Characterization of Acne-Prone Skin with Reflectance Confocal Microscopy and Optical Coherence Tomography and Modifications Induced by Topical Treatment and Probiotic Supplementation
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hay, R.J.; Johns, N.E.; Williams, H.C.; Bolliger, I.W.; Dellavalle, R.P.; Margolis, D.J.; Marks, R.; Naldi, L.; Weinstock, M.A.; Wulf, S.K.; et al. The Global Burden of Skin Disease in 2010: An Analysis of the Prevalence and Impact of Skin Conditions. J. Investig. Dermatol. 2014, 134, 1527–1534. [Google Scholar] [CrossRef]
- Tan, J.K.L.; Bhate, K. A Global Perspective on the Epidemiology of Acne. Br. J. Dermatol. 2015, 172, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Zahra Ghodsi, S.; Orawa, H.; Zouboulis, C.C. Prevalence, Severity, and Severity Risk Factors of Acne in High School Pupils: A Community-Based Study. J. Investig. Dermatol. 2009, 129, 2136–2141. [Google Scholar] [CrossRef] [PubMed]
- Williams, H.C.; Dellavalle, R.P.; Garner, S. Acne Vulgaris. Lancet 2012, 379, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Bettoli, V.; Borghi, A.; Zauli, S.; Toni, G.; Ricci, M.; Giari, S.; Virgili, A. Maintenance Therapy for Acne Vulgaris: Efficacy of a 12-Month Treatment with Adapalene-Benzoyl Peroxide after Oral Isotretinoin and a Review of the Literature. Dermatology 2013, 227, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Manfredini, M.; Mazzaglia, G.; Ciardo, S.; Farnetani, F.; Mandel, V.D.; Longo, C.; Zauli, S.; Bettoli, V.; Virgili, A.; Pellacani, G. Acne: In Vivo Morphologic Study of Lesions and Surrounding Skin by Means of Reflectance Confocal Microscopy. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Manfredini, M.; Bettoli, V.; Sacripanti, G.; Farnetani, F.; Bigi, L.; Puviani, M.; Corazza, M.; Pellacani, G. The Evolution of Healthy Skin to Acne Lesions: A Longitudinal, in Vivo Evaluation with Reflectance Confocal Microscopy and Optical Coherence Tomography. J. Eur. Acad. Derm. Venereol. 2019, 33, 1768–1774. [Google Scholar] [CrossRef]
- Fontao, F.; von Engelbrechten, M.; Seilaz, C.; Sorg, O.; Saurat, J.H. Microcomedones in Non-Lesional Acne Prone Skin New Orientations on Comedogenesis and Its Prevention. J. Eur. Acad. Derm. Venereol. 2020, 34, 357–364. [Google Scholar] [CrossRef]
