Computed Tomography Lung Density Analysis: An Imaging Biomarker Predicting Physical Inactivity in Chronic Obstructive Pulmonary Disease: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Subjects
2.2. PA Evaluations
2.3. CT Scanning and Lung Density Analysis
2.4. Statistical Analyses
3. Results
3.1. Healthy Subjects vs. Patients with COPD
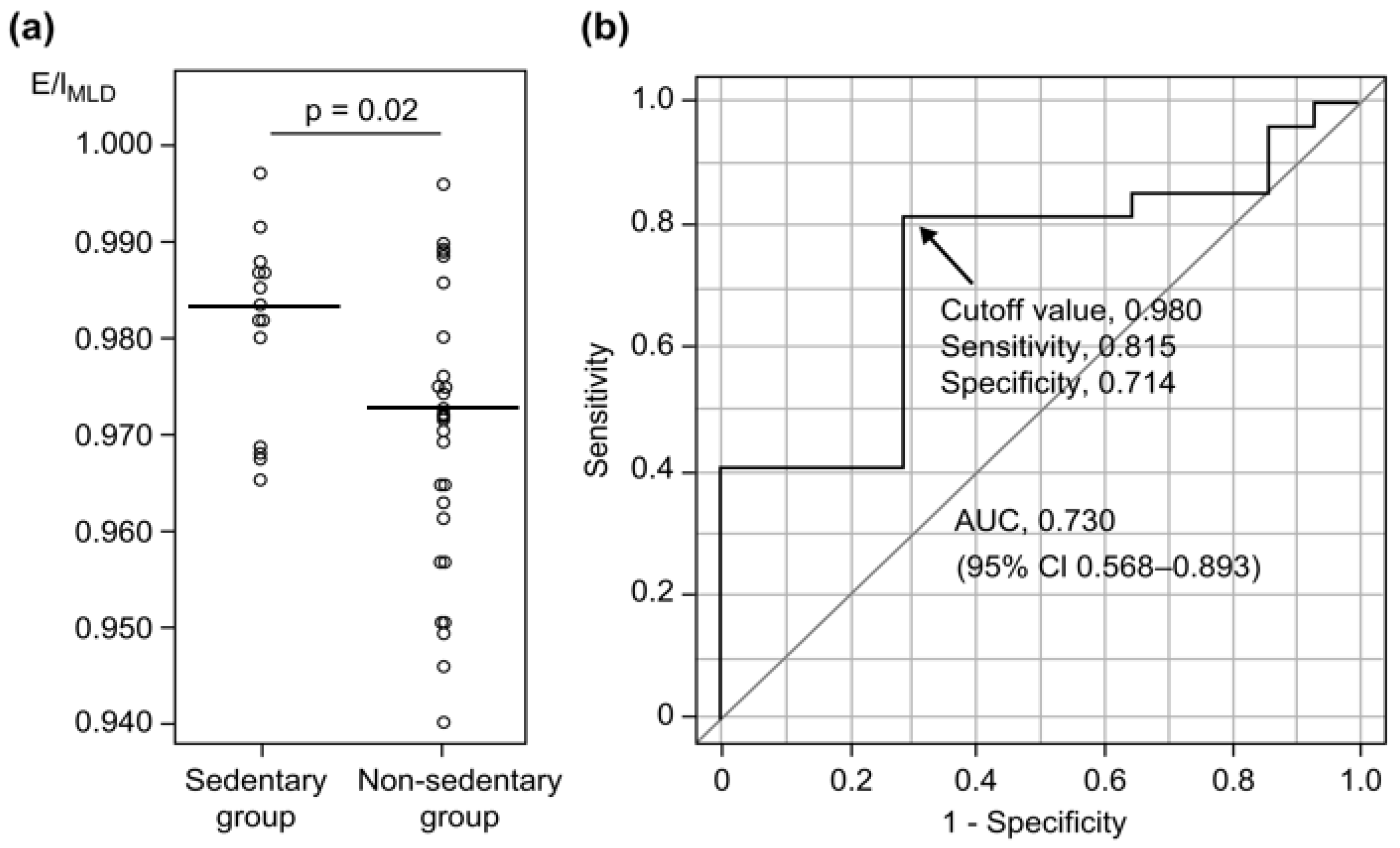
3.2. Predictive Factors for Detecting Sedentary Behavior in COPD
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pitta, F.; Troosters, T.; Spruit, M.A.; Probst, V.S.; Decramer, M.; Gosselink, R. Characteristics of physical activities in daily life in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2005, 171, 972–977. [Google Scholar] [CrossRef] [PubMed]
- Pitta, F.; Troosters, T.; Probst, V.S.; Spruit, M.A.; Decramer, M.; Gosselink, R. Physical activity and hospitalization for exacerbation of COPD. Chest 2006, 129, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Waschki, B.; Kirsten, A.; Holz, O.; Müller, K.C.; Meyer, T.; Watz, H.; Magnussen, H. Physical activity is the strongest predictor of all-cause mortality in patients with COPD: A prospective cohort study. Chest 2011, 140, 331–342. [Google Scholar] [CrossRef]
- Garcia-Rio, F.; Rojo, B.; Casitas, R.; Lores, V.; Madero, R.; Romero, D.; Galera, R.; Villasante, C. Prognostic value of the objective measurement of daily physical activity in patients with COPD. Chest 2012, 142, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Gimeno-Santos, E.; Frei, A.; Steurer-Stey, C.; de Batlle, J.; Rabinovich, R.A.; Raste, Y.; Hopkinson, N.S.; Polkey, M.I.; van Remoortel, H.; Troosters, T.; et al. Determinants and outcomes of physical activity in patients with COPD: A systematic review. Thorax 2014, 69, 731–739. [Google Scholar] [CrossRef]
- Hirano, T.; Matsunaga, K.; Hamada, K.; Uehara, S.; Suetake, R.; Yamaji, Y.; Oishi, K.; Asami, M.; Edakuni, N.; Ogawa, H.; et al. Combination of assist use of short-acting beta-2 agonists inhalation and guidance based on patient-specific restrictions in daily behaviour: Impact on physical activity of Japanese patients with chronic obstructive pulmonary disease. Respir. Investig. 2019, 57, 133–139. [Google Scholar] [CrossRef]
