa, Y.; et al. Congenital Deficiency of Conventional Dendritic Cells Promotes the Development of Atopic Dermatitis-Like Inflammation. Front. Immunol. 2021, 12, 712676. [Google Scholar] [CrossRef]
- Mashiko, S.; Mehta, H.; Bissonnette, R.; Sarfati, M. Increased Frequencies of Basophils, Type 2 Innate Lymphoid Cells and Th2 Cells in Skin of Patients with Atopic Dermatitis but Not Psoriasis. J. Dermatol. Sci. 2017, 88, 167–174. [Google Scholar] [CrossRef]
- Kim, B.S.; Siracusa, M.C.; Saenz, S.A.; Noti, M.; Monticelli, L.A.; Sonnenberg, G.F.; Hepworth, M.R.; Van Voorhees, A.S.; Comeau, M.R.; Artis, D. TSLP Elicits IL-33–Independent Innate Lymphoid Cell Responses to Promote Skin Inflammation. Sci. Transl. Med. 2013, 5, 170ra16. [Google Scholar] [CrossRef]
- Salimi, M.; Barlow, J.L.; Saunders, S.P.; Xue, L.; Gutowska-Owsiak, D.; Wang, X.; Huang, L.-C.; Johnson, D.; Scanlon, S.T.; McKenzie, A.N.J.; et al. A Role for IL-25 and IL-33-Driven Type-2 Innate Lymphoid Cells in Atopic Dermatitis. J. Exp. Med. 2013, 210, 2939–2950. [Google Scholar] [CrossRef]
- Brüggen, M.-C.; Bauer, W.M.; Reininger, B.; Clim, E.; Captarencu, C.; Steiner, G.E.; Brunner, P.M.; Meier, B.; French, L.E.; Stingl, G. In Situ Mapping of Innate Lymphoid Cells in Human Skin: Evidence for Remarkable Differences between Normal and Inflamed Skin. J. Invest. Dermatol. 2016, 136, 2396–2405. [Google Scholar] [CrossRef] [PubMed]
- Teunissen, M.B.M.; Munneke, J.M.; Bernink, J.H.; Spuls, P.I.; Res, P.C.M.; te Velde, A.; Cheuk, S.; Brouwer, M.W.D.; Menting, S.P.; Eidsmo, L.; et al. Composition of Innate Lymphoid Cell Subsets in the Human Skin: Enrichment of NCR + ILC3 in Lesional Skin and Blood of Psoriasis Patients. J. Investig. Dermatol. 2014, 134, 2351–2360. [Google Scholar] [CrossRef] [PubMed]
- Hardman, C.S.; Chen, Y.-L.; Salimi, M.; Jarrett, R.; Johnson, D.; Järvinen, V.J.; Owens, R.J.; Repapi, E.; Cousins, D.J.; Barlow, J.L.; et al. CD1a Presentation of Endogenous Antigens by Group 2 Innate Lymphoid Cells. Sci. Immunol. 2017, 2, eaan5918. [Google Scholar] [CrossRef]
- Salimi, M.; Xue, L.; Jolin, H.; Hardman, C.; Cousins, D.J.; McKenzie, A.N.J.; Ogg, G.S. Group 2 Innate Lymphoid Cells Express Functional NKp30 Receptor Inducing Type 2 Cytokine Production. J. Immunol. 2016, 196, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Bonanno, A.; Gangemi, S.; La Grutta, S.; Malizia, V.; Riccobono, L.; Colombo, P.; Cibella, F.; Profita, M. 25-Hydroxyvitamin D, IL-31, and IL-33 in Children with Allergic Disease of the Airways. Mediat. Inflamm 2014, 2014, 520241. [Google Scholar] [CrossRef]
- Murdaca, G.; Greco, M.; Tonacci, A.; Negrini, S.; Borro, M.; Puppo, F.; Gangemi, S. IL-33/IL-31 Axis in Immune-Mediated and Allergic Diseases. Int. J. Mol. Sci. 2019, 20, 5856. [Google Scholar] [CrossRef] [PubMed]
- Imai, Y.; Yasuda, K.; Nagai, M.; Kusakabe, M.; Kubo, M.; Nakanishi, K.; Yamanishi, K. IL-33–Induced Atopic Dermatitis–Like Inflammation in Mice Is Mediated by Group 2 Innate Lymphoid Cells in Concert with Basophils. J. Investig. Dermatol. 2019, 139, 2185–2194.e3. [Google Scholar] [CrossRef]
- Imai, Y.; Yasuda, K.; Sakaguchi, Y.; Haneda, T.; Mizutani, H.; Yoshimoto, T.; Nakanishi, K.; Yamanishi, K. Skin-Specific Expression of IL-33 Activates Group 2 Innate Lymphoid Cells and Elicits Atopic Dermatitis-like Inflammation in Mice. Proc. Natl. Acad. Sci. USA 2013, 110, 13921–13926. [Google Scholar] [CrossRef]