- Cunliffe, W.J.; Holland, D.B.; Clark, S.M.; Stables, G.I. Comedogenesis: Some Aetiological, Clinical and Therapeutic Strategies. Dermatology 2003, 206, 11–16. [Google Scholar] [CrossRef]
- Baek, J.H.; Ahn, H.-J.; Koh, J.S.; Kwon, H.; Shin, M.K. Early Detection of Microcomedones Induced by Cocoa Butter Using Reflectance Confocal Microscopy. J. Cosmet. Derm. 2022, 21, 3016–3021. [Google Scholar] [CrossRef]
- Li, A.; Dubey, S.; Varney, M.L.; Dave, B.J.; Singh, R.K. IL-8 Directly Enhanced Endothelial Cell Survival, Proliferation, and Matrix Metalloproteinases Production and Regulated Angiogenesis. J. Immunol. 2003, 170, 3369–3376. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.K.L.; Stein Gold, L.F.; Alexis, A.F.; Harper, J.C. Current Concepts in Acne Pathogenesis: Pathways to Inflammation. Semin. Cutan. Med. Surg. 2018, 37, S60–S62. [Google Scholar] [CrossRef] [PubMed]
- Platsidaki, E.; Dessinioti, C. Recent Advances in Understanding Propionibacterium Acnes (Cutibacterium Acnes) in Acne. F1000Res 2018, 7, F1000 Faculty Rev-1953. [Google Scholar] [CrossRef] [PubMed]
- Manfredini, M.; Greco, M.; Farnetani, F.; Mazzaglia, G.; Ciardo, S.; Bettoli, V.; Virgili, A.; Pellacani, G. In Vivo Monitoring of Topical Therapy for Acne with Reflectance Confocal Microscopy. Ski. Res. Technol. 2017, 23, 36–40. [Google Scholar] [CrossRef]
- Fuchs, C.S.K.; Ortner, V.K.; Mogensen, M.; Philipsen, P.A.; Haedersdal, M. Transfollicular Delivery of Gold Microparticles in Healthy Skin and Acne Vulgaris, Assessed by in Vivo Reflectance Confocal Microscopy and Optical Coherence Tomography. Lasers Surg. Med. 2019, 51, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Villani, A.; Annunziata, M.C.; Cinelli, E.; Donnarumma, M.; Milani, M.; Fabbrocini, G. Efficacy and Safety of a New Topical Gel Formulation Containing Retinol Encapsulated in Glycospheres and Hydroxypinacolone Retinoate, an Antimicrobial Peptide, Salicylic Acid, Glycolic Acid and Niacinamide for the Treatment of Mild Acne: Preliminary Results of a 2-Month Prospective Study. G. Ital. Derm. Venereol. 2020, 155, 676–679. [Google Scholar] [CrossRef]