- Gouzi, F.; Préfaut, C.; Abdellaoui, A.; Vuillemin, A.; Molinari, N.; Ninot, G.; Caris, G.; Hayot, M. Evidence of an early physical activity reduction in chronic obstructive pulmonary disease patients. Arch. Phys. Med. Rehabil. 2011, 92, 1611–1617.e2. [Google Scholar] [CrossRef]
- Gawlitza, J.; Henzler, T.; Trinkmann, F.; Nekolla, E.; Haubenreisser, H.; Brix, G. COPD imaging on a 3rd generation dual-source CT: Acquisition of paired inspiratory-expiratory chest scans at an overall reduced radiation risk. Diagnostics 2020, 10, 1106. [Google Scholar] [CrossRef]
- Eda, S.; Kubo, K.; Fujimoto, K.; Matsuzawa, Y.; Sakai, F. The relations between expiratory chest CT using helical CT and pulmonary function tests in emphysema. Am. J. Respir. Crit. Care Med. 1997, 155, 1290–1294. [Google Scholar] [CrossRef]
- Kubo, K.; Eda, S.; Yamamoto, H.; Fujimoto, K.; Matsuzawa, Y.; Hasegawa, Y.; Sone, S.; Sakai, F. Expiratory and inspiratory chest computed tomography and pulmonary function tests in cigarette smokers. Eur. Respir. J. 1999, 13, 252–256. [Google Scholar] [CrossRef]
- O’Donnell, R.A.; Peebles, C.; Ward, J.A.; Daraker, A.; Angco, G.; Broberg, P.; Pierrou, S.; Lund, J.; Holgate, S.T.; Davies, D.E.; et al. Relationship between peripheral airway dysfunction, airway obstruction, and neutrophilic inflammation in COPD. Thorax 2004, 59, 837–842. [Google Scholar] [CrossRef]
- Mets, O.M.; Murphy, K.; Zanen, P.; Gietema, H.A.; Lammers, J.W.; van Ginneken, B.; Prokop, M.; de Jong, P.A. The relationship between lung function impairment and quantitative computed tomography in chronic obstructive pulmonary disease. Eur. Radiol. 2012, 22, 120–128. [Google Scholar] [CrossRef]
- Hersh, C.P.; Washko, G.R.; Estépar, R.S.J.; Lutz, S.; Friedman, P.J.; Han, M.K.; Hokanson, J.E.; Judy, P.F.; Lynch, D.A.; Make, B.J.; et al. Paired inspiratory-expiratory chest CT scans to assess for small airways disease in COPD. Respir. Res. 2013, 14, 42. [Google Scholar] [CrossRef]