- Schwartz, C.; Moran, T.; Saunders, S.P.; Kaszlikowska, A.; Floudas, A.; Bom, J.; Nunez, G.; Iwakura, Y.; O’Neill, L.; Irvine, A.D.; et al. Spontaneous Atopic Dermatitis in Mice with a Defective Skin Barrier Is Independent of ILC2 and Mediated by IL-1β. Allergy 2019, 74, 1920–1933. [Google Scholar] [CrossRef]
- Borgia, F.; Li Pomi, F.; Vaccaro, M.; Alessandrello, C.; Papa, V.; Gangemi, S. Oxidative Stress and Phototherapy in Atopic Dermatitis: Mechanisms, Role, and Future Perspectives. Biomolecules 2022, 12, 1904. [Google Scholar] [CrossRef]
- Li Pomi, F.; Papa, V.; Borgia, F.; Vaccaro, M.; Allegra, A.; Cicero, N.; Gangemi, S. Rosmarinus Officinalis and Skin: Antioxidant Activity and Possible Therapeutical Role in Cutaneous Diseases. Antioxidants 2023, 12, 680. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Seok, J.K.; Cho, I.; Yang, G.; Kim, K.-B.; Kwack, S.J.; Kang, H.C.; Cho, Y.-Y.; Lee, H.S.; Lee, J.Y. Topical Application of Celastrol Alleviates Atopic Dermatitis Symptoms Mediated through the Regulation of Thymic Stromal Lymphopoietin and Group 2 Innate Lymphoid Cells. J. Toxicol. Environ. Health A 2021, 84, 922–931. [Google Scholar] [CrossRef] [PubMed]
- Natsume, C.; Aoki, N.; Aoyama, T.; Senda, K.; Matsui, M.; Ikegami, A.; Tanaka, K.; Azuma, Y.-T.; Fujita, T. Fucoxanthin Ameliorates Atopic Dermatitis Symptoms by Regulating Keratinocytes and Regulatory Innate Lymphoid Cells. Int. J. Mol. Sci. 2020, 21, 2180. [Google Scholar] [CrossRef] [PubMed]
- Perrone Sibilia, M.D.; de los Ängeles Aldirico, M.; Soto, A.S.; Picchio, M.S.; Sánchez, V.R.; Arcón, N.; Moretta, R.; Martín, V.; Vanzulli, S.; Fenoy, I.M.; et al. Chronic Infection with the Protozoan Toxoplasma Gondii Prevents the Development of Experimental Atopic Dermatitis in Mice. J. Dermatol. Sci. 2019, 96, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.O.; Roh, J.Y.; Jung, Y. Oral Tolerance Inhibits Atopic Dermatitis-like Type 2 Inflammation in Mice by Modulating Immune Microenvironments. Allergy 2017, 72, 397–406. [Google Scholar] [CrossRef]
- Liu, S.; Verma, M.; Michalec, L.; Liu, W.; Sripada, A.; Rollins, D.; Good, J.; Ito, Y.; Chu, H.; Gorska, M.M.; et al. Steroid Resistance of Airway Type 2 Innate Lymphoid Cells from Patients with Severe Asthma: The Role of Thymic Stromal Lymphopoietin. J. Allergy Clin. Immunol. 2018, 141, 257–268.e6. [Google Scholar] [CrossRef]
- Hoy, S.M. Tezepelumab: First Approval. Drugs 2022, 82, 461–468. [Google Scholar] [CrossRef]
- Pelaia, C.; Pelaia, G.; Longhini, F.; Crimi, C.; Calabrese, C.; Gallelli, L.; Sciacqua, A.; Vatrella, A. Monoclonal Antibodies Targeting Alarmins: A New Perspective for Biological Therapies of Severe Asthma. Biomedicines 2021, 9, 1108. [Google Scholar] [CrossRef]
- Kelsen, S.G.; Agache, I.O.; Soong, W.; Israel, E.; Chupp, G.L.; Cheung, D.S.; Theess, W.; Yang, X.; Staton, T.L.; Choy, D.F.; et al. Astegolimab (Anti-ST2) Efficacy and Safety in Adults with Severe Asthma: A Randomized Clinical Trial. J. Allergy Clin. Immunol. 2021, 148, 790–798. [Google Scholar] [CrossRef]
| Authors | Year | Topic | Study Characteristics |
|---|---|---|---|
| Laurent et al. [41] | 2021 | Sistemic sclerosis | Killer Cell Lectin-Like Receptor G (KLRG1)-Group 2 Innate lympohid cells (ILC2s) have the following:
|