- Garofalo, V.; Cannizzaro, M.V.; Mazzilli, S.; Bianchi, L.; Campione, E. Clinical Evidence on the Efficacy and Tolerability of a Topical Medical Device Containing Benzoylperoxide 4%, Retinol 0.5%, Mandelic Acid 1% and Lactobionic Acid 1% in the Treatment of Mild Facial Acne: An Open Label Pilot Study. Clin. Cosmet. Investig. Derm. 2019, 12, 363–369. [Google Scholar] [CrossRef]
- Fuchs, C.S.K.; Andersen, A.J.B.; Ardigo, M.; Philipsen, P.A.; Haedersdal, M.; Mogensen, M. Acne Vulgaris Severity Graded by in Vivo Reflectance Confocal Microscopy and Optical Coherence Tomography. Lasers Surg. Med. 2019, 51, 104–113. [Google Scholar] [CrossRef]
- Guida, S.; Longhitano, S.; Ardigò, M.; Pampena, R.; Ciardo, S.; Bigi, L.; Mandel, V.D.; Vaschieri, C.; Manfredini, M.; Pezzini, C.; et al. Dermoscopy, Confocal Microscopy and Optical Coherence Tomography Features of Main Inflammatory and Autoimmune Skin Diseases: A Systematic Review. Australas. J. Derm. 2021, 63, 15–26. [Google Scholar] [CrossRef]
- Guida, S.; Longo, C.; Casari, A.; Ciardo, S.; Manfredini, M.; Reggiani, C.; Pellacani, G.; Farnetani, F. Update on the Use of Confocal Microscopy in Melanoma and Non-Melanoma Skin Cancer. G. Ital. Dermatol. Venereol. 2015, 150, 547–563. [Google Scholar]
- Farnetani, F.; Manfredini, M.; Chester, J.; Ciardo, S.; Gonzalez, S.; Pellacani, G. Reflectance Confocal Microscopy in the Diagnosis of Pigmented Macules of the Face: Differential Diagnosis and Margin Definition. Photochem. Photobiol. Sci. 2019, 18, 963–969. [Google Scholar] [CrossRef]
- Lupu, M.; Malciu, A.M.; Voiculescu, V.M. Feasibility of Reflectance Confocal Microscopy Monitoring in Oily, Acne-Prone Facial Skin Treated with a Topical Combination of Alpha and Beta-Hydroxy Acids, Anti-Inflammatory Molecules, and Herculane Thermal Water: A Blinded, One-Month Study. Life 2022, 12, 1973. [Google Scholar] [CrossRef] [PubMed]
- Ciardo, S.; Pezzini, C.; Guida, S.; Del Duca, E.; Ungar, J.; Guttman-Yassky, E.; Manfredini, M.; Farnetani, F.; Longo, C.; Pellacani, G. A Plea for Standardization of Confocal Microscopy and Optical Coherence Tomography Parameters to Evaluate Physiological and Para-Physiological Skin Conditions in Cosmetic Science. Exp. Derm. 2021, 30, 911–922. [Google Scholar] [CrossRef] [PubMed]