- Mets, O.M.; Zanen, P.; Lammers, J.W.; Isgum, I.; Gietema, H.A.; van Ginneken, B.; Prokop, M.; de Jong, P.A. Early identification of small airways disease on lung cancer screening CT: Comparison of current air trapping measures. Lung 2012, 190, 629–633. [Google Scholar] [CrossRef]
- Hogg, J.C.; Chu, F.; Utokaparch, S.; Woods, R.; Elliott, W.M.; Buzatu, L.; Cherniack, R.M.; Rogers, R.M.; Sciurba, F.C.; Coxson, H.O.; et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N. Engl. J. Med. 2004, 24, 2645–2653. [Google Scholar] [CrossRef]
- Hogg, J.C.; Paré, P.D.; Hackett, T.L. The contribution of small airway obstruction to the pathogenesis of chronic obstructive pulmonary disease. Physiol. Rev. 2017, 97, 529–552. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Rio, F.; Lores, V.; Mediano, O.; Rojo, B.; Hernanz, A.; López-Collazo, E.; Alvarez-Sala, R. Daily physical activity in patients with chronic obstructive pulmonary disease is mainly associated with dynamic hyperinflation. Am. J. Respir. Crit. Care Med. 2009, 180, 506–512. [Google Scholar] [CrossRef]
- Lahaije, A.J.; ven Helvoort, H.A.; Dekhuijzen, P.N.; Vercoulen, J.H.; Heijdra, Y.F. Resting and ADL-induced dynamic hyperinflation explain physical inactivity in COPD better than FEV1. Respir. Med. 2013, 107, 834–840. [Google Scholar] [CrossRef]
- Hartman, J.E.; Boezen, H.M.; de Greef, M.H.; Ten Hacken, N.H. Physical and psychosocial factors associated with physical activity in patients with chronic obstructive pulmonary disease. Arch. Phys. Med. Rehabil. 2013, 94, 2396–2402.e7. [Google Scholar] [CrossRef]
- Troosters, T.; van der Molen, T.; Polkey, M.; Rabinovich, R.A.; Vogiatzis, I.; Weisman, I.; Kulich, K. Improving physical activity in COPD: Towards a new paradigm. Respir. Res. 2013, 14, 115. [Google Scholar] [CrossRef] [PubMed]
- Watz, H.; Waschki, B.; Meyer, T.; Magnussen, H. Physical activity in patients with COPD. Eur. Respir. J. 2009, 33, 262–272. [Google Scholar] [CrossRef]
- Haskell, W.L.; Lee, I.M.; Pate, R.R.; Powell, K.E.; Blair, S.N.; Franklin, B.A.; Macera, C.A.; Heath, G.W.; Thompson, P.D.; Bauman, A. Physical activity and public health: Updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation 2007, 116, 1081–1093. [Google Scholar] [CrossRef]