| Guggino et al. [42] | 2017 | Sistemic sclerosis | Following the addition of IL-9 in in vitro experiments, mast cells have undergone proliferation and activation with IL-2 production, which is known to activate dermal ILC2s. |
| Wohlfahrt et al. [39] | 2016 | Sistemic sclerosis | Detection of ILC2s in diffuse form and in forms with lung involvement (pulmonary fibrosis). |
| Petrasca et al. [46] | 2023 | Hidradenitis suppurativa | Hidradenitis suppurativa lesional and non-lesional skin significantly increased in the absolute innatel lymphoid cell (ILC) numbers compared to control skin, suggesting the role of ILCs in hidradenitis pathogenesis. |
| Ricardo-Gonzalez et al. [44] | 2022 | Demodex folliculorum | ILC2s elaborated IL-13 that attenuated air follicles and epithelial proliferation; in their absence, Demodex colonization led to increased epithelial proliferation and replacement of gene programs for repair by aberrant inflammation, leading to the loss of barrier function and follicle depletion. |
| Wilhelm et al. [43] | 2016 | Helminthic infections | ILC2s have a dependent fatty acid metabolism for IL-13 production, especially in conditions of vitamin A deficiency. |
| Rak et al. [47] | 2016 | Wound healing | A mouse model and human skin samples model demonstrated that skin injury promotes an IL-33-dependent ILC2 response while the abrogation of this response impairs re-epithelialization and efficient wound closure. |
| Li et al. [48] | 2022 | Wound healing in diabetic mice | IL-33-driven ILC2 response promotes wound repair by activating pro-inflammatory processes during the first phase and subsequently limiting inflammation by promoting the transition to reparative mechanisms. |
| Walton et al. [50] | 2021 | Skin graft rejection | The dual blockade of janus kinase (JAK)-2 and Aurora kinase A reduced rejection due to ILC2s, which are involved in tolerance, tissue regeneration, and repair. AJI-100 maintains adequate levels of phosphorylated Signal transducer and activator of transcription 5 (pSTAT5), which in turn regulates the expression of GATA-binding protein 3 (GATA3), which is required for ILC2 development. |
| Munneke et al. [51] | 2014 | Graft versus host disease | In 51 patient affected by leukemia, ILC2 reduced the susceptibility to therapy-induced mucositis and acute graft versus host disease. |
| Bruce et al. [52] | 2021 | Graft versus host disease | A mouse model highlighted that third-party ILCs significantly improve the survival of mice after allo-hematopoietic stem cell transplantation (HSCT) when given at the time of transplantation. |
| Van der Ploeg et al. [53] | 2020 | Mastocytosis | A total of 21 patients with systemic mastocytosis were compared to 18 healthy controls and it was found that patients with skin involvement and pruritic symptoms had higher ILC2 levels, independent of serum tryptase levels. |
| Authors | Year | Topic | Study Characteristics |
|---|---|---|---|
| Roediger et al. [54] | 2013 | Cutaneous type 2 inflammation | Two functional states of dermal group 2 innate lymphoid cells (ILC2s): steady-state ILC2s with immuno-regulatory action, and stimulated ILC2s with a pro-inflammatory activity. |
| Nakatani-Kusakabe et al. [55] | 2021 | Cutaneous type 2 inflammation |
|
| Leyva-Castillo et al. [56] | 2020 | Cutaneous type 2 inflammation |
|
| Ricardo-Gonzalez et al. [57] | 2018 | Cutaneous type 2 inflammation | Subpopulations of ILC2s expressing receptors for IL-18 could be partly responsible for the most severe forms of allergic skin inflammation and increased levels of IL-18 that correlate with severity of atopic dermatitis. |