- Manfredini, M.; Liberati, S.; Ciardo, S.; Bonzano, L.; Guanti, M.; Chester, J.; Kaleci, S.; Pellacani, G. Microscopic and Functional Changes Observed with Dynamic Optical Coherence Tomography for Severe Refractory Atopic Dermatitis Treated with Dupilumab. Ski. Res. Technol. 2020, 26, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Muguet Guenot, L.; Vourc’h Jourdain, M.; Saint-Jean, M.; Corvec, S.; Gaultier, A.; Khammari, A.; Le Moigne, M.; Boisrobert, A.; Paugam, C.; Dréno, B. Confocal Microscopy in Adult Women with Acne. Int. J. Dermatol. 2018, 57, 278–283. [Google Scholar] [CrossRef]
- Alma, A.; Sticchi, A.; Chello, C.; Guida, S.; Farnetani, F.; Chester, J.; Bettoli, V.; Pellacani, G.; Manfredini, M. Dermoscopy, Reflectance Confocal Microscopy and Optical Coherence Tomography Features of Acne: A Systematic Review. J. Clin. Med. 2022, 11, 1783. [Google Scholar] [CrossRef]
- Barnard, E.; Shi, B.; Kang, D.; Craft, N.; Li, H. The Balance of Metagenomic Elements Shapes the Skin Microbiome in Acne and Health. Sci. Rep. 2016, 6, 39491. [Google Scholar] [CrossRef]
- Yan, H.-M.; Zhao, H.-J.; Guo, D.-Y.; Zhu, P.-Q.; Zhang, C.-L.; Jiang, W. Gut Microbiota Alterations in Moderate to Severe Acne Vulgaris Patients. J. Derm. 2018, 45, 1166–1171. [Google Scholar] [CrossRef]
- Kwon, H.-K.; Lee, C.-G.; So, J.-S.; Chae, C.-S.; Hwang, J.-S.; Sahoo, A.; Nam, J.H.; Rhee, J.H.; Hwang, K.-C.; Im, S.-H. Generation of Regulatory Dendritic Cells and CD4+Foxp3+ T Cells by Probiotics Administration Suppresses Immune Disorders. Proc. Natl. Acad. Sci. USA 2010, 107, 2159–2164. [Google Scholar] [CrossRef]
- Resta-Lenert, S.; Barrett, K.E. Probiotics and Commensals Reverse TNF-Alpha- and IFN-Gamma-Induced Dysfunction in Human Intestinal Epithelial Cells. Gastroenterology 2006, 130, 731–746. [Google Scholar] [CrossRef]
- Marasca, C.; Ruggiero, A.; Cacciapuoti, S.; Fabbrocini, G.; Annunziata, M.C. Probiotic Supplement Combined with Topical Therapy in the Treatment of Mild to Moderate Acne: Results from an Italian Single Centre Interventional Study. Ital. J. Derm. Venerol. 2022, 157, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Fabbrocini, G.; Bertona, M.; Picazo, Ó.; Pareja-Galeano, H.; Monfrecola, G.; Emanuele, E. Supplementation with Lactobacillus Rhamnosus SP1 Normalises Skin Expression of Genes Implicated in Insulin Signalling and Improves Adult Acne. Benef. Microbes 2016, 7, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Werner, R.N.; Stockfleth, E.; Connolly, S.M.; Correia, O.; Erdmann, R.; Foley, P.; Gupta, A.K.; Jacobs, A.; Kerl, H.; Lim, H.W.; et al. Evidence- and Consensus-Based (S3) Guidelines for the Treatment of Actinic Keratosis—International League of Dermatological Societies in Cooperation with the European Dermatology Forum—Short Version. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 2069–2079. [Google Scholar] [CrossRef] [PubMed]
- Alsulaimani, H.; Kokandi, A.; Khawandanh, S.; Hamad, R. Severity of Acne Vulgaris: Comparison of Two Assessment Methods. Clin. Cosmet. Investig. Derm. 2020, 13, 711–716. [Google Scholar] [CrossRef]
- Manfredini, M.; Greco, M.; Farnetani, F.; Ciardo, S.; De Carvalho, N.; Mandel, V.D.; Starace, M.; Pellacani, G. Acne: Morphologic and Vascular Study of Lesions and Surrounding Skin by Means of Optical Coherence Tomography. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1541–1546. [Google Scholar] [CrossRef] [PubMed]
- Thiboutot, D.M.; Shalita, A.R.; Yamauchi, P.S.; Dawson, C.; Kerrouche, N.; Arsonnaud, S.; Kang, S. Adapalene Gel, 0.1%, as Maintenance Therapy for Acne Vulgaris: A Randomized, Controlled, Investigator-Blind Follow-up of a Recent Combination Study. Arch. Derm. 2006, 142, 597–602. [Google Scholar] [CrossRef]
- Samuels, D.V.; Rosenthal, R.; Lin, R.; Chaudhari, S.; Natsuaki, M.N. Acne Vulgaris and Risk of Depression and Anxiety: A Meta-Analytic Review. J. Am. Acad. Dermatol. 2020, 83, 532–541. [Google Scholar] [CrossRef]
- Katsambas, A.; Graupe, K.; Stratigos, J. Clinical Studies of 20% Azelaic Acid Cream in the Treatment of Acne Vulgaris. Comparison with Vehicle and Topical Tretinoin. Acta Derm. Venereol. Suppl. 1989, 143, 35–39. [Google Scholar]
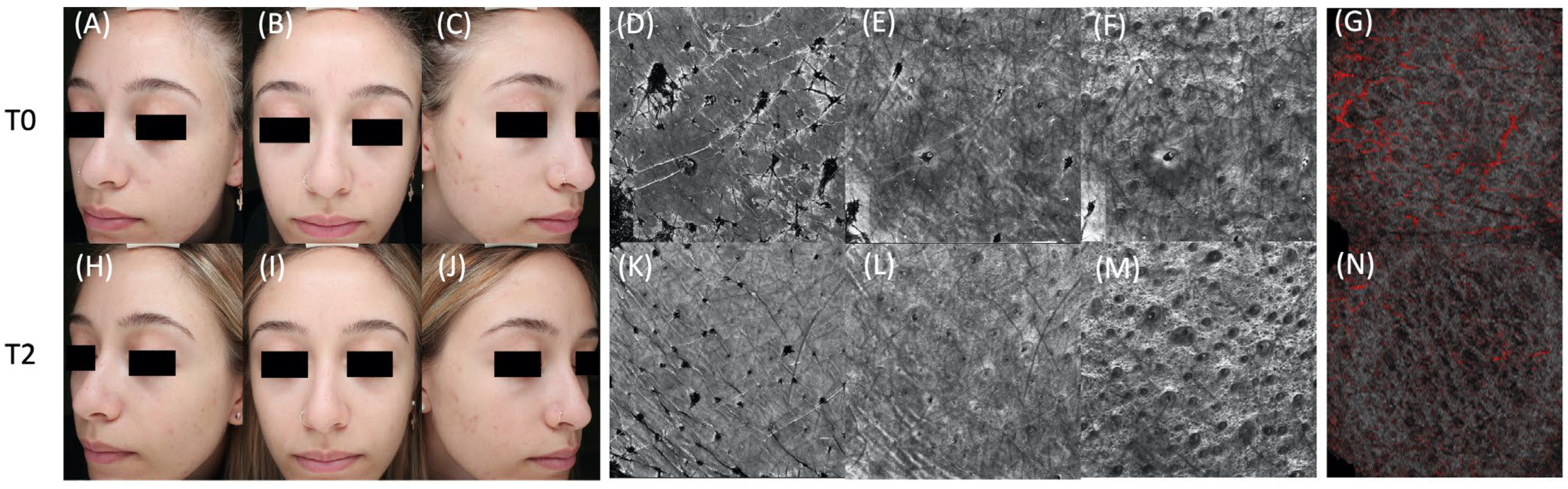
| Characteristic, Mean (SD) | T0, Baseline | T1, 4 Weeks | T2, 12 Weeks | ||||
|---|---|---|---|---|---|---|---|
| Revised Leeds Score | 1.45 | (0.51) | 1.35 | (0.49) | 1.25 | (0.44) | |
| IGA grade | 0.90 | (0.31) | 0.90 | (0.31) | 0.85 | (0.36) | |
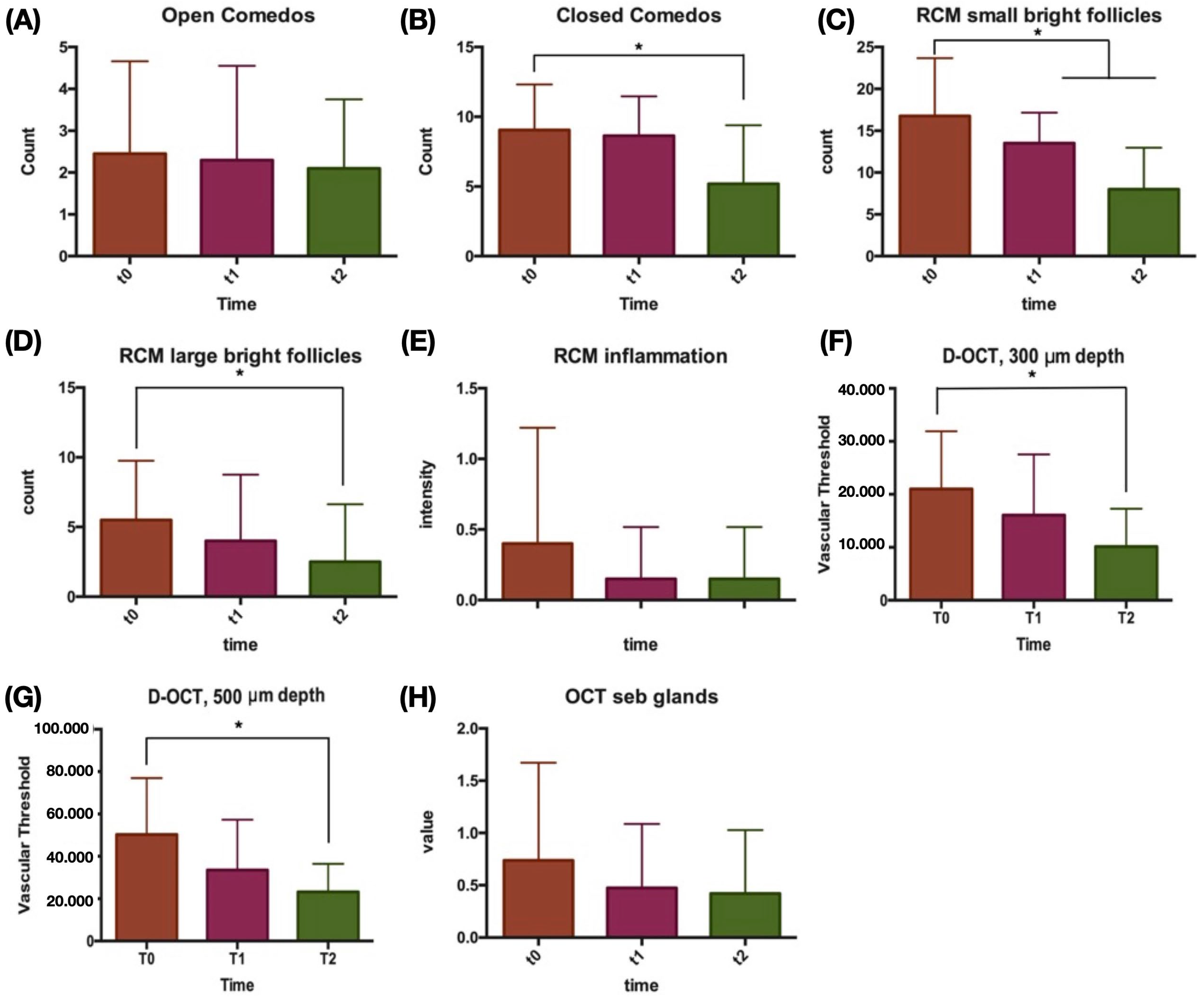
| Non-inflammatory lesions | 11.50 | (4.25) | 10.95 | (3.05) | 7.30 *§ | (5.18) | |
| Open comedos | 2.45 | (2.21) | 2.30 | (2.25) | 2.10 | (1.65) | |
| Closed comedos | 9.05 | (3.87) | 8.65 | (2.81) | 5.20 *§ | (4.19) | |
| RCM features | |||||||