- Tremblay, M.S.; Aubert, S.; Barnes, J.D.; Saunders, T.J.; Carson, V.; Latimer-Cheung, A.E.; Chastin, S.F.M.; Altenburg, T.M.; Chinapaw, M.J.M. Sedentary Behavior Research Network (SBRN)—Terminology Consensus Project process and outcome. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 75. [Google Scholar] [CrossRef] [PubMed]
- Bodduluri, S.; Reinhardt, J.M.; Hoffman, E.A.; Newell, J.D., Jr.; Nath, H.; Dransfield, M.T.; Bhatt, S.P. Signs of gas trapping in normal lung density Regions in smokers. Am. J. Respir. Crit. Care Med. 2017, 196, 1404–1410. [Google Scholar] [CrossRef] [PubMed]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Bommart, S.; Marin, G.; Bourdin, A.; Molinari, N.; Klein, F.; Hayot, M.; Vachier, I.; Chanez, P.; Mercier, J.; Vernhet-Kovacsik, H. Relationship between CT air trapping criteria and lung function in small airway impairment quantification. BMC Pulm. Med. 2014, 14, 29. [Google Scholar] [CrossRef]
- Hayata, A.; Minakata, Y.; Matsunaga, K.; Nakanishi, M.; Yamamoto, N. Differences in physical activity according to mMRC grade in patients with COPD. Int. J. Chronic Obstr. Pulm. Dis. 2016, 11, 2203–2208. [Google Scholar]
- van Gestel, A.J.; Clarenbach, C.F.; Stöwhas, A.C.; Rossi, V.A.; Sievi, N.A.; Camen, G.; Russi, E.W.; Kohler, M. Predicting daily physical activity in patients with chronic obstructive pulmonary disease. PLoS ONE 2012, 7, e48081. [Google Scholar] [CrossRef]
- Minakata, Y.; Sugino, A.; Kanda, M.; Ichikawa, T.; Akamatsu, K.; Koarai, A.; Hirano, T.; Nakanishi, M.; Sugiura, H.; Matsunaga, K.; et al. Reduced level of physical activity in Japanese patients with chronic obstructive pulmonary disease. Respir. Investig. 2014, 52, 41–48. [Google Scholar] [CrossRef]
- Hamakawa, Y.; Tanabe, N.; Shima, H.; Terada, K.; Shiraishi, Y.; Maetani, T.; Kubo, T.; Kozawa, S.; Koizumi, K.; Kanezaki, M.; et al. Associations of pulmonary and extrapulmonary computed tomographic manifestations with impaired physical activity in symptomatic patients with chronic obstructive pulmonary disease. Sci. Rep. 2022, 12, 5608. [Google Scholar] [CrossRef]