| Hsu et al. [59] | 2020 | Cutaneous type 2 inflammation |
|
| Kim et al. [58] | 2014 | Cutaneous type 2 inflammation |
|
| Malhotra et al. [60] | 2018 | Cutaneous type 2 inflammation | Certain polymorphisms in the gene encoding for RORalpha may underlie the genesis of Th2/ILC2-mediated skin inflammatory diseases. |
| Rafei-Shamsabadi et al. [61] | 2018 | Allergic contact dermatitis | ILC2 activation would be critical for type 2 skin inflammation in allergic contact dermatitis, and its lack would promote a type 1 immune response. |
| Shane et al. [62] | 2019 | Skin allergic disease to didecyldimethylammonium chloride | ILC2s could be the potential mediator of immunologic reaction skin allergic diseases with an underlying mixed IgE-mediated and T-cell-mediated immunologic mechanism. |
| Tsai et al. [63] | 2019 | Drug Rash with Eosinophilia and Systemic Symptoms |
|
| Numazaki et al. [64] | 2019 | Cutaneous inflammation | Monoclonal anti-TSLP antibody reduces ILC2 activation and resolves the resistance that ILC2s develop toward steroids. |
| Authors | Year | Study Characteristics |
|---|---|---|
| Nishikawa et al. [67] | 2021 | A mouse model using transgenic binary mice that are constitutively deficient in conventional dendritic cells (cDCs) demonstrated that congenital cDCs deficiency not only exacerbated atopic dermatitis (AD)-related inflammation but also resulted in immune alterations as the composition and function of the cells increased granulocytes and group 2 innate lymphoid cells (ILC2s). |
| Früh et al. [66] | 2020 | In a canine model, levated frequencies and numbers of T-helper 2 cells but not ILC2s in peripheral blood were associated with chronic canine atopy. |
| Imai et al. [78] | 2019 | In a mouse model, ILC2 cells were depleted in IL33tg mice via bone marrow transplantation; in these mice, the development of inflammation was almost completely suppressed, demonstrating the central role of ILC2s in immune processes. |
| Salimi et al. [70] | 2013 | The binding of E-cadherin to human ILC2s dramatically inhibited interleukin (IL)-5 and IL-13 release. In the absence of E-cadherin, which is characteristic of filaggrin insufficiency and feature of AD, ILC2s resulted in increased production of type 2 cytokines. |
| Salimi et al. [74] | 2015 | The expression of NKp30 ILC2s ex vivo and on cultured ILC2s and the interaction with its ligand B7-H6 induced the production of type 2 cytokines in AD. |
| Baek et al. [85] | 2017 | In a mouse model, immune tolerance was induced by oral administration of ovalbumin with subsequent epicutaneous sensitization by repeated application of ovalbumin. The induction of oral tolerance resulted in a reduction in the inflammatory response through the inhibition of ILC2 levels. |
| Perrone Sibilia et al. [84] | 2019 | Chronic infection with Toxoplasma gondii appeared to prevent the development of atopic dermatitis through a reduced susceptibility that appeared to result from changes in the type II innate immune response, with reductions in IL-4, IL-5, and ILC2s. |
| Lee et al. [82] | 2021 | Celastrol decreased the levels of thymic stromal lymphopoietin (TSLP), ILC2s, and T-helper 2-cytokines in AD lesions of house dust mite-stimulated NC/Nga mice. |