| Small bright follicles | 16.75 | (6.93) | 13.50 * | (3.66) | 8.00 *§ | (4.97) | |
| Large bright follicles | 5.50 | (4.26) | 4.00 | (4.76) | 2.50 * | (4.14) | |
| Inflammatory features (0–3) | 0.40 | (0.82) | 0.15 | (0.37) | 0.15 | (0.37) | |
| D-OCT features | |||||||
| Vascular threshold density | |||||||
| 300 um | 21,030.63 | (10,887.40) | 16,078.06 | (11,465.61) | 10,152.80 * | (7132.32) | |
| 500 um | 50,319.21 | (26,633.76) | 33,548.19 | (23,747.34) | 23,238.80 * | (13,232.31) | |
| Sebaceous gland hypertrophy (0–3) | 0.74 | (0.93) | 0.47 | (0.61) | 0.42 | (0.61) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manfredini, M.; Sticchi, A.; Lippolis, N.; Pedroni, G.; Giovani, M.; Ciardo, S.; Chello, C.; Guida, S.; Farnetani, F.; Pellacani, G. Characterization of Acne-Prone Skin with Reflectance Confocal Microscopy and Optical Coherence Tomography and Modifications Induced by Topical Treatment and Probiotic Supplementation. J. Clin. Med. 2023, 12, 4787. https://doi.org/10.3390/jcm12144787
Manfredini M, Sticchi A, Lippolis N, Pedroni G, Giovani M, Ciardo S, Chello C, Guida S, Farnetani F, Pellacani G. Characterization of Acne-Prone Skin with Reflectance Confocal Microscopy and Optical Coherence Tomography and Modifications Induced by Topical Treatment and Probiotic Supplementation. Journal of Clinical Medicine. 2023; 12(14):4787. https://doi.org/10.3390/jcm12144787
Chicago/Turabian StyleManfredini, Marco, Alberto Sticchi, Nicola Lippolis, Gioia Pedroni, Matteo Giovani, Silvana Ciardo, Camilla Chello, Stefania Guida, Francesca Farnetani, and Giovanni Pellacani. 2023. "Characterization of Acne-Prone Skin with Reflectance Confocal Microscopy and Optical Coherence Tomography and Modifications Induced by Topical Treatment and Probiotic Supplementation" Journal of Clinical Medicine 12, no. 14: 4787. https://doi.org/10.3390/jcm12144787
APA StyleManfredini, M., Sticchi, A., Lippolis, N., Pedroni, G., Giovani, M., Ciardo, S., Chello, C., Guida, S., Farnetani, F., & Pellacani, G. (2023). Characterization of Acne-Prone Skin with Reflectance Confocal Microscopy and Optical Coherence Tomography and Modifications Induced by Topical Treatment and Probiotic Supplementation. Journal of Clinical Medicine, 12(14), 4787. https://doi.org/10.3390/jcm12144787