- Mantoani, L.C.; Rubio, N.; McKinstry, B.; MacNee, W.; Rabinovich, R.A. Interventions to modify physical activity in patients with COPD: A systematic review. Eur. Respir. J. 2016, 48, 69–81. [Google Scholar] [CrossRef]
- O’Donnell, D.E.; Casaburi, R.; Vincken, W.; Puente-Maestu, L.; Swales, J.; Lawrence, D.; Kramer, B. Effect of indacaterol on exercise endurance and lung hyperinflation in COPD. Respir. Med. 2011, 105, 1030–1036. [Google Scholar] [CrossRef]
- Hataji, O.; Naito, M.; Ito, K.; Watanabe, F.; Gabazza, E.C.; Taguchi, O. Indacaterol improves daily physical activity in patients with chronic obstructive pulmonary disease. Int. J. Chronic Obstr. Pulm. Dis. 2013, 8, 1–5. [Google Scholar]
- Troosters, T.; Sciurba, F.C.; Decramer, M.; Siafakas, N.M.; Klioze, S.S.; Sutradhar, S.C.; Weisman, I.M.; Yunis, C. Tiotropium in patients with moderate COPD naive to maintenance therapy: A randomised placebo-controlled trial. NPJ Prim. Care Respir. Med. 2014, 24, 14003. [Google Scholar] [CrossRef]
- Beeh, K.M.; Watz, H.; Puente-Maestu, L.; de Teresa, L.; Jarreta, D.; Caracta, C.; Garcia, G.E.; Magnussen, H. Aclidinium improves exercise endurance, dyspnea, lung hyperinflation, and physical activity in patients with COPD: A randomized, placebo-controlled, crossover trial. BMC Pulm. Med. 2014, 14, 209. [Google Scholar] [CrossRef]
- Nishijima, Y.; Minami, S.; Yamamoto, S.; Ogata, Y.; Koba, T.; Futami, S.; Komuta, K. Influence of indacaterol on daily physical activity in patients with untreated chronic obstructive pulmonary disease. Int. J. Chronic Obstr. Pulm. Dis. 2015, 10, 439–444. [Google Scholar]
- Watz, H.; Mailänder, C.; Baier, M.; Kirsten, A. Effects of indacaterol/glycopyrronium (QVA149) on lung hyperinflation and physical activity in patients with moderate to severe COPD: A randomised, placebo-controlled, crossover study (The MOVE Study). BMC Pulm. Med. 2016, 16, 95. [Google Scholar] [CrossRef]
- Ichinose, M.; Minakata, Y.; Motegi, T.; Ueki, J.; Gon, Y.; Seki, T.; Anzai, T.; Nakamura, S.; Hirata, K. Efficacy of tiotropium/olodaterol on lung volume, exercise capacity, and physical activity. Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 1407–1419. [Google Scholar] [CrossRef]