| Mashiko et al. [68] | 2017 | AD lesional skin, but not psoriasis skin, was infiltrated by increased numbers of basophils, which produced IL-4,T-helper 2 cells and ILC2s. |
| Schwartz et al. [79] | 2019 | ILC2 deficiency did not improve AD in Flgft/ft mice, which was also independent of IL-4, IL-5, IL-9, IL-13, IL-17A, and IL-22, but required the signaling of IL-1β and IL-1R1, which therefore, also play a fundamental role in the development of inflammation. |
| Natsume et al. [83] | 2020 | A study comparing the anti-itch effect of xanthophyll with tacrolimus observed that the former significantly reduced symptoms compared to the latter through the presence of ILCreg suppressing the immune response. |
| Hardman et al. [73] | 2017 | In a human AD-based model, TSLP-induced ILC2s expressed Cluster of Differentiation 1a (CD1a), which, in turn, activated TH lymphocytes. In addition, ILC2s presented endogenous lipid antigens to CD1a-reactive T cells. |
| Authors | Target | Disease | Immunological Mechanism |
|---|---|---|---|
| Okuyama et al. [38] | IL-33/ST2 interaction | melanoma | Group 2 innate lymphoid cells (ILC2s), once stimulated by interleukin (IL)-33 after binding with their ST2 receptor, play a tumor role activating CD8+ T-cell expression and infiltration into the tumor and inducing an unfavorable tumor environment. |
| Jacquelot et al. [36] | PD1 | melanoma | Inhibition of programmed cell death protein (PD-1) results in increased total ILC2s number. High ILC2s infiltration in human melanoma is associated with a good clinical prognosis. |
| Wagner et al. [37] | Lactic acid | melanoma | Acid lactic inhibits ILC2 proliferation and cytokine production, exerting immunosuppressive activity against ILC2s. Conversely. in knockdown lactate dehydrogenase A B16F10 melanomas (LDHAlow), the number of intratumoral ILC2s increased proportionally. |
| Ricardo-Gonzalez et al. [57] | IL-18 | Cutaneous type 2 inflammation |
|
| Numazaki et al. [64] | TSLP | Cutaneous inflammation | In vitro study demonstrated that monoclonal anti-thymic stromal lymphopoietin (TSLP) receptor antibodies managed to reduce both type 2 and non-type 2 inflammation. |
| Salimi et al. [74] | NKp30 | AD | Cultured ILC2s subset expressing NKp30, after interaction with its activator ligand B7-H6, rapidly produce type 2 cytokines. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borgia, F.; Li Pomi, F.; Alessandrello, C.; Vaccaro, M.; Gangemi, S. Potential Role of Innate Lymphoid Cells in the Pathogenesis and Treatment of Skin Diseases. J. Clin. Med. 2023, 12, 3043. https://doi.org/10.3390/jcm12083043
Borgia F, Li Pomi F, Alessandrello C, Vaccaro M, Gangemi S. Potential Role of Innate Lymphoid Cells in the Pathogenesis and Treatment of Skin Diseases. Journal of Clinical Medicine. 2023; 12(8):3043. https://doi.org/10.3390/jcm12083043
Chicago/Turabian StyleBorgia, Francesco, Federica Li Pomi, Clara Alessandrello, Mario Vaccaro, and Sebastiano Gangemi. 2023. "Potential Role of Innate Lymphoid Cells in the Pathogenesis and Treatment of Skin Diseases" Journal of Clinical Medicine 12, no. 8: 3043. https://doi.org/10.3390/jcm12083043
APA StyleBorgia, F., Li Pomi, F., Alessandrello, C., Vaccaro, M., & Gangemi, S. (2023). Potential Role of Innate Lymphoid Cells in the Pathogenesis and Treatment of Skin Diseases. Journal of Clinical Medicine, 12(8), 3043. https://doi.org/10.3390/jcm12083043