- Takahashi, K.; Uchida, M.; Kato, G.; Takamori, A.; Kinoshita, T.; Yoshida, M.; Tajiri, R.; Kojima, K.; Inoue, H.; Kobayashi, H.; et al. First-line treatment with tiotropium/olodaterol improves physical activity in patients with treatment naive chronic obstructive pulmonary disease. Int. J. Chronic Obstr. Pulm. Dis. 2020, 15, 2115–2126. [Google Scholar] [CrossRef]
- Nici, L.; Mammen, M.J.; Charbek, E.; Alexander, P.E.; Au, D.H.; Boyd, C.M.; Criner, G.J.; Donaldson, G.C.; Dreher, M.; Fan, V.S.; et al. Pharmacologic management of chronic obstructive pulmonary disease. An Official American Thoracic Society Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2020, 201, e56–e69. [Google Scholar] [CrossRef]
- Pitta, F.; Troosters, T.; Probst, V.S.; Langer, D.; Decramer, M.; Gosselink, R. Are patients with COPD more active after pulmonary rehabilitation? Chest 2008, 134, 273–280. [Google Scholar] [CrossRef]
- Wats, H.; Pitta, F.; Rochester, C.L.; Garcia-Aymerich, J.; ZuWallack, R.; Troosters, T.; Vaes, A.W.; Puhan, M.A.; Jehn, M.; Polkey, M.I.; et al. An official European Respiratory Society statement on physical activity in COPD. Eur. Repsir. J. 2014, 44, 1521–1537. [Google Scholar] [CrossRef] [PubMed]
- Celli, B.; Vestbo, J.; Jenkins, C.R.; Jones, P.W.; Ferguson, G.T.; Calverley, P.M.; Yates, J.C.; Anderson, J.A.; Willits, L.R.; Wise, R.A. Sex differences in mortality and clinical expressions of patients with chronic obstructive pulmonary disease. The TORCH experience. Am. J. Respir. Crit. Care Med. 2011, 183, 317–322. [Google Scholar] [CrossRef]
- Dransfield, M.T.; Washko, G.R.; Foreman, M.G.; Estepar, R.S.; Reilly, J.; Bailey, W.C. Gender differences in the severity of CT emphysema in COPD. Chest 2007, 132, 464–470. [Google Scholar] [CrossRef]
- Sánchez Castillo, S.; Smith, L.; Díaz Suárez, A.; López Sánchez, G.F. Physical activity behaviour in people with COPD residing in Spain: A cross-sectional analysis. Lung 2019, 197, 769–775. [Google Scholar] [CrossRef] [PubMed]
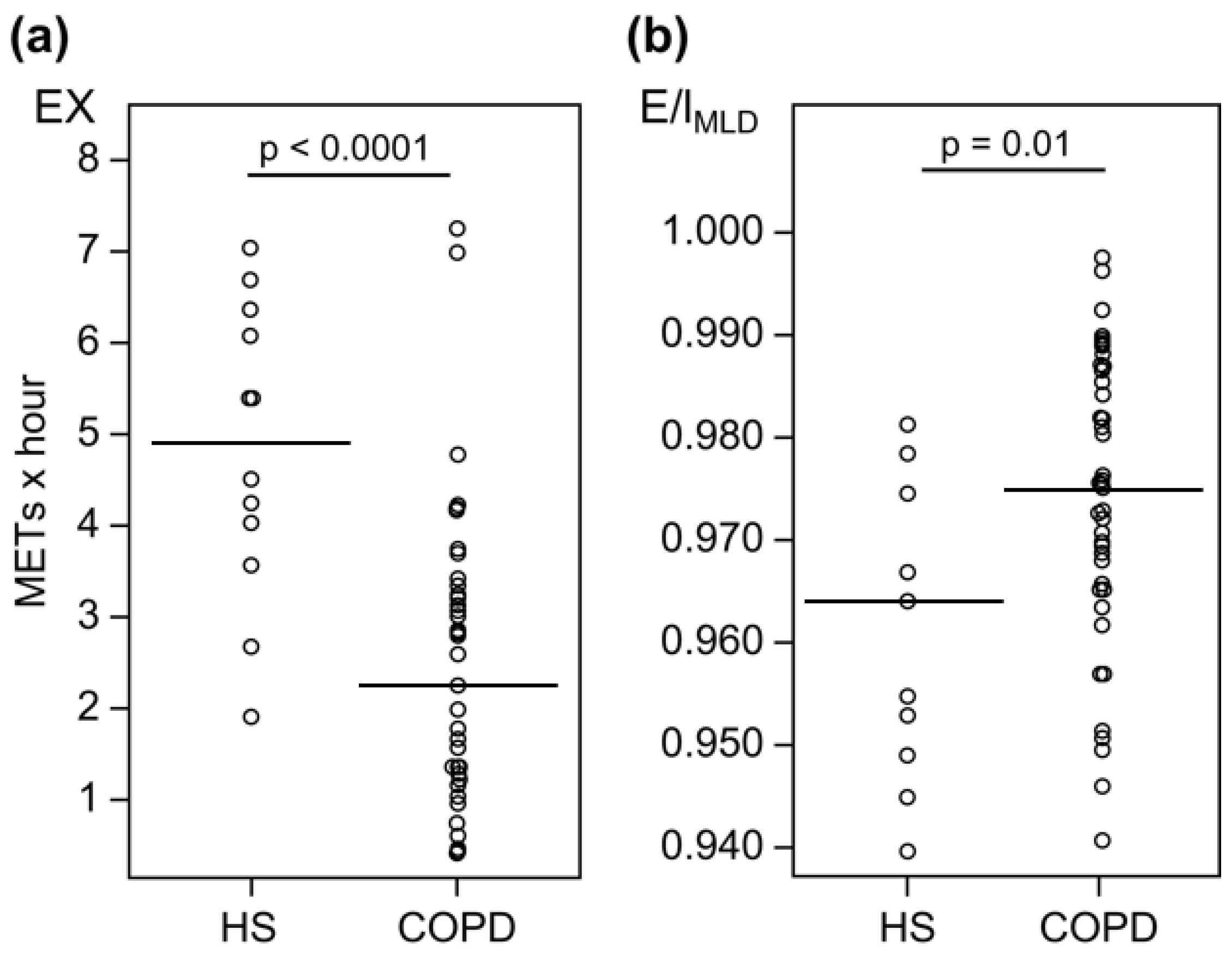

| HS (n = 12) | COPD (n = 41) | p-Value | |
|---|---|---|---|
| Sex (M/F) | 6/6 | 41/0 | <0.0001 |
| Age (year) | 62 (56–70) | 71 (67–74) | 0.02 |
| BMI (kg/m2) | 21.4 (19.8–23.8) | 22.8 (20.4–24.4) | 0.33 |
| Smoking index (pack-year) | 10 (0.0–31) | 45 (29–103) | 0.0004 |
| CAT | 4 (3.5–6.3) | 11 (7.8–14.5) | 0.001 |
| mMRC Dyspnea Scale (0/1/2/3/4) | 6/6/0/0/0 | 17/15/5/3/0 | 0.62 |
| FEV1 (L) | 2.65 (2.39–3.16) | 2.21 (1.90–2.46) | 0.002 |
| FEV1/FVC (%) | 78.2 (75.8–86.9) | 63.6 (58.0–66.4) | <0.0001 |
| FEV1 % pred (%) | 110 (103–115) | 77.0 (65.9–85.6) | <0.0001 |
| GOLD stage (1/2/3/4) | - | 19/20/2/0 | - |
| FVC % pred (%) | 110 (105–120) | 103 (88.6–118) | 0.08 |
| RV % pred (%) | 114 (103–117) | 105 (98.7–128) | 0.84 |
| RV/TLC % pred (%) | 100 (90.7–113) | 90.7 (80.7–102) | 0.46 |
| IC/TLC (%) | 44.4 (42.5–49.6) | 41.4 (34.1–45.8) | 0.18 |
| %DLCO (%) | 105 (96.8–125) | 90.1 (75.8–109) | 0.09 |
| %DLCO/VA (%) | 94.6 (89.9–105) | 75.0 (65.1–102) | 0.18 |
| E/IMLD | |||
|---|---|---|---|
| All Subjects (n = 53) | Healthy (n = 12) | COPD (n = 41) | |
| >1 MET duration (min) | −0.18 | −0.02 | −0.03 |
| >2 MET duration (min) | −0.21 | −0.31 | −0.15 |
| >3 MET duration (min) | −0.37 † | −0.31 | −0.31 * |
| >4 MET duration (min) | −0.37 † | −0.46 | −0.36 * |
| Exercise (METs × hour) | −0.36 † | −0.32 | −0.32 * |
| Sedentary Group (n = 14) | Nonsedentary Group (n = 27) | p-Value | |
|---|---|---|---|
| Sex (M/F) | 14/0 | 27/0 | - |
| Age (years) | 75.5 (72.0–80.8) | 68.0 (66.0–72.o) | 0.01 |
| BMI (kg/m2) | 24.1 (22.0–24.7) | 22.7 (20.4–23.9) | 0.42 |
| Smoking index (pack-year) | 52.9 (41.3–61.5) | 38.0 (22.8–51.5) | 0.04 |
| CAT | 9.5 (7.0–11.8) | 12.0 (9.3–16.0) | 0.16 |
| mMRC Dyspnea Scale (0/1/2/3/4) | 3/7/4/0/0 | 14/8/1/3/0 | 0.02 |
| FEV1/FVC (%) | 61.6 (58.1–66.2) | 63.7 (58.5–66.8) | 0.69 |
| FEV1 % pred (%) | 74.4 (58.6–83.8) | 80.9 (69.8–90.2) | 0.26 |
| GOLD stage (1/2/3/4) | 5/9/0/0 | 14/11/2/0 | 0.33 |
| FVC % pred (%) | 98.0 (80.6–121) | 104 (93.3–113) | 0.67 |
| RV % pred (%) | 115 (103–132) | 104 (96.1–121) | 0.34 |
| RV/TLC % pred (%) | 94.3 (81.2–107) | 88.8 (80.7–102) | 0.87 |
| IC/TLC (%) | 39.0 (33.5–41.8) | 44.3 (35.8–47.4) | 0.10 |
| %DLCO (%) | 92.2 (76.3–109) | 89.5 (76.2–109) | 0.90 |
| %DLCO/VA (%) | 68.0 (60.6–85.8) | 85.1 (70.1–107) | 0.11 |
| Step per hour | 59.1 (49.0–61.5) | 64.4 (52.9–73.3) | 0.17 |
| 6MWD (m) | 386 (375–417) | 412 (372–459) | 0.21 |
| LAA (%) | 22.2 (20.9–22.8) | 22.5 (20.9–25.6) | 0.75 |
| E/IMLD | 0.983 (0.972–0.987) | 0.972 (0.959–0.976) | 0.02 |
| Variable | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Age (years) | 0.89 (0.80–0.99) | 0.03 | 0.87 (0.75–1.00) | 0.05 |
| CAT | 1.05 (0.95–1.15) | 0.34 | 1.15 (0.99–1.33) | 0.07 |
| FEV1 % pred (%) | 1.02 (0.98–1.05) | 0.38 | 1.04 (0.92–1.10) | 0.21 |
| %DLCO (%) | 1.00 (0.98–1.03) | 0.78 | 0.96 (0.92–1.01) | 0.11 |
| E/IMLD (%) | 0.47 (0.25–0.87) | 0.02 | 0.39 (0.16–0.95) | 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murata, Y.; Hirano, T.; Doi, K.; Fukatsu-Chikumoto, A.; Hamada, K.; Oishi, K.; Kakugawa, T.; Yano, M.; Matsunaga, K. Computed Tomography Lung Density Analysis: An Imaging Biomarker Predicting Physical Inactivity in Chronic Obstructive Pulmonary Disease: A Pilot Study. J. Clin. Med. 2023, 12, 2959. https://doi.org/10.3390/jcm12082959
Murata Y, Hirano T, Doi K, Fukatsu-Chikumoto A, Hamada K, Oishi K, Kakugawa T, Yano M, Matsunaga K. Computed Tomography Lung Density Analysis: An Imaging Biomarker Predicting Physical Inactivity in Chronic Obstructive Pulmonary Disease: A Pilot Study. Journal of Clinical Medicine. 2023; 12(8):2959. https://doi.org/10.3390/jcm12082959
Chicago/Turabian StyleMurata, Yoriyuki, Tsunahiko Hirano, Keiko Doi, Ayumi Fukatsu-Chikumoto, Kazuki Hamada, Keiji Oishi, Tomoyuki Kakugawa, Masafumi Yano, and Kazuto Matsunaga. 2023. "Computed Tomography Lung Density Analysis: An Imaging Biomarker Predicting Physical Inactivity in Chronic Obstructive Pulmonary Disease: A Pilot Study" Journal of Clinical Medicine 12, no. 8: 2959. https://doi.org/10.3390/jcm12082959
APA StyleMurata, Y., Hirano, T., Doi, K., Fukatsu-Chikumoto, A., Hamada, K., Oishi, K., Kakugawa, T., Yano, M., & Matsunaga, K. (2023). Computed Tomography Lung Density Analysis: An Imaging Biomarker Predicting Physical Inactivity in Chronic Obstructive Pulmonary Disease: A Pilot Study. Journal of Clinical Medicine, 12(8), 2959. https://doi.org/10.3390/jcm12082959





